Efficacy of 177Lu-Dotatate Induction and Maintenance Therapy of Various Types of Neuroendocrine Tumors: A Phase II Registry Study
Abstract
1. Introduction
2. Experimental Section
2.1. Protocol
2.2. Statistics
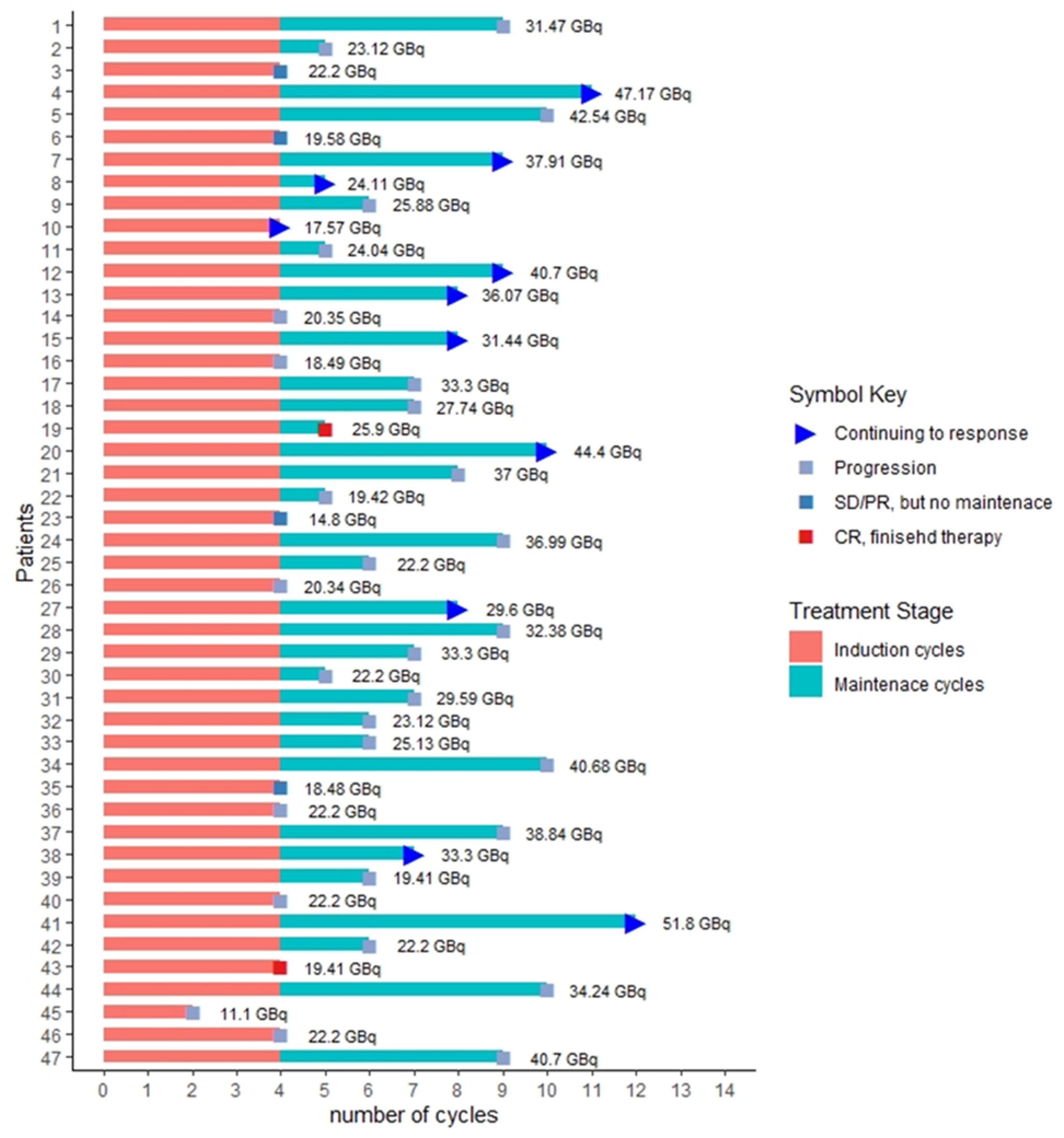
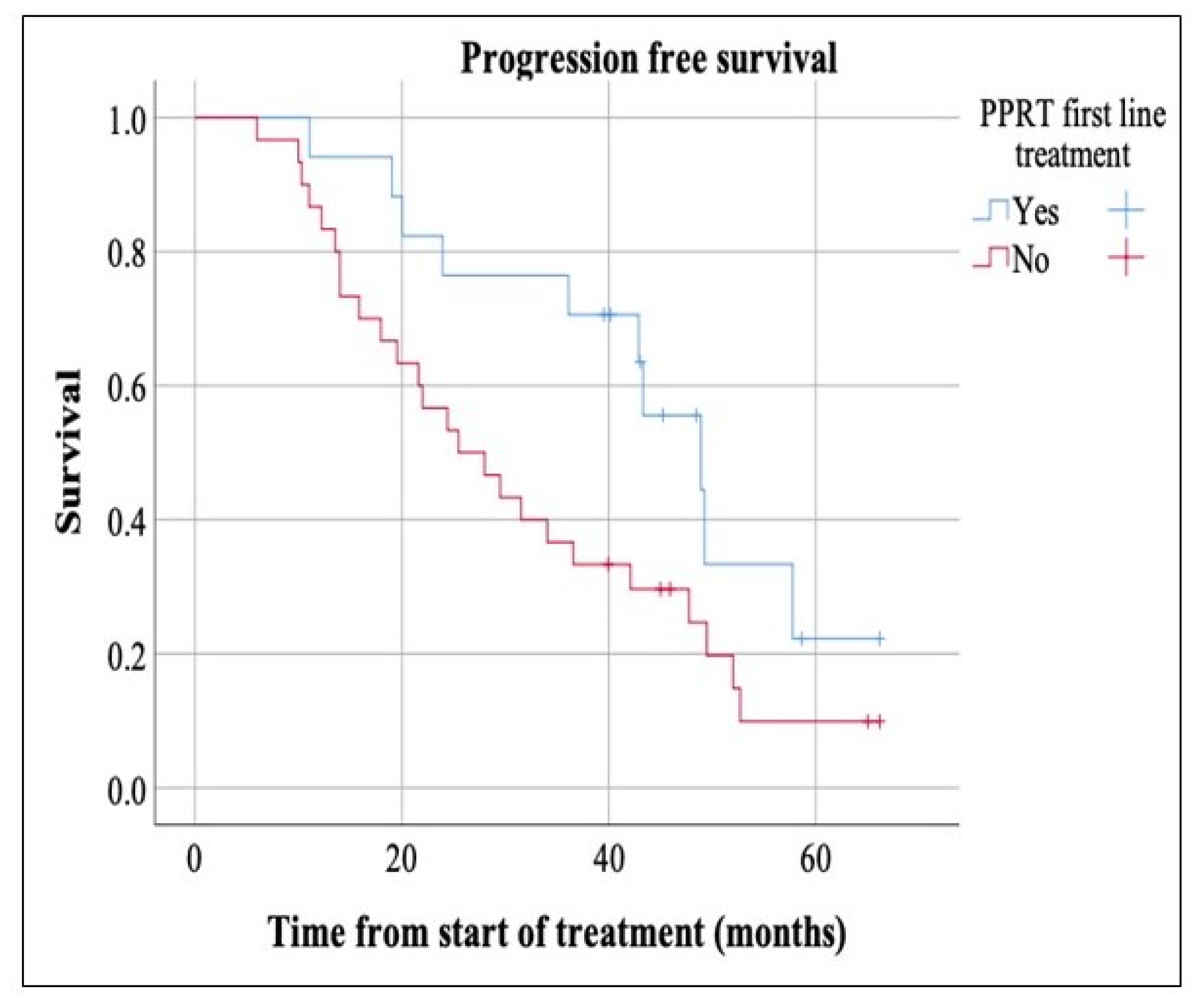
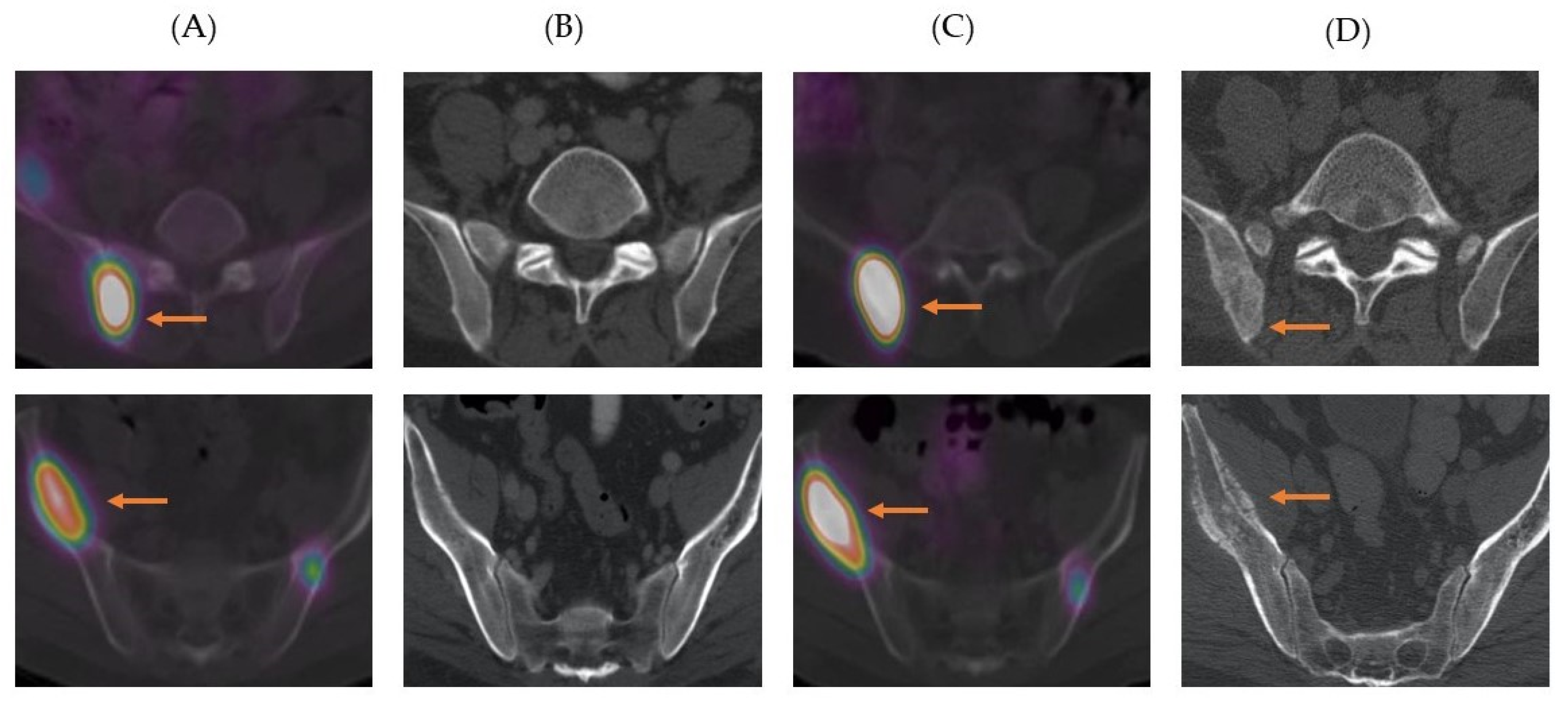
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Oronsky, B.; Ma, P.C.; Morgensztern, D.; Carter, C.A. Nothing But NET: A Review of Neuroendocrine Tumors and Carcinomas. Neoplasia 2017, 19, 991–1002. [Google Scholar] [CrossRef] [PubMed]
- Hallet, J.; Law, C.H.L.; Cukier, M.; Saskin, R.; Liu, N.; Singh, S. Exploring the rising incidence of neuroendocrine tumors: A population-based analysis of epidemiology, metastatic presentation, and outcomes. Cancer 2015, 121, 589–597. [Google Scholar] [CrossRef] [PubMed]
- Cavalcoli, F.; Garrahy, A.; Castellaneta, M.; Tamagno, G. Epidemiology of neuroendocrine tumours. Neuroendocrinology 2004, 80 (Suppl. 1), 3–7. [Google Scholar]
- Man, D.; Wu, J.; Shen, Z.; Zhu, X. Prognosis of patients with neuroendocrine tumor: A SEER database analysis. Cancer Manag. Res. 2018, 10, 5629–5638. [Google Scholar] [CrossRef] [PubMed]
- Bodei, L.; Kwekkeboom, D.J.; Kidd, M.; Modlin, I.M.; Krenning, E.P. Radiolabeled Somatostatin Analogue Therapy of Gastroenteropancreatic Cancer. Semin. Nucl. Med. 2016, 46, 225–238. [Google Scholar] [CrossRef]
- Strosberg, J.; Halfdanarson, T.R.; Bellizzi, A.M.; Chan, J.A.; Dillon, J.S.; Heaney, A.P.; Kunz, P.L.; O’Dorisio, T.M.; Salem, R.; Segelov, E.; et al. The North American Neuroendocrine Tumor Society Consensus Guidelines for Surveillance and Medical Management of Midgut Neuroendocrine Tumors. Pancreas 2017, 46, 707–714. [Google Scholar] [CrossRef]
