Oncolytic Viruses and Hematological Malignancies: A New Class of Immunotherapy Drugs
Abstract
1. Introduction
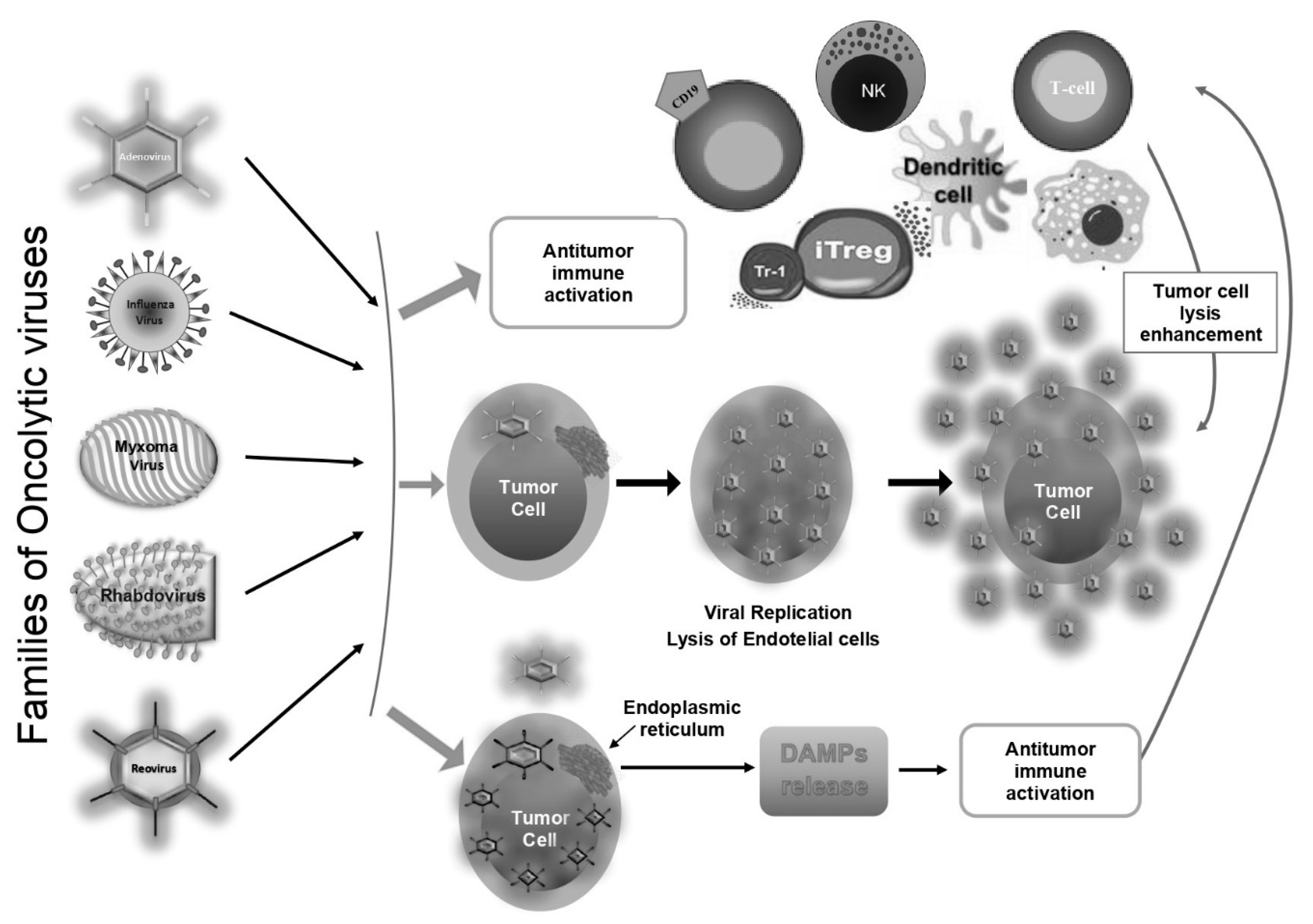
1.1. General Considerations on Oncolytic Viruses
1.2. Families of Oncolytic Viruses
2. Antitumoral Action of Oncolytic Viruses
2.1. Oncolytic Viruses and Haematological Malignancies
Oncolytic Viruses and Multiple Myeloma
2.2. Oncolytic Viruses and Acute Myeloid Leukemia
2.3. Oncolytic Viruses, Chronic Myeloid Leukemia, and Chronic Lymphocytic Leukemia
2.4. Oncolytic Viruses and Lymphomas
2.5. Oncolytic Viruses in Transplantation and Graft vs. Host
3. Oncolytic Viruses: Possible Combination Therapies
4. Limitations of Oncolytic Virus Therapy
| Study | Disease | Virus | Possible Disadvantages | Ref. |
|---|---|---|---|---|
| In vitro | Multiple Myeloma cell line | Measles virus | [78] | |
| Multiple myeloma and breast cancer cells | Adenovirus | [81,82,83] | ||
| Multiple myeloma cell lines | Reovirus | [85] | ||
| In vivo | Multiple Myeloma | Adenovirus | Induction of proinflammatory cytokines. Neutralization by serum factors Sequestration in liver and spleen | [83,182] |
| Multiple myeloma | Reovirus | Unknown | [85] | |
| Multiple myeloma | Measles virus | Increased unwanted pathology | [80,182] | |
| In vitro | Acute Myeloid Leukemia | Measles virus | [93] | |
| Acute myeloid leukemia | Reovirus | [95] | ||
| Acute myeloid leukemia, FLT3 mutant acute myeloid leukemia cells | Myxoma virus | [98,99,100] | ||
| Kasumi-1 (AML), SD-1 (BCR-ABL-positive ALL) | Cytomegalovirus | [101] | ||
| Acute myeloid leukemia cells | Coxsackievirus | [105] | ||
| Kasumi-1, KG-1, HL-60, U937 AML cell lines | Adenovirus | [106,107] | ||
| High-burden multidrug-resistant AML cells A549, HEPG2, Huh-7 cell lines | Non-replicating rhabdovirus-derived particles, Vesicular Stomatitis Virus | [109,111] | ||
| Wild-type leukemia cells, Multiple myeloma cell lines | Vaccinia virus | [115] | ||
| Baby hamster kidney-21 cells | Herpes Simplex Virus-1 | [118] | ||
| In vivo | Acute Myeloid Leukemia | Adenovirus | Sequestration in liver and spleen | [107] |
| Acute myeloid leukemia | Vesicular Stomatitis Virus | Neurotoxicity | [111,182] | |
| Acute myeloid leukemia cells | Measles virus | Increased unwanted pathology | [116] | |
| In vitro | Chronic Myeloid Leukemia cells | Adenovirus | [119] | |
| Human and canine lymphomas | Newcastle disease virus | [126] | ||
| Burkitt lymphoma cells, Cutaneous T-cell lymphoma | Measles virus | [128,142] | ||
| Burkitt’s tumor cells, Chronic lymphocytic leukemia | Reovirus type 3 | [130,134] | ||
| Chronic lymphocytic leukemia | Adenovirus | [137] | ||
| In vivo | Mantle cell lymphoma, Cutaneous T-cell lymphoma | Measles virus | Increased unwanted pathology | [140,142,182] |
| Non Hodgkin lymphoma- A20 lymphoma | Sindbis virus | Unknown | [144] |
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| OVs | Oncolytic viruses |
| IFNs | Interferons |
| TLR | Toll-like receptors |
| Ad | Adenovirus |
| MYXV | Myxoma virus |
| VSV | Vesicular Stomatitis Virus |
| MYC | Myelocytomatosis |
| eIF2B | Eukaryotic translation initiation factor 2B subunit alpha |
| Rae1 | ribonucleic acid export 1 |
| Nup98 | Nucleoporin 98 |
| eIF4E | Eukaryotic translation initiation factor 4E |
| KEAP1 | Kelch-like ECH-associated protein 1 |
| ROS | Reactive oxygen species |
| NRF2 | nuclear factor erythroid 2-related factor 2 |
| PGAM5 | Phosphoglycerate mutase 5 |
| ER | Endoplasmic reticulum |
| DAMPs | Damage associate molecular pattern |
| APCs | Antigen presenting cells |
| DCs | Dendritic cells |
| TNF-α | Tumour necrosis factor-α |
| IL-1 | Interleukin 1 |
| NK | Natural killer |
| RIPK3 | Receptor Interacting Protein Kinase 3 |
| ZBP1 | Z-DNA binding protein 1 |
| cIAP2 | cellular inhibitor of apoptosis protein 2 |
| CAR | Coxsackie adenovirus receptor |
| CRAds | Conditionally Replicative Adenoviruses |
| T-VEC | Talimogene laherparepvec |
| ECHO | 7 ECHO virus type 7 |
| HSV-1 | Herpes simplex virus 1 |
| GM-CSF | Granulocyte-macrophage colony-stimulating factor |
| MM | multiple myeloma |
| MV | Measles virus |
| MVNIS | Measles virus encoding the human thyroidal sodium iodide symporter |
| MTD | Maximal tolerated dose |
| RRMM | Multiple myeloma relapsed or refractory |
| CR | Complete remission |
| MUC1 | Mucin 1 |
| TK | thymidine kinase |
| AdEHCD40L | CD40 ligand transgene |
| SCID | Severe combined immunodeficiency disease (SCID) |
| NF-κB | nuclear factor kappa-light-chain-enhancer of activated B cells |
| TAAs | Tumour-associated antigens |
| PBMC | peripheral blood mononuclear cell |
| MAGE | Melanoma-associated antigen |
| AML | Acute myeloid leukemia |
| GFP | Green fluorescent protein |
| BM | Bone marrow |
| MOI | Minimum multiplicity of infection |
| CMV | Cytomegalovirus |
| CVA21 | Coxsackievirus A21 |
| DAF | Decay Accelerating Factor |
| ICAM-1 | Intercellular Adhesion Molecule 1 |
| TRAIL | TNF-related apoptosis-inducing ligand (TRAIL) |
| A4 | rAd5pz-zTRAIL-RFP-SΔ24E1a |
| ZA4 | Zipper-like dimerization domain |
| RVs | Rhabdoviruses |
| NRRPs | Non-proliferating rhabdovirus-originated particles |
| PD-L1 | Programmed death ligand 1 |
| Ab | Antibody |
| Mcl-1 | Myeloid cell leukemia 1 protein |
| Bcl-2 | B-cell lymphoma 2 |
| Bax | Bcl-2-associated X protein |
| Bak | Bcl-2 homologous antagonist killer |
| OVV | Oncolytic vaccinia virus |
| ALT | Alternative lengthening of telomeres |
| PML NBs | Promyelocytic leukemia nuclear bodies |
| CML | Chronic myeloid leukaemia |
| NDV | Newcastle disease virus |
| CLL | Chronic lymphocytic leukemia |
| ADCC | Antibody-dependent cellular cytotoxicity |
| MCL | Mantle cell lymphoma |
| CPA | Cyclophosphamide |
| CTCLs | Cutaneous T-cell lymphomas |
| EBV | Epstein-Barr Virus |
| ICP0 | Infected cell protein 0 |
| GvHD | Graft-versus-host disease |
| allo-HSCT | Allogeneic Hematopoietic Cell Transplantation |
| HDAC | Histone deacetylase HDAC |
| VPA | Valproic acid |
| NKG2D | Natural Killer-gene complex 2D |
| CTLA-4 | Cytotoxic T lymphocyte antigen 4 |
| CAR | Chimeric Antigen Receptor |
| EMA | European Medicines Agency |
| FDA | Food and Drug Administration |
References
- Kelly, E.; Russell, S.J. History of oncolytic viruses: Genesis to genetic engineering. Mol. Ther. 2007, 15, 651–659. [Google Scholar] [CrossRef] [PubMed]