- Parra-Medina, R.; Moreno-Lucero, P.; Jimenez-Moreno, J.; Parra-Morales, A.M.; Romero-Rojas, A. Neuroendocrine neoplasms of gastrointestinal tract and secondary primary synchronous tumors: A systematic review of case reports. Casualty or causality? PLoS ONE 2019, 14, e0216647. [Google Scholar] [CrossRef]
- Frilling, A.; Sotiropoulos, G.C.; Li, J.; Kornasiewicz, O.; Plöckinger, U. Multimodal management of neuroendocrine liver metastases. HPB 2010, 12, 361–379. [Google Scholar] [CrossRef]
- Chen, H.; Hardacre, J.M.; Uzar, A.; Cameron, J.L.; A Choti, M. Isolated liver metastases from neuroendocrine tumors: Does resection prolong survival? J. Am. Coll. Surg. 1998, 187, 88–92. [Google Scholar] [CrossRef]
- Nave, H.; Mössinger, E.; Feist, H.; Lang, H.; Raab, H.-R. Surgery as primary treatment in patients with liver metastases from carcinoid tumors: A retrospective, unicentric study over 13 years. Surgery 2001, 129, 170–175. [Google Scholar] [CrossRef]
- Gupta, S.; Johnson, M.M.; Murthy, R.; Ahrar, K.; Wallace, M.J.; Madoff, D.C.; McRae, S.E.; Hicks, M.E.; Rao, S.; Vauthey, J.-N.; et al. Hepatic arterial embolization and chemoembolization for the treatment of patients with metastatic neuroendocrine tumors: Variables affecting response rates and survival. Cancer 2005, 104, 1590–1602. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, A.S.; Dezarn, W.A.; McNeillie, P.; Coldwell, D.; Nutting, C.; Carter, D.; Murthy, R.; Rose, S.; Warner, R.R.P.; Liu, D.; et al. Radioembolization for unresectable neuroendocrine hepatic metastases using resin 90Y-microspheres: Early results in 148 patients. Am. J. Clin. Oncol. 2008, 31, 271–279. [Google Scholar] [CrossRef] [PubMed]
- Mazzaglia, P.J.; Berber, E.; Milas, M.; Siperstein, A.E. Laparoscopic radiofrequency ablation of neuroendocrine liver metastases: A 10-year experience evaluating predictors of survival. Surgery 2007, 142, 10–19. [Google Scholar] [CrossRef] [PubMed]