- Lawler, S.E.; Speranza, M.C.; Cho, C.F.; Chiocca, E.A. Oncolytic Viruses in Cancer Treatment: A Review. JAMA Oncol. 2017, 3, 841–849. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.H.; Pabon, C.; Alsayed, Y.; Huang, P.P.; Jandeska, S.; Uddin, S.; Platanias, L.C.; Rosen, S.T. Interferon-alpha resistance in a cutaneous T-cell lymphoma cell line is associated with lack of STAT1 expression. Blood 1998, 91, 570–576. [Google Scholar] [CrossRef] [PubMed]
- Wong, L.H.; Krauer, K.G.; Hatzinisiriou, I.; Estcourt, M.J.; Hersey, P.; Tam, N.D.; Edmondson, S.; Devenish, R.J.; Ralph, S.J. Interferon-resistant human melanoma cells are deficient in ISGF3 components, STAT1, STAT2, and p48-ISGF3gamma. Interferon-resistant human melanoma cells are deficient in ISGF3 components, STAT1, STAT2, and p48-ISGF3gamma. J. Biol. Chem. 1997, 272, 28779–28785. [Google Scholar] [CrossRef] [PubMed]
- Kaufman, J.L.; Kohlhapp, F.J.; Zloza, A. Oncolytic viruses: A new class of immunotherapy drugs. Nat. Rev. Drug Discov. 2015, 14, 642–662. [Google Scholar] [CrossRef]
- Coffey, M.C.; Strong, J.E.; Forsyth, P.A.; Lee, P.W. Reovirus therapy of tumors with activated Ras pathway. Science 1998, 282, 1332–1334. [Google Scholar] [CrossRef]
- MacLean, A.R.; Ul-Fareed, M.; Robertson, L.; Harland, J.; Brown, S.M. Herpes simplex virus type 1 deletion Variants 1714 and 1716 pinpoint neurovirulence-related sequences in Glasgow strain 17+ between immediate early gene 1 and the ‘a’ sequence. J. Gen. Virol. 1991, 72, 631–639. [Google Scholar] [CrossRef]
- Bolovan, C.A.; Sawtell, N.M.; Thompson, R.L. ICP34.5 mutants of herpes simplex virus type 1 strain 17syn+ are attenuated for neurovirulence in mice and for replication in confluent primary mouse embryo cell cultures. J. Virol. 1994, 68, 48–55. [Google Scholar] [CrossRef]
- Mineta, T.; Rabkin, S.D.; Yazaki, T.; Hunter, W.D.; Martuza, R.L. Attenuated multi-mutated herpes simplex virus-1 for the treatment of malignant gliomas. Nat. Med. 1995, 1, 938–943. [Google Scholar] [CrossRef]
- Wein, L.M.; Wu, J.T.; Kirn, D.H. Validation and analysis of a mathematical model of a replication-competent oncolytic virus for cancer treatment: Implications for virus design and delivery. Cancer Res. 2003, 63, 1317–1324. [Google Scholar]
- Sato-Dahlman, M.; Yamamoto, M. The development of oncoltyic adenovirus therapy in the past and future—For the case of pancreatic cancer. Curr. Cancer Drug Targets 2018, 18, 153–161. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.S.; Bishop, E.S.; Zhang, R.; Yu, X.; Farina, E.M.; Yan, S.; Zhao, C.; Zeng, Z.; Shu, Y.; Wu, X.; et al. Adenovirus-mediated gene delivery: Potential applications for gene and cell-based therapies in the new era of personalized medicine. Genes Dis. 2017, 4, 43–63. [Google Scholar] [CrossRef] [PubMed]
- Shenk, T. Adenoviridae: The viruses and their replication. In Virology; Fields, B., Knipe, D., Howley, P., Eds.; Lipponcott-Raven: Philadelphia, PA, USA, 1996; Volume 2, pp. 2111–2148. [Google Scholar]
- Robert-Guroff, M. Replicating and non-replicating viral vectors for vaccine development. Curr. Opin. Biotechnol. 2007, 18, 546–555. [Google Scholar] [CrossRef] [PubMed]
- Bouvier, N.M.; Palese, P. The biology of influenza viruses. Vaccine 2008, 2, D49–D53. [Google Scholar] [CrossRef]
- Su, S.; Fu, X.; Li, G.; Kerlin, F.; Veit, M. Novel influenza D virus: Epidemiology, pathology, evolution and biological characteristics. Virulence 2017, 8, 1580–1591. [Google Scholar] [CrossRef]
- Bardsley, S.A. An account of the epidemic catarrhal fever, or influenza, in Manchester, together with some general remarks on this and similar epidemics. Med. Phys. J. 1803, 9, 522–531. [Google Scholar]
- Talon, J.; Salvatore, M.; O’Neill, R.E.; Nakaya, Y.; Zheng, H.; Muster, T.; García-Sastre, A.; Palese, P. Influenza A and B viruses expressing altered NS1 proteins: A vaccine approach. Proc. Natl. Acad. Sci. USA 2000, 97, 4309–4314. [Google Scholar] [CrossRef]
- Kim, M.; Williamson, C.T.; Prudhomme, J.; Bebb, D.G.; Riabowol, K.; Lee, P.W.; Lees-Miller, S.P.; Mori, Y.; Rahman, M.M.; McFadden, G.; et al. The viral tropism of two distinct oncolytic viruses, reovirus and myxoma virus, is modulated by cellular tumor suppressor gene status. Oncogene 2010, 29, 3990–3996. [Google Scholar] [CrossRef]
- Barber, G.N. Vesicular stomatitis virus as an oncolytic vector. Viral. Immunol. 2004, 17, 516–527. [Google Scholar] [CrossRef]
- Balachandran, S.; Porosnicu, M.; Barber, G.N. Oncolytic activity of vesicular stomatitis virus is effective against tumors exhibiting aberrant p53, Ras, or myc function and involves the induction of apoptosis. J. Virol. 2001, 75, 3474–3479. [Google Scholar] [CrossRef]
- Stojdl, D.F.; Lichty, B.; Knowles, S.; Marius, R.; Atkins, H.; Sonenberg, N.; Bell, J.C. Exploiting tumor-specific defects in the interferon pathway with a previously unknown oncolytic virus. Nat. Med. 2000, 6, 821–825. [Google Scholar] [CrossRef] [PubMed]
- Balachandran, S.; Barber, G.N. Defective translational control facilitates vesicular stomatitis virus oncolysis. Cancer Cell 2004, 5, 51–65. [Google Scholar] [CrossRef]
- Barber, G.N. VSV-tumor selective replication and protein translation. Oncogene 2005, 24, 7710–7719. [Google Scholar] [CrossRef] [PubMed]
- Renukaradhya, G.J.; Khan, M.A.; Shaji, D.; Brutkiewicz, R.R. The VSV matrix protein impairs CD1d-mediated antigen presentation through activation of the p38 MAPK pathway. J. Virol. 2008, 82, 12535–12542. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Black, B.L.; Lyles, D.S. Vesicular stomatitis virus matrix protein inhibits host cell-directed transcription of target genes in vivo. J. Virol. 1992, 66, 4058–4064. [Google Scholar] [CrossRef]
- Ahmed, M.; Lyles, D.S. Effect of vesicular stomatitis virus matrix protein on transcription directed by host RNA polymerases I, II and III. J. Virol. 1998, 72, 8413–8419. [Google Scholar] [CrossRef] [PubMed]
- Faria, P.A.; Chakraborty, P.; Levay, A.; Barber, G.N.; Ezelle, H.J.; Enninga, J.; Arana, C.; van Deursen, J.; Fontoura, B.M.A. VSV disrupts the Rae1/mrnp41 mRNA nuclear export pathway. Mol. Cell 2005, 17, 93–102. [Google Scholar] [CrossRef]
- Petersen, J.M.; Her, L.S.; Varvel, V.; Lund, E.; Dahlberg, J.E. The matrix protein of vesicular stomatitis virus inhibits nucleocytoplasmic transport when it is in the nucleus and associated with nuclear pore complexes. Mol. Cell Biol. 2000, 20, 8590–8601. [Google Scholar] [CrossRef]
- Petersen, J.M.; Her, L.S.; Dahlberg, J.E. Multiple vesiculoviral matrix proteins inhibit both nuclear export and import. Proc. Natl. Acad. Sci. USA 2001, 98, 8590–8595. [Google Scholar] [CrossRef]
- Von Kobbe, C.; van Deursen, J.M.; Rodrigues, J.P.; Sitterlin, D.; Bachi, A.; Wu, X.; Wilm, M.; Carmo-Fonseca, M.; Izaurralde, E. Vesicular stomatitis virus matrix protein inhibits host cell gene expression by targeting the nucleoporin Nup98. Mol. Cell 2000, 6, 1243–1252. [Google Scholar] [CrossRef]
- Enninga, J.; Levy, D.E.; Blobel, G.; Fontoura, B.M. Role of nucleoporin induction in releasing an mRNA nuclear export block. Science 2002, 295, 1523–1525. [Google Scholar] [CrossRef]
- Connor, J.H.; Lyles, D.S. Vesicular stomatitis virus infection alters the eIF4F translation initiation complex and causes dephosphorylation of the eIF4E binding protein 4E-BP1. J. Virol. 2002, 76, 10177–10187. [Google Scholar] [CrossRef]
- Chen, A.; Zhang, Y.; Meng, G.; Jiang, D.; Zhang, H.; Zheng, M.; Xia, M.; Jiang, A.; Wu, J.; Beltinger, C.; et al. Oncolytic measles virus enhances antitumour responses of adoptive CD8+NKG2D+ cells in hepatocellular carcinoma treatment. Sci. Rep. 2017, 7, 5170. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Liu, T.; Chen, J. Oncolytic Measles Virus Encoding Interleukin-12 Mediated Antitumor Activity and Immunologic Control of Colon Cancer In Vivo and Ex Vivo. Cancer Biother. Radiopharm. 2020. [Google Scholar] [CrossRef] [PubMed]
- Rafailidis, P.I.; Mourtzoukou, E.G.; Varbobitis, I.C.; Falagas, M.E. Severe cytomegalovirus infection in apparently immunocompetent patients: A systematic review. Virol. J. 2008, 5, 47. [Google Scholar] [CrossRef] [PubMed]
- Herbein, G.; Nehme, Z. Tumor Control by Cytomegalovirus: A Door Open for Oncolytic Virotherapy? Mol. Ther. Oncolytics. 2020, 17, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Gao, L.; Yeagy, B.; Reid, T. Virus combinations and chemotherapy for the treatment of human cancers. Curr. Opin. Mol. Ther. 2008, 10, 371–379. [Google Scholar] [PubMed]
- Burman, B.; Pesci, G.; Zamarin, D. Newcastle Disease Virus at the Forefront of Cancer Immunotherapy. Cancers 2020, 12, 3552. [Google Scholar] [CrossRef]
- Hviid, A.; Rubin, S.; Muhlemann, K. Mumps. Lancet 2008, 371, 932–944. [Google Scholar] [CrossRef]
- Son, H.A.; Zhang, L.; Cuong, B.K.; Van Tong, H.; Cuong, L.D.; Hang, N.T.; Nhung, H.T.M.; Yamamoto, N.; Toan, N.L. Combination of Vaccine-Strain Measles and Mumps Viruses Enhances Oncolytic Activity against Human Solid Malignancies. Cancer Investig. 2018, 36, 106–117. [Google Scholar] [CrossRef]
- Ilkow, C.S.; Marguerie, M.; Batenchuk, C.; Mayer, J.; Ben Neriah, D.; Cousineau, S.; Falls, T.; Jennings, V.A.; Boileau, M.; Bellamy, D.; et al. Reciprocal cellular cross-talk within the tumor microenvironment promotes oncolytic virus activity. Nat. Med. 2015, 21, 530–536. [Google Scholar] [CrossRef] [PubMed]
- Simpson, G.R.; Relph, K.; Harrington, K.; Melcher, A.; Pandha, H. Cancer immunotherapy via combining oncolytic virotherapy with chemotherapy: Recent advances. Oncolytic Virother. 2016, 5, 1–13. [Google Scholar] [PubMed]
- Imbesi, S.; Musolino, C.; Allegra, A.; Saija, A.; Morabito, F.; Calapai, G.; Gangemi, S. Oxidative stress in oncohematologic diseases: An update. Expert. Rev. Hematol. 2013, 6, 317–325. [Google Scholar] [CrossRef] [PubMed]
- Musolino, C.; Allegra, A.; Saija, A.; Alonci, A.; Russo, S.; Spatari, G.; Penna, G.; Gerace, D.; Cristani, M.; David, A.; et al. Changes in advanced oxidation protein products, advanced glycation end products, and s-nitrosylated proteins, in patients affected by polycythemia vera and essential thrombocythemia. Clin. Biochem. 2012, 45, 1439–1443. [Google Scholar] [CrossRef] [PubMed]
- Allegra, A.; Pioggia, G.; Tonacci, A.; Musolino, C.; Gangemi, S. Oxidative Stress and Photodynamic Therapy of Skin Cancers: Mechanisms, Challenges and Promising Developments. Antioxidants 2020, 9, 448. [Google Scholar] [CrossRef]
- Gangemi, S.; Allegra, A.; Alonci, A.; Cristani, M.; Russo, S.; Speciale, A.; Penna, G.; Spatari, G.; Cannavò, A.; Bellomo, G.; et al. Increase of novel biomarkers for oxidative stress in patients with plasma cell disorders and in multiple myeloma patients with bone lesions. Inflamm. Res. 2012, 61, 1063–1067. [Google Scholar] [CrossRef]
- Musolino, C.; Allegra, A.; Alonci, A.; Saija, A.; Russo, S.; Cannavò, A.; Cristani, M.; Centorrino, R.; Saitta, S.; Alibrandi, A.; et al. Carbonyl group serum levels are associated with CD38 expression in patients with B chronic lymphocytic leukemia. Clin. Biochem. 2011, 44, 1487–1490. [Google Scholar] [CrossRef]
- Gangemi, S.; Allegra, A.; Aguennouz, M.; Alonci, A.; Speciale, A.; Cannavò, A.; Cristani, M.; Russo, S.; Spatari, G.; Alibrandi, A.; et al. Relationship between advanced oxidation protein products, advanced glycation end products, and S-nitrosylated proteins with biological risk and MDR-1 polymorphisms in patients affected by B-chronic lymphocytic leukemia. Cancer Investig. 2012, 30, 20–26. [Google Scholar] [CrossRef]
- Holze, C.; Michaudel, C.; Mackowiak, C.; Haas, D.A.; Benda, C.; Hubel, P.; Pennemann, F.L.; Schnepf, D.; Wettmarshausen, J.; Braun, M.; et al. Oxeiptosis, a ROS-induced caspase-independent apoptosis-like cell-death pathway. Nat. Immunol. 2018, 19, 130–140. [Google Scholar] [CrossRef]
- Malfitano, A.M.; Di Somma, S.; Iannuzzi, C.A.; Pentimalli, F.; Portella, G. Virotherapy: From single agents to combinatorial treatments. Biochem. Pharmacol. 2020, 177, 113986. [Google Scholar] [CrossRef]
- Garg, A.D.; Galluzzi, L.; Apetoh, L.; Baert, T.; Birge, R.B.; Bravo-San Pedro, J.M.; Breckpot, K.; Brough, D.; Chaurio, R.; Cirone, M.; et al. Molecular and Translational Classifications of DAMPs in Immunogenic Cell Death. Front. Immunol. 2015, 6, 588. [Google Scholar] [CrossRef]
- Berghe, T.V.; Linkermann, A.; Jouan-Lanhouet, S.; Walczak, H.; Vandenabeele, P. Regulated necrosis: The expanding network of non-apoptotic cell death pathways. Nat. Rev. Mol. Cell Biol. 2014, 15, 135–147. [Google Scholar] [CrossRef] [PubMed]
- Kabiljo, J.; Harpain, F.; Carotta, S.; Bergmann, M. Radiotherapy as a backbone for novel concepts in cancer immunotherapy. Cancers 2019, 12, 79. [Google Scholar] [CrossRef] [PubMed]
- Snyder, A.G.; Hubbard, N.W.; Messmer, M.N.; Kofman, S.B.; Hagan, C.E.; Orozco, S.L.; Chiang, K.; Daniels, B.P.; Baker, D.; Oberst, A. Intratumoral activation of the necroptotic pathway components RIPK1 and RIPK3 potentiates antitumor immunity. Sci. Immunol. 2019, 4, eaaw2004. [Google Scholar] [CrossRef] [PubMed]
- Kuriakose, T.; Man, S.M.; Malireddi, R.K.; Karki, R.; Kesavardhana, S.; Place, D.E.; Neale, G.; Vogel, P.; Kanneganti, T.D. ZBP1/DAI is an innate sensor of influenza virus triggering the NLRP3 inflammasome and programmed cell death pathways. Sci. Immunol. 2016, 1, aag2045. [Google Scholar] [CrossRef]