- Mohan, H.M.; Nicholson, P.; Winter, D.C.; O’Shea, D.; O’Toole, D.; Geoghegan, J.; Maguire, D.; Hoti, E.; Traynor, O.; Cantwell, C.P. Radiofrequency ablation for neuroendocrine liver metastases: A systematic review. J. Vasc. Interv. Radiol. 2015, 26, 935–942. [Google Scholar] [CrossRef]
- Peixoto, R.D.; Noonan, K.L.; Pavlovich, P.; Kennecke, H.F.; Lim, H.J. Outcomes of patients treated with capecitabine and temozolamide for advanced pancreatic neuroendocrine tumors (PNETs) and non-PNETs. J. Gastrointest. Oncol. 2014, 5, 247–252. [Google Scholar] [CrossRef]
- Yao, J.C.; Fazio, N.; Singh, S.; Buzzoni, R.; Carnaghi, C.; Wolin, E.; Tomasek, J.; Raderer, M.; Lahner, H.; Voi, M.; et al. Everolimus for the treatment of advanced, non-functional neuroendocrine tumours of the lung or gastrointestinal tract (RADIANT-4): A randomised, placebo-controlled, phase 3 study. Lancet 2016, 387, 968–977. [Google Scholar] [CrossRef]
- Caplin, M.E.; Pavel, M.; Ćwikła, J.B.; Phan, A.T.; Raderer, M.; Sedláčková, E.; Cadiot, G.; Wolin, E.M.; Capdevila, J.; Wall, L.; et al. Lanreotide in metastatic enteropancreatic neuroendocrine tumors. N. Engl. J. Med. 2014, 371, 224–233. [Google Scholar] [CrossRef]
- Rinke, A.; Müller, H.-H.; Schade-Brittinger, C.; Klose, K.-J.; Barth, P.; Wied, M.; Mayer, C.; Aminossadati, B.; Pape, U.-F.; Bläker, M.; et al. Placebo-controlled, double-blind, prospective, randomized study on the effect of octreotide LAR in the control of tumor growth in patients with metastatic neuroendocrine midgut tumors: A report from the PROMID Study Group. J. Clin. Oncol. 2009, 27, 4656–4663. [Google Scholar] [CrossRef]
- Zou, Y.; Tan, H.; Zhao, Y.; Zhou, Y.; Cao, L. Expression and selective activation of somatostatin receptor subtypes induces cell cycle arrest in cancer cells. Oncol. Lett. 2019, 17, 1723–1731. [Google Scholar] [CrossRef]