- Rodrigue-Gervais, I.G.; Labbé, K.; Dagenais, M.; Dupaul-Chicoine, J.; Champagne, C.; Morizot, A.; Skeldon, A.; Brincks, E.L.; Vidal, S.M.; Griffith, T.S.; et al. Cellular inhibitor of apoptosis protein cIAP2 protects against pulmonary tissue necrosis during influenza virus infection to promote host survival. Cell Host Microbe 2014, 15, 23–35. [Google Scholar] [CrossRef]
- Larson, C.; Oronsky, B.; Scicinski, J.; Fanger, G.R.; Stirn, M.; Oronsky, A.; Reid, T.R. Going viral: A review of replication-selective oncolytic adenoviruses. Oncotarget 2015, 6, 19976–19989. [Google Scholar] [CrossRef]
- Short, J.J.; Curiel, D.T. Oncolytic adenoviruses targeted to cancer stem cells. Mol. Cancer Ther. 2009, 8, 2096–2102. [Google Scholar] [CrossRef]
- Rivera, A.A.; Davydova, J.; Schierer, S.; Wang, M.; Krasnykh, V.; Yamamoto, M.; Curiel, D.T.; Nettelbeck, D.M. Combining high selectivity of replication with fiber chimerism for effective adenoviral oncolysis of CAR-negative melanoma cells. Gene Ther. 2004, 11, 1694–1702. [Google Scholar] [CrossRef]
- Ranki, T.; Hemminki, A. Serotype chimeric human adenoviruses for cancer gene therapy. Viruses 2010, 2, 2196–2212. [Google Scholar] [CrossRef]
- Hermiston, T. Gene delivery from replication-selective viruses: Arming guided missiles in the war against cancer. J. Clin. Investig. 2000, 105, 1169–1172. [Google Scholar] [CrossRef]
- Liu, X.Y.; Qiu, S.B.; Zou, W.G.; Pei, Z.F.; Gu, J.F.; Luo, C.X.; Ruan, H.M.; Chen, Y.; Qi, Y.P.; Qian, C. Effective genevirotherapy for complete eradication of tumor mediated by the combination of hTRAIL (TNFSF10) and plasminogen k5. Mol. Ther. 2005, 11, 531–541. [Google Scholar] [CrossRef] [PubMed]
- De Graaf, J.F.; de Vor, L.; Fouchier, R.A.M.; van den Hoogen, B.G. Armed oncolytic viruses: A kick-start for anti-tumor immunity. Cytokine Growth Factor Rev. 2018, 41, 28–39. [Google Scholar] [CrossRef] [PubMed]
- Weiss, R.; Sachet, M.; Zinngrebe, J.; Aschacher, T.; Krainer, M.; Hegedus, B.; Walczak, H.; Bergmann, M. IL-24 sensitizes tumor cells to TLR3-mediated apoptosis. Cell Death Differ. 2013, 20, 823–833. [Google Scholar] [CrossRef] [PubMed]
- Weiss, R.; Laengle, J.; Sachet, M.; Shurygina, A.P.; Kiselev, O.; Egorov, A.; Bergmann, M. Interleukin-24 inhibits influenza A virus replication in vitro through induction of toll-like receptor 3 dependent apoptosis. Antivir. Res. 2015, 123, 93–104. [Google Scholar] [CrossRef] [PubMed]
- Cattaneo, R.; Miest, T.; Shashkova, E.V.; Barry, M.A. Reprogrammed viruses as cancer therapeutics: Targeted, armed and shielded. Nat. Rev. Microbiol. 2008, 6, 529–540. [Google Scholar] [CrossRef]
- Harrington, K.J.; Puzanov, I.; Hecht, J.R.; Hodi, F.S.; Szabo, Z.; Murugappan, S.; Kaufman, H.L. Clinical development of talimogene laherparepvec (TVEC): A modified herpes simplex virus type-1-derived oncolytic immunotherapy. Expert Rev. Anticancer Ther. 2015, 15, 1389–1403. [Google Scholar] [CrossRef]
- Alberts, P.; Tilgase, A.; Rasa, A.; Bandere, K.; Venskus, D. The advent of oncolytic virotherapy in oncology: The Rigvir(R) story. Eur. J. Pharmacol. 2018, 837, 117–126. [Google Scholar] [CrossRef]
- Conry, R.M.; Westbrook, B.; McKee, S.; Norwood, T.G. Talimogene laherparepvec: First in class oncolytic Virotherapy. Human Vaccines Immunother. 2018, 14, 839–846. [Google Scholar] [CrossRef]
- Ettari, R.; Zappalà, M.; Grasso, S.; Musolino, C.; Innao, V.; Allegra, A. Immunoproteasome-selective and non-selective inhibitors: A promising approach for the treatment of multiple myeloma. Pharmacol. Ther. 2018, 182, 176–192. [Google Scholar] [CrossRef]
- Allegra, A.; Alonci, A.; Gerace, D.; Russo, S.; Innao, V.; Calabrò, L.; Musolino, C. New orally active proteasome inhibitors in multiple myeloma. Leuk Res. 2014, 38, 1–9. [Google Scholar] [CrossRef]
- Allegra, A.; Penna, G.; Alonci, A.; Russo, S.; Greve, B.; Innao, V.; Minardi, V.; Musolino, C. Monoclonal antibodies: Potential new therapeutic treatment against multiple myeloma. Eur. J. Haematol. 2013, 90, 441–468. [Google Scholar] [CrossRef]
- Allegra, A.; Sant’antonio, E.; Penna, G.; Alonci, A.; D’Angelo, A.; Russo, S.; Cannavò, A.; Gerace, D.; Musolino, C. Novel therapeutic strategies in multiple myeloma: Role of the heat shock protein inhibitors. Eur. J. Haematol. 2011, 86, 93–110. [Google Scholar] [CrossRef]
- Musolino, C.; Allegra, A.; Profita, M.; Alonci, A.; Saitta, S.; Russo, S.; Bonanno, A.; Innao, V.; Gangemi, S. Reduced IL-33 plasma levels in multiple myeloma patients are associated with more advanced stage of disease. Br. J. Haematol. 2013, 160, 709–710. [Google Scholar] [CrossRef]
- Miller, A.; Asmann, Y.; Cattaneo, L.; Braggio, E.; Keats, J.; Auclair, D.; Lonial, S.; MMRF CoMMpass Network; Russell, S.J.; Stewart, A.K. High somatic mutation and neoantigen burden are correlated with decreased progression-free survival in multiple myeloma. Blood Cancer J. 2017, 7, e612. [Google Scholar] [CrossRef]
- Russell, L.; Peng, K.W. The emerging role of oncolytic virus therapy against cancer. Chin. Clin. Oncol. 2018, 7, 16. [Google Scholar] [CrossRef]
- Dingli, D.; Peng, K.W.; Harvey, M.E.; Greipp, P.R.; O’Connor, M.K.; Cattaneo, R.; Morris, J.C.; Russell, S.J. Image-guided radiovirotherapy for multiple myeloma using a recombinant measles virus expressing the thyroidal sodium iodide symporter. Blood 2004, 103, 1641–1646. [Google Scholar] [CrossRef]
- Ong, H.T.; Timm, M.M.; Greipp, P.R.; Witzig, T.E.; Dispenzieri, A.; Russell, S.J.; Peng, K.W. Oncolytic measles virus targets high CD46 expression on multiple myeloma cells. Exp. Hematol. 2006, 34, 713–720. [Google Scholar] [CrossRef]
- Dispenzieri, A.; Tong, C.; LaPlant, B.; Lacy, M.Q.; Laumann, K.; Dingli, D.; Zhou, Y.; Federspiel, M.J.; Gertz, M.A.; Hayman, S.; et al. Phase I trial of systemic administration of Edmonston strain of measles virus genetically engineered to express the sodium iodide symporter in patients with recurrent or refractory multiple myeloma. Leukemia 2017, 31, 2791–2798. [Google Scholar] [CrossRef]
- Chen, L.; Pulsipher, M.; Chen, D.; Sieff, C.; Elias, A.; Fine, H.A.; Kufe, D.W. Selective transgene expression for detection and elimination of contaminating carcinoma cells in hematopoietic stem cell sources. J. Clin. Investig. 1996, 98, 2539–2548. [Google Scholar] [CrossRef]