- Kwekkeboom, D.J.; De Herder, W.W.; Kam, B.L.; Van Eijck, C.H.; Van Essen, M.; Kooij, P.P.; Feelders, R.A.; Van Aken, M.O.; Krenning, E.P. Treatment with the radiolabeled somatostatin analog [177 Lu-DOTA 0,Tyr3]octreotate: Toxicity, efficacy, and survival. J. Clin. Oncol. 2008, 26, 2124–2130. [Google Scholar] [CrossRef]
- Strosberg, J.; El-Haddad, G.; Wolin, E.; Hendifar, A.; Yao, J.; Chasen, B.; Mittra, E.; Kunz, P.L.; Kulke, M.H.; Jacene, H.; et al. Phase 3 Trial of (177)Lu-Dotatate for Midgut Neuroendocrine Tumors. N. Engl. J. Med. 2017, 376, 125–135. [Google Scholar] [CrossRef] [PubMed]
- Valkema, R.; Pauwels, S.; Kvols, L.K.; Barone, R.; Jamar, F.; Bakker, W.H.; Kwekkeboom, D.J.; Bouterfa, H.; Krenning, E.P. Survival and response after peptide receptor radionuclide therapy with [90Y-DOTA0,Tyr3]octreotide in patients with advanced gastroenteropancreatic neuroendocrine tumors. Semin. Nucl. Med. 2006, 36, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Van Essen, M.; Krenning, E.P.; Kam, B.L.; De Jong, M.; Valkema, R.; Kwekkeboom, D.J. Peptide-receptor radionuclide therapy for endocrine tumors. Nat. Rev. Endocrinol. 2009, 5, 382–393. [Google Scholar] [CrossRef] [PubMed]
- Zaknun, J.J.; Bodei, L.; Mueller-Brand, J.; Pavel, M.E.; Baum, R.P.; Hörsch, D.; O’Dorisio, M.S.; O’Dorisiol, T.M.; Howe, J.R.; Cremonesi, M.; et al. The joint IAEA, EANM, and SNMMI practical guidance on peptide receptor radionuclide therapy (PRRNT) in neuroendocrine tumours. Eur. J. Nucl. Med. Mol. Imaging 2013, 40, 800–816. [Google Scholar] [CrossRef] [PubMed]
- Hosono, M.; Ikebuchi, H.; Nakamura, Y.; Nakamura, N.; Yamada, T.; Yanagida, S.; Kitaoka, A.; Kojima, K.; Sugano, H.; Kinuya, S.; et al. Manual on the proper use of lutetium-177-labeled somatostatin analogue (Lu-177-DOTA-TATE) injectable in radionuclide therapy (2nd ed.). Ann. Nucl. Med. 2018, 32, 217–235. [Google Scholar] [CrossRef]
- Tirkes, T.; Hollar, M.A.; Tann, M.; Kohli, M.D.; Akisik, F.; Sandrasegaran, K. Response criteria in oncologic imaging: Review of traditional and new criteria. Radiographics 2013, 33, 1323–1341. [Google Scholar] [CrossRef]
- Ezziddin, S.; Khalaf, F.; Vanezi, M.; Haslerud, T.; Mayer, K.; Al Zreiqat, A.; Willinek, W.; Biersack, H.-J.; Sabet, A. Outcome of peptide receptor radionuclide therapy with 177Lu-octreotate in advanced grade 1/2 pancreatic neuroendocrine tumours. Eur. J. Nucl. Med. Mol. Imaging 2014, 41, 925–933. [Google Scholar] [CrossRef]
- Paganelli, G.; Sansovini, M.; Ambrosetti, A.; Severi, S.; Monti, M.; Scarpi, E.; Donati, C.; Ianniello, A.; Matteucci, F.; Amadori, D. 177 Lu-Dota-octreotate radionuclide therapy of advanced gastrointestinal neuroendocrine tumors: Results from a phase II study. Eur. J. Nucl. Med. Mol. Imaging 2014, 41, 1845–1851. [Google Scholar] [CrossRef]
- Sabet, A.; Dautzenberg, K.; Haslerud, T.; Aouf, A.; Sabet, A.; Simon, B.; Mayer, K.; Biersack, H.-J.; Ezziddin, S. Specific efficacy of peptide receptor radionuclide therapy with (177)Lu-octreotate in advanced neuroendocrine tumours of the small intestine. Eur. J. Nucl. Med. Mol. Imaging 2015, 42, 1238–1246. [Google Scholar] [CrossRef]
- Strosberg, J.; Wolin, E.; Chasen, B.; Kulke, M.; Bushnell, D.; Caplin, M.; Baum, R.P.; Hobday, T.; Hendifar, A.; Öberg, K.; et al. Overall survival, progression-free survival, and quality of life updates from the NETTER-1 study: 177Lu-Dotatate vs. high dose octreotide in progressive midgut neuroendocrine tumors. In Proceedings of the 15th Annual ENETS Conference, Barcelona, Spain, 7–9 March 2018. [Google Scholar]
- Bodei, L.; Schöder, H.; Baum, R.P.; Herrmann, K.; Strosberg, J.; Caplin, M.; Öberg, K.; Modlin, I.M. Molecular profiling of neuroendocrine tumours to predict response and toxicity to peptide receptor radionuclide therapy. Lancet Oncol. 2020, 21, e431–e443. [Google Scholar] [CrossRef]