- Teoh, G.; Chen, L.; Urashima, M.; Tai, Y.T.; Celi, L.A.; Chen, D.; Chauhan, D.; Ogata, A.; Finberg, R.W.; Webb, I.J.; et al. Adenovirus vector-based purging of multiple myeloma cells. Blood 1998, 92, 4591–4601. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, M.S.; Gomes, E.M.; Butcher, L.D.; Hernandez-Alcoceba, R.; Chang, D.; Kansopon, J.; Newman, J.; Stone, M.J.; Tong, A.W. Growth inhibition of human multiple myeloma cells by an oncolytic adenovirus carrying the CD40 ligand transgene. Clin. Cancer Res. 2009, 15, 4847–4856. [Google Scholar] [CrossRef]
- Raus, S.; Coin, S.; Monsurrò, V. Adenovirus as a new agent for multiple myeloma therapies: Opportunities and restrictions. Korean J. Hematol. 2011, 46, 229–238. [Google Scholar] [CrossRef] [PubMed]
- Thirukkumaran, C.M.; Morris, D.G. Oncolytic virotherapy for multiple myeloma: Past, present, and future. Bone Marrow Res. 2011, 2011, 632948. [Google Scholar] [CrossRef] [PubMed]
- Thirukkumaran, C.M.; Shi, Z.Q.; Luider, J.; Kopciuk, K.; Gao, H.; Bahlis, N.; Neri, P.; Pho, M.; Stewart, D.; Mansoor, A.; et al. Reovirus as a viable therapeutic option for the treatment of multiple myeloma. Clin. Cancer Res. 2012, 18, 4962–4972. [Google Scholar] [CrossRef]
- Mendoza-Maldonado, R.; Zentilin, L.; Fanin, R.; Giacca, M. Purging of chronic myelogenous leukemia cells by retrovirally expressed anti-bcr-abl ribozymes with specific cellular compartmentalization. Cancer Gene Ther. 2002, 9, 71–86. [Google Scholar] [CrossRef][Green Version]
- Yarde, D.N.; Nace, R.A.; Russell, S.J. Oncolytic vesicular stomatitis virus and bortezomib are antagonistic against myeloma cells in vitro but have additive anti-myeloma activity in vivo. Exp. Hematol. 2013, 41, 1038–1049. [Google Scholar] [CrossRef]
- Allegra, A.; Penna, G.; Innao, V.; Greve, B.; Maisano, V.; Russo, V.; Musolino, C. Vaccination of multiple myeloma: Current strategies and future prospects. Crit. Rev. Oncol. Hematol. 2015, 96, 339–354. [Google Scholar] [CrossRef]
- Delaunay, T.; Violland, M.; Boisgerault, N.; Dutoit, S.; Vignard, V.; Munz, C.; Gannage, M.; Dréno, B.; Vaivode, K.; Pjanova, D.; et al. Oncolytic viruses sensitize human tumor cells for NY-ESO-1 tumor antigen recognition by CD4+ effector T cells. Oncoimmunology 2018, 7, e1407897. [Google Scholar] [CrossRef]
- Packiriswamy, N.; Upreti, D.; Zhou, Y.; Khan, R.; Miller, A.; Diaz, R.M.; Rooney, C.M.; Dispenzieri, A.; Peng, K.W.; Russell, S.J.; et al. Oncolytic measles virus therapy enhances tumor antigen-specific T-cell responses in patients with multiple myeloma. Leukemia 2020. [Google Scholar] [CrossRef]
- Kadia, T.M. Release the hounds: Virotherapy with immunotherapy. Blood 2016, 127, 1381–1383. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Maurer, S.; Salih, H.R.; Smirnow, I.; Lauer, U.M.; Berchtold, S. Suicide gene-armed measles vaccine virus for the treatment of AML. Int. J. Oncol. 2019, 55, 347–358. [Google Scholar] [CrossRef] [PubMed]
- Hashiguchi, T.; Maenaka, K.; Yanagi, Y. Measles virus hemagglutinin: Structural insights into cell entry and measles vaccine. Front. Microbiol. 2011, 2, 247. [Google Scholar] [CrossRef]
- Hall, K.; Scott, K.J.; Rose, A.; Desborough, M.; Harrington, K.; Pandha, H.; Parrish, C.; Vile, R.; Coffey, M.; Bowen, D.; et al. Reovirus-mediated cytotoxicity and enhancement of innate immune responses against acute myeloid leukemia. Biores Open Access. 2012, 1, 3–15. [Google Scholar] [CrossRef]
- Errington, F.; Steele, L.; Prestwich, R.; Harrington, K.J.; Pandha, H.S.; Vidal, L.; de Bono, J.; Selby, P.; Coffey, M.; Vile, R.; et al. Reovirus activates human dendritic cells to promote innate antitumor immunity. J. Immunol. 2008, 180, 6018–6026. [Google Scholar] [CrossRef]
- Andrews, D.M.; Andoniou, C.E.; Scalzo, A.A.; van Dommelen, S.L.; Wallace, M.E.; Smyth, M.J.; Degli-Esposti, M.A. Cross-talk between dendritic cells and natural killer cells in viral infection. Mol. Immunol. 2005, 42, 547–555. [Google Scholar] [CrossRef]
- Madlambayan, G.J.; Bartee, E.; Kim, M.; Rahman, M.M.; Meacham, A.; Scott, E.W.; McFadden, G.; Cogle, C.R. Acute myeloid leukemia targeting by myxoma virus in vivo depends on cell binding but not permissiveness to infection in vitro. Leuk. Res. 2012, 36, 619–624. [Google Scholar] [CrossRef]
- Kim, M.; Madlambayan, G.J.; Rahman, M.M.; Smallwood, S.E.; Meacham, A.M.; Hosaka, K.; Scott, E.W.; Cogle, C.R.; McFadden, G. Myxoma virus targets primary human leukemic stem and progenitor cells while sparing normal hematopoietic stem and progenitor cells. Leukemia 2009, 23, 2313–2317. [Google Scholar] [CrossRef]
- Sauter, C.; Baumberger, U.; Ekenbark, S.; Lindenmann, J. Vermehrung eines tierischen Myxovirus in Primärkulturen menschlicher Leukämiezellen: Voraussetzung für eine Immunotherapie akuter Leukämien mit Virusonkolysaten. Vorläufige. Schweiz Med Wochenschr. 1974, 104, 150. [Google Scholar] [PubMed]
- Koldehoff, M.; Lindemann, M.; Opalka, B.; Bauer, S.; Ross, R.S.; Elmaagacli, A.H. Cytomegalovirus induces apoptosis in acute leukemia cells as a virus-versus leukemia function. Leuk. Lymphoma 2015, 56, 3189–3197. [Google Scholar] [CrossRef]
- Coughlan, L.; Alba, R.; Parker, A.L.; Bradshaw, A.C.; McNeish, I.A.; Nicklin, S.A.; Baker, A.H. Tropism-modification strategies for targeted gene delivery using adenoviral vectors. Viruses 2010, 2, 2290–2355. [Google Scholar] [CrossRef] [PubMed]
- Shafren, D.R.; Dorahy, D.J.; Ingham, R.A.; Burns, G.F.; Barry, R.D. Coxsackievirus A21 binds to decay-accelerating factor but requires intercellular adhesion molecule 1 for cell entry. J. Virol. 1997, 71, 4736–4743. [Google Scholar] [CrossRef] [PubMed]
- Au, G.G.; Lincz, L.F.; Enno, A.; Shafren, D.R. Oncolytic Coxsackievirus A21 as a novel therapy for multiple myeloma. Br. J. Haematol. 2007, 37, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Müller, L.M.E.; Holmes, M.; Michael, J.L.; Scott, G.B.; West, E.J.; Scott, K.J.; Parrish, C.; Hall, K.; Stäble, S.; Jennings, V.A.; et al. Plasmacytoid dendritic cells orchestrate innate and adaptive anti-tumor immunity induced by oncolytic coxsackievirus A21. J. Immunother. Cancer 2019, 7, 164. [Google Scholar] [CrossRef] [PubMed]
- Meng, H.T.; Li, L.; Liu, H.; Wang, Y.; Li, G.C.; Qian, W. Homoharringtonine acts synergistically with SG235-TRAIL, a conditionally replicating adenovirus, in the treatment of leukemia. Acta Pharmacol. Sin. 2009, 11, 1529–1536. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, W.; Wang, L.; Gao, P.; Li, Z.; Wu, J.; Zhang, H.; Wu, H.; Kong, W.; Yu, B.; et al. Enhancing the antitumor activity of an engineered TRAIL-coated oncolytic adenovirus for treating acute myeloid leukemia. Signal Transduct. Target. Ther. 2020, 5, 40. [Google Scholar] [CrossRef]
- Bais, S.; Bartee, E.; Rahman, M.M.; McFadden, G.; Cogle, C.R. Oncolytic virotherapy for hematological malignancies. Adv. Virol. 2012, 2012, 186512. [Google Scholar] [CrossRef]
- Batenchuk, C.; Le Boeuf, F.; Stubbert, L.; Falls, T.; Atkins, H.L.; Bell, J.C.; Conrad, D.P. Non-replicating rhabdovirus-derived particles (NRRPs) eradicate acute leukemia by direct cytolysis and induction of antitumor immunity. Blood Cancer J. 2014, 4, e201. [Google Scholar] [CrossRef]
- Shen, W.; Patnaik, M.M.; Ruiz, A.; Russell, S.J.; Peng, K.-W. Immunovirotherapy with vesicular stomatitis virus and PD-L1 blockade enhances therapeutic outcome in murine acute myeloid leukemia. Blood 2016, 127, 1449–1458. [Google Scholar] [CrossRef]
- Schache, P.; Gürlevik, E.; Strüver, N.; Woller, N.; Malek, N.; Zender, L.; Manns, M.; Wirth, T.; Kühnel, F.; Kubicka, S. VSV virotherapy improves chemotherapy by triggering apoptosis due to proteasomal degradation of Mcl-1. Gene Ther. 2009, 16, 849–861. [Google Scholar] [CrossRef]
- Liang, X.H.; Jackson, S.; Seaman, M.; Brown, K.; Kempkes, B.; Hibshoosh, H.; Levine, B. Induction of autophagy and inhibition of tumorigenesis by beclin 1. Nature 1999, 402, 672–676. [Google Scholar] [CrossRef] [PubMed]
- Gong, C.; Bauvy, C.; Tonelli, G.; Yue, W.; Deloménie, C.; Nicolas, V.; Zhu, Y.; Domergue, V.; Marin-Esteban, V.; Tharinger, H.; et al. Beclin 1 and autophagy are required for the tumorigenicity of breast cancer stem-like/progenitor cells. Oncogene 2013, 32, 2261–2272. [Google Scholar] [CrossRef] [PubMed]
- Yue, Z.; Jin, S.; Yang, C.; Levine, A.J.; Heintz, N. Beclin 1, an autophagy gene essential for early embryonic development, is a haploinsufficient tumor suppressor. Proc. Natl. Acad. Sci. USA 2003, 100, 15077–15082. [Google Scholar] [CrossRef] [PubMed]
- Lei, W.; Wang, S.; Xu, N.; Chen, Y.; Wu, G.; Zhang, A.; Chen, X.; Tong, Y.; Qian, W. Enhancing therapeutic efficacy of oncolytic vaccinia virus armed with Beclin-1, an autophagic Gene in leukemia and myeloma. Biomed. Pharmacother. 2020, 125, 110030. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.F.; Tan, D.Q.; Jeyasekharan, A.D.; Hsieh, W.S.; Ho, A.S.; Ichiyama, K.; Ye, M.; Pang, B.; Ohba, K.; Liu, X.; et al. Combination of vaccine-strain measles and mumps virus synergistically kills a wide range of human hematological cancer cells: Special focus on acute myeloid leukemia. Cancer Lett. 2014, 354, 272–280. [Google Scholar] [CrossRef]
- Allegra, A.; Innao, V.; Penna, G.; Gerace, D.; Allegra, A.G.; Musolino, C. Telomerase and telomere biology in hematological diseases: A new therapeutic target. Leuk. Res. 2017, 56, 60–74. [Google Scholar] [CrossRef]
- Han, M.; Napier, C.E.; Frölich, S.; Teber, E.; Wong, T.; Noble, J.R.; Choi, E.H.Y.; Everett, R.D.; Cesare, A.J.; Reddel, R.R. Synthetic lethality of cytolytic HSV-1 in cancer cells with ATRX and PML deficiency. J. Cell Sci. 2019, 132, jcs222349. [Google Scholar] [CrossRef]
- Li, L.; You, L.S.; Mao, L.P.; Jin, S.H.; Chen, X.H.; Qian, W.B. Combing oncolytic adenovirus expressing Beclin-1 with chemotherapy agent doxorubicin synergistically enhances cytotoxicity in human CML cells in vitro. Acta Pharmacol. Sin. 2018, 39, 251–260. [Google Scholar] [CrossRef]
- Weiss, R.A. Introducing viruses and cancer. In Viruses and Human Cancer; Arrand, J.R., Harper, D.R., Eds.; Bios Scientific Publishers: Oxford, UK, 1998; pp. 1–15. [Google Scholar]
- De Vita, V.T., Jr.; Canellos, G.P. The lymphomas. Semin. Hematol. 1999, 36, 84–94. [Google Scholar]
- Taqi, A.M.; Abdurrahman, M.B.; Yakubu, A.M.; Fleming, A.F. Regression of Hodgkin’s disease after measles. Lancet 1981, 1, 1112. [Google Scholar] [CrossRef]
- Ziegler, J.L. Spontaneous remission in Burkitt’s lymphoma. Natl. Cancer Inst. Monogr. 1976, 44, 61–65. [Google Scholar] [PubMed]
- Shabplessg, R.; Daviesm, C.; Cox, H.R. Antagonistic Action of Certain Neurotropic Viruses toward a Lymphoid Tumor in Chickens with Resulting Immunity. Proc. Soc. Exper. Bid. Med. 1950, 73, 270–275. [Google Scholar]
- Love, R.; Sharpless, G. Studies on a transplantable chicken tumor (RPL-12 lymphoma). III. Cytological changes during virus-induced oncolysis. Cancer Res. 1954, 14, 758–767. [Google Scholar] [PubMed]
- Sánchez, D.; Pelayo, R.; Medina, L.A.; Vadillo, E.; Sánchez, R.; Núñez, L.; Cesarman-Maus, G.; Sarmiento-Silva, R.E. Newcastle Disease Virus: Potential Therapeutic Application for Human and Canine Lymphoma. Viruses 2015, 8, 3. [Google Scholar] [CrossRef]
- Eaton, M.D.; Levinthal, J.D.; Scala, A.R. Contribution of antiviral immunity to oncolysis by Newcastle disease virus in a murine lymphoma. J. Natl. Cancer Inst. 1967, 39, 1089–1097. [Google Scholar]
- Chung, M.; Ma, B.I.; Murphy, W.H. Multiplication of viruses in Burkitt lymphoma cells. J. Natl. Cancer Inst. 1970, 44, 1231–1239. [Google Scholar]
- Peng, K.W.; Ahmann, G.J.; Pham, L.; Greipp, P.R.; Cattaneo, R.; Russell, S.J. Systemic therapy of myeloma xenografts by an attenuated measles virus. Blood 2001, 98, 2002–2007. [Google Scholar] [CrossRef]
- Levy, J.A.; Henle, G.; Henle, W.; Zajac, B.A. Effect of reovirus type 3 on cultured Burkitt’s tumour cells. Nature 1968, 220, 607–608. [Google Scholar] [CrossRef]
- Schattner, E.J. CD40 ligand in CLL pathogenesis and therapy. Leuk. Lymphoma 2000, 37, 461–472. [Google Scholar] [CrossRef]
- Gulbins, E.; Brenner, B.; Schlottmann, K.; Koppenhoefer, U.; Linderkamp, O.; Coggeshall, K.M.; Lang, F. Activation of the Ras-signaling pathway by the CD40 receptor. J. Immunol. 1996, 157, 2844–2850. [Google Scholar]
- Hangaishi, A.; Ogawa, S.; Imamura, N.; Miyawaki, S.; Miura, Y.; Uike, N.; Shimazaki, C.; Emi, N.; Takeyama, K.; Hirosawa, S.; et al. Inactivation of multiple tumor-suppressor genes involved in negative regulation of the cell cycle, MTS1/p16INK4A/CDKN2, MTS2/ p15INK4B, p53, and Rb genes in primary lymphoid malignancies. Blood 1996, 87, 4949–4958. [Google Scholar] [CrossRef] [PubMed]
- Parrish, C.; Scott, G.B.; Migneco, G.; Scott, K.J.; Steele, L.P.; Ilett, E.; West, E.J.; Hall, K.; Selby, P.J.; Buchanan, D.; et al. Oncolytic reovirus enhances rituximab-mediated antibody-dependent cellular cytotoxicity against chronic lymphocytic leukaemia. Leukemia. 2015, 29, 1799–1810. [Google Scholar] [CrossRef] [PubMed]
- Alain, T.; Hirasawa, K.; Pon, K.J.; Nishikawa, S.G.; Urbanski, S.J.; Auer, Y.; Luider, J.; Martin, A.; Johnston, R.N.; Janowska-Wieczorek, A.; et al. Reovirus therapy of lymphoid malignancies. Blood 2002, 100, 4146–4153. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Thirukkumaran, C.M.; Luider, J.M.; Stewart, D.A.; Cheng, T.; Lupichuk, S.M.; Nodwell, M.J.; Russell, J.A.; Auer, I.A.; Morris, D.G. Reovirus oncolysis as a novel purging strategy for autologous stem cell transplantation. Blood 2003, 102, 377–387. [Google Scholar] [CrossRef] [PubMed]
- Medina, D.J.; Sheay, W.; Goodell, L.; Kidd, P.; White, E.; Rabson, A.B.; Strair, R.K. Adenovirus-mediated cytotoxicity of chronic lymphocytic leukemia cells. Blood 1999, 94, 3499–3508. [Google Scholar] [PubMed]
- Tumilasci, V.F.; Oliere, S.; Nguyen, T.L.; Shamy, A.; Bell, J.; Hiscott, J. Targeting the apoptotic pathway with BCL-2 inhibitors sensitizes primary chronic lymphocytic leukemia cells to vesicular stomatitis virus-induced oncolysis. J. Virol. 2008, 82, 8487–8499. [Google Scholar] [CrossRef]
- Samuel, S.; Tumilasci, V.F.; Oliere, S.; Nguyen, T.L.; Shamy, A.; Bell, J.; Hiscott, J. VSV oncolysis in combination with the BCL-2 inhibitor obatoclax overcomes apoptosis resistance in chronic lymphocytic leukemia. Mol. Ther. 2010, 18, 2094–2103. [Google Scholar] [CrossRef]
- Ungerechts, G.; Frenzke, M.E.; Yaiw, K.C.; Miest, T.; Johnston, P.B.; Cattaneo, R. Mantle cell lymphoma salvage regimen: Synergy between a reprogrammed oncolytic virus and two chemotherapeutics. Gene Ther. 2010, 17, 1506–1516. [Google Scholar] [CrossRef]
- Saxon, M. Denileukin diftitox. Clin. J. Oncol. Nurs. 2000, 4, 289–293. [Google Scholar]
- Künzi, V.; Oberholzer, P.A.; Heinzerling, L.; Dummer, R.; Naim, H.Y. Recombinant measles virus induces cytolysis of cutaneous T-cell lymphoma in vitro and in vivo. J. Investig. Dermatol. 2006, 126, 2525–2532. [Google Scholar] [CrossRef]
- La Pointe, A.T.; Landers, V.D.; Westcott, C.E.; Sokoloski, K.J. Production of Noncapped Genomic RNAs Is Critical to Sindbis Virus Disease and Pathogenicity. mBio 2020, 11, e02675-20. [Google Scholar] [CrossRef]
- Yu, M.; Scherwitzl, I.; Opp, S.; Tsirigos, A.; Meruelo, D. Molecular and metabolic pathways mediating curative treatment of a non-Hodgkin B cell lymphoma by Sindbis viral vectors and anti-4-1BB monoclonal antibody. J. Immunother. Cancer 2019, 7, 185. [Google Scholar] [CrossRef] [PubMed]
- Kiseleva, I.V.; Egorov, A.I.; Aleksandrova, G.I.; Aksenov, O.A.; Osipova, Z.A.; Maĭorova, L.P.; Klimov, A.I. Antitumor activity of cold-adapted strains of influenza virus in a model transformed murine tumor cells. Vopr. Virusol. 1999, 4429–4432. [Google Scholar]
- Cooper, L.J.; Al-Kadhimi, Z.; Serrano, L.M.; Pfeiffer, T.; Olivares, S.; Castro, A.; Chang, W.C.; Gonzalez, S.; Smith, D.; Forman, S.J.; et al. Enhanced antilymphoma efficacy of CD19-redirected influenza MP1-specific CTLs by cotransfer of T cells modified to present influenza MP1. Blood 2005, 105, 1622–1631. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Rahman, M.M.; Cogle, C.R.; McFadden, G. Prevention of EBV lymphoma development by oncolytic myxoma virus in a murine xenograft model of post-transplant lymphoproliferative disease. Biochem. Biophys. Res. Commun. 2015, 462, 283–287. [Google Scholar] [CrossRef]
- Pasquini, M.C.; Wang, Z.; Horowitz, M.M.; Gale, R.P. 2010 report from Center for International Blood and Marrow Transplant Research (CIBMTR): Current uses and outcomes of hematopoietic cell transplants for blood and bone marrow disorders. Clin. Transplant. 2010, 2010, 87–105. [Google Scholar]
- Arai, S.A.M.; Wang, T.; Spellman, S.R.; He, W.; Couriel, D.R.; Urbano-Ispizua, A.; Cutler, C.S.; Bacigalupo, A.A.; Battiwalla, M.; Flowers, M.E.; et al. Graft-vs-Host Disease Working Committee of the CIBMTR. Graft-vs-Host Disease Working Committee of the CIBMTR, Increasing incidence of chronic graft-versus-host disease in allogeneic transplantation: A report from the Center for International Blood and Marrow Transplant Research. Biol. Blood Marrow Transplant. 2015, 21, 266–274. [Google Scholar]
- Ferrara, J.L.Y.G. Acute graft versus host disease: Pathophysiology, risk factors, and prevention strategies. Clin. Adv. Hematol. Oncol. 2005, 3, 415–419. [Google Scholar]
- Villa, N.Y.; McFadden, G. Virotherapy as Potential Adjunct Therapy for Graft-Vs-Host Disease. Curr. Pathobiol. Rep. 2018, 6, 247–263. [Google Scholar] [CrossRef]
- Wodarz, D. Use of oncolytic viruses for the eradication of drug-resistant cancer cells. J. R. Soc. Interface 2009, 6, 179–186. [Google Scholar] [CrossRef][Green Version]
- Eager, R.M.; Nemunaitis, J. Clinical development directions in oncolytic viral therapy. Cancer Gene Ther. 2011, 18, 305–317. [Google Scholar] [CrossRef]
- Zhang, Q.; Chen, G.; Peng, L.; Wang, X.; Yang, Y.; Liu, C.; Shi, W.; Su, C.; Wu, H.; Liu, X.; et al. Increased safety with preserved antitumoral efficacy on hepatocellular carcinoma with dual-regulated oncolytic adenovirus. Clin. Cancer Res. 2006, 12, 6523–6531. [Google Scholar] [CrossRef] [PubMed]
- Harrington, K.; Freeman, D.J.; Kelly, B.; Harper, J.; Soria, J.C. Optimizing oncolytic virotherapy in cancer Treatment. Nat. Rev. Drug Discov. 2019, 18, 689–706. [Google Scholar] [CrossRef] [PubMed]
- Phan, M.; Watson, M.F.; Alain, T.; Diallo, J.S. Oncolytic Viruses on Drugs: Achieving Higher TherapeuticEfficacy. ACS Infect. Dis. 2018, 4, 1448–1467. [Google Scholar] [CrossRef] [PubMed]
- Musolino, C.; Sant’antonio, E.; Penna, G.; Alonci, A.; Russo, S.; Granata, A.; Allegra, A. Epigenetic therapy in myelodysplastic syndromes. Eur. J. Haematol. 2010, 84, 463–473. [Google Scholar] [CrossRef]
- Nakashima, H.; Nguyen, T.; Chiocca, E.A. Combining HDAC inhibitors with oncolytic virotherapy for cancer therapy. Oncol. Virother. 2015, 4, 183–191. [Google Scholar]
- Otsuki, A.; Patel, A.; Kasai, K.; Suzuki, M.; Kurozumi, K.; Chiocca, E.A.; Saeki, Y. Histone deacetylase inhibitors augment antitumor efficacy of herpes-based oncolytic viruses. Mol. Ther. 2008, 16, 1546–1555. [Google Scholar] [CrossRef]
- Poggi, A.; Catellani, S.; Garuti, A.; Pierri, I.; Gobbi, M.; Zocchi, M.R. Effective in vivo induction of NKG2D ligands in acute myeloid leukaemias by all-transretinoic acid or sodium valproate. Leukemia 2009, 23, 641–648. [Google Scholar] [CrossRef]
- MacTavish, H.; Diallo, J.S.; Huang, B.; Stanford, M.; Le Boeuf, F.; De Silva, N.; Cox, J.; Simmons, J.G.; Guimond, T.; Falls, T.; et al. Enhancement of vaccinia virus based oncolysis with histone deacetylase inhibitors. PLoS ONE 2010, 5, e14462. [Google Scholar] [CrossRef]
- Yao, Y.; Zhou, J.; Wang, L.; Gao, X.; Ning, Q.; Jiang, M.; Wang, J.; Wang, L.; Yu, L. Increased PRAME specific CTL killing of acute myeloid leukemia cells by either a novel histone deacetylase inhibitor chidamide alone or combined treatment with decitabine. PLoS ONE 2013, 8, e70522. [Google Scholar] [CrossRef]
- Thomas, S.; Kuncheria, L.; Roulstone, V.; Kyula, J.N.; Mansfield, D.; Bommareddy, P.K.; Smith, H.; Kaufman, H.L.; Harrington, K.J.; Coffin, R.S. Development of a new fusion-enhanced oncolytic immunotherapy platform based on herpes simplex virus type 1. J. Immunother. Cancer 2019, 7, 214. [Google Scholar] [CrossRef] [PubMed]
- Galon, J.; Bruni, D. Approaches to treat immune hot, altered and cold tumours with combination immunotherapies. Nat. Rev. Drug Discov. 2019, 18, 197–218. [Google Scholar] [CrossRef] [PubMed]
- Chaurasiya, S.; Chen, N.G.; Fong, Y. Oncolytic viruses and immunity. Curr. Opin. Immunol. 2018, 51, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Samson, A.; Scott, K.J.; Taggart, D.; West, E.J.; Wilson, E.; Nuovo, G.J.; Thomson, S.; Corns, R.; Mathew, R.K.; Fuller, M.J.; et al. Intravenous delivery of oncolytic reovirus to brain tumor patients immunologically primes for subsequent checkpoint blockade. Sci. Transl. Med. 2018, 10, eaam7577. [Google Scholar] [CrossRef]
- Newman, J.H.; Chesson, C.B.; Herzog, N.L.; Bommareddy, P.K.; Aspromonte, S.M.; Pepe, R.; Estupinian, R.; Aboelatta, M.M.; Buddhadev, S.; Tarabichi, S.; et al. Intratumoral injection of the seasonal flu shot converts immunologically cold tumors to hot and serves as an immunotherapy for cancer. Proc. Natl. Acad. Sci. USA 2020, 117, 1119–1128. [Google Scholar] [CrossRef]
- Engeland, C.E.; Grossardt, C.; Veinalde, R.; Bossow, S.; Lutz, D.; Kaufmann, J.K.; Shevchenko, I.; Umansky, V.; Nettelbeck, D.M.; Weichert, W.; et al. CTLA-4 and PD-L1 checkpoint blockade enhances oncolytic measles virus therapy. Mol. Ther. 2014, 22, 1949–1959. [Google Scholar] [CrossRef]
- Kabiljo, J.; Laengle, J.; Bergmann, M. From threat to cure: Understanding of virus-induced cell death leads to highly immunogenic oncolytic influenza viruses. Cell Death Discov. 2020, 6, 48. [Google Scholar] [CrossRef]
- Zamarin, D.; Holmgaard, R.B.; Subudhi, S.K.; Park, J.S.; Mansour, M.; Palese, P.; Merghoub, T.; Wolchok, J.D.; Allison, J.P. Localized oncolytic virotherapy overcomes systemic tumor resistance to immune checkpoint blockade immunotherapy. Sci. Transl. Med. 2014, 6, 226ra32. [Google Scholar] [CrossRef]
- Allegra, A.; Innao, V.; Gerace, D.; Vaddinelli, D.; Musolino, C. Adoptive immunotherapy for hematological malignancies: Current status and new insights in chimeric antigen receptor T cells. Blood Cells Mol. Dis. 2016, 62, 49–63. [Google Scholar] [CrossRef]
- Sato-Dahlman, M.; LaRocca, C.J.; Yanagiba, C.; Yamamoto, M. Adenovirus and Immunotherapy: Advancing Cancer Treatment by Combination. Cancers 2020, 12, 1295. [Google Scholar] [CrossRef]
- Watanabe, K.; Luo, Y.; Da, T.; Guedan, S.; Ruella, M.; Scholler, J.; Keith, B.; Young, R.M.; Engels, B.; Sorsa, S.; et al. Pancreatic cancer therapy with combined mesothelin-redirected chimeric antigen receptor T cells and cytokine-armed oncolytic adenoviruses. JCI Insight 2018, 3, e99573. [Google Scholar] [CrossRef] [PubMed]
- Tanoue, K.; Rosewell Shaw, A.; Watanabe, N.; Porter, C.; Rana, B.; Gottschalk, S.; Brenner, M.; Suzuki, M. Armed oncolytic adenovirus-expressing PD-L1 Mini-Body enhances antitumor effects of chimeric antigen receptor T Cells in Solid Tumors. Cancer Res. 2017, 77, 2040–2051. [Google Scholar] [CrossRef] [PubMed]
- Ylösmäki, E.; Cerullo, V. Design and application of oncolytic viruses for cancer immunotherapy. Curr. Opin. Biotechnol. 2020, 65, 25–36. [Google Scholar] [CrossRef] [PubMed]
- Nwanegbo, E.; Vardas, E.; Gao, W.; Whittle, H.; Sun, H.; Rowe, D.; Robbins, P.D.; Gambotto, A. Prevalence of neutralizing antibodies to adenoviral serotypes 5 and 35 in the adult populations of The Gambia, South Africa, and the United States. Clin. Diagn. Lab. Immunol. 2004, 11, 351–357. [Google Scholar] [CrossRef]
- Vogels, R.; Zuijdgeest, D.; van Rijnsoever, R.; Hartkoorn, E.; Damen, I.; de Béthune, M.P.; Kostense, S.; Penders, G.; Helmus, N.; Koudstaal, W.; et al. Replication-deficient human adenovirus Type 35 vectors for gene transfer and vaccination: Efficient human cell infection and bypass of preexisting adenovirus immunity. J. Virol. 2003, 77, 8263–8271. [Google Scholar] [CrossRef]
- Roy, S.; Shirley, P.S.; McClelland, A.; Kaleko, M. Circumvention of immunity to the adenovirus major coat protein hexon. J. Virol. 1998, 72, 6875–6879. [Google Scholar] [CrossRef]
- Fisher, K.; Stallwood, Y.; Green, N.; Ulbrich, K.; Mautner, V.; Seymour, L. Polymer-coated adenovirus permits efficient retargeting and evades neutralising antibodies. Gene Ther. 2001, 8, 341–348. [Google Scholar] [CrossRef]
- Niemann, J.; Woller, N.; Brooks, J.; Fleischmann-Mundt, B.; Martin, N.T.; Kloos, A.; Knocke, S.; Ernst, A.M.; Manns, M.P.; Kubicka, S.; et al. Molecular retargeting of antibodies converts immune defense against oncolytic viruses into cancer immunotherapy. Nat. Commun. 2019, 10, 3236. [Google Scholar] [CrossRef]
- Kemp, V.; Lamfers, M.L.M.; van der Pluijm, G.; van den Hoogen, B.G.; Hoeben, R.C. Developing oncolytic viruses for clinical use: A consortium approach. Cytokine Growth Factor Rev. 2020. [Google Scholar] [CrossRef]
- Russell, S.J.; Peng, K.W.; Bell, J.C. Oncolytic virotherapy. Nat. Biotechnol. 2012, 30, 658–670. [Google Scholar] [CrossRef]
- Dautzenberg, I.J.C.; van den Wollenberg, D.J.M.; van den Hengel, S.K.; Limpens, R.W.A.; Bárcena, M.; Koster, A.J.; Hoeben, R.C. Mammalian orthoreovirus T3D infects U-118 MG cell spheroids independent of junction adhesion molecule-A. Gene Ther. 2014, 21, 609–617. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Innao, V.; Rizzo, V.; Allegra, A.G.; Musolino, C.; Allegra, A. Oncolytic Viruses and Hematological Malignancies: A New Class of Immunotherapy Drugs. Curr. Oncol. 2021, 28, 159-183. https://doi.org/10.3390/curroncol28010019
Innao V, Rizzo V, Allegra AG, Musolino C, Allegra A. Oncolytic Viruses and Hematological Malignancies: A New Class of Immunotherapy Drugs. Current Oncology. 2021; 28(1):159-183. https://doi.org/10.3390/curroncol28010019
Chicago/Turabian StyleInnao, Vanessa, Vincenzo Rizzo, Andrea Gaetano Allegra, Caterina Musolino, and Alessandro Allegra. 2021. "Oncolytic Viruses and Hematological Malignancies: A New Class of Immunotherapy Drugs" Current Oncology 28, no. 1: 159-183. https://doi.org/10.3390/curroncol28010019
APA StyleInnao, V., Rizzo, V., Allegra, A. G., Musolino, C., & Allegra, A. (2021). Oncolytic Viruses and Hematological Malignancies: A New Class of Immunotherapy Drugs. Current Oncology, 28(1), 159-183. https://doi.org/10.3390/curroncol28010019