- Yao, J.C.; Shah, M.H.; Ito, T.; Bohas, C.L.; Wolin, E.M.; Van Cutsem, E.; Hobday, T.J.; Okusaka, T.; Capdevila, J.; De Vries, E.G.; et al. Everolimus for Advanced Pancreatic Neuroendocrine Tumors. N. Engl. J. Med. 2011, 364, 514–523. [Google Scholar] [CrossRef] [PubMed]
- Faivre, S.; Niccoli, P.; Castellano, D.; Valle, J.W.; Hammel, P.; Raoul, J.-L.; Vinik, A.; Van Cutsem, E.; Bang, Y.-J.; Lee, S.-H.; et al. Sunitinib in pancreatic neuroendocrine tumors: Updated progression-free survival and final overall survival from a phase III randomized study. Ann. Oncol. 2017, 28, 339–343. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.F.; Lin, L.; Wang, M.J.; Li, Y. The therapeutic efficacy of 177Lu-DOTATATE/DOTATOC in advanced neuroendocrine tumors: A meta-analysis. Medicine 2020, 99, e19304. [Google Scholar] [CrossRef] [PubMed]
- Del Prete, M.; Buteau, F.-A.; Arsenault, F.; Saighi, N.; Bouchard, L.-O.; Beaulieu, A.; Beauregard, J.-M. Personalized (177)Lu-octreotate peptide receptor radionuclide therapy of neuroendocrine tumours: Initial results from the P-PRRT trial. Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 728–742. [Google Scholar] [CrossRef] [PubMed]
- Reubi, J.C.; Schär, J.-C.; Waser, B.; Wenger, S.; Heppeler, A.; Schmitt, J.S.; Mäcke, H.R. Affinity profiles for human somatostatin receptor subtypes SST1-SST5 of somatostatin radiotracers selected for scintigraphic and radiotherapeutic use. Eur. J. Nucl. Med. 2000, 27, 273–282. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; Hong, S.M.; Ro, J.Y. Recent updates on grading and classification of neuroendocrine tumors. Ann. Diagn. Pathol. 2017, 29, 11–16. [Google Scholar] [CrossRef]
- Choe, J.; Kim, K.W.; Kim, H.J.; Ki, D.W.; Kim, K.P.; Hong, S.-M.; Ryu, J.-S.; Tirumani, S.H.; Krajewski, K.; Ramaiya, N. What Is New in the 2017 World Health Organization Classification and 8th American Joint Committee on Cancer Staging System for Pancreatic Neuroendocrine Neoplasms? Korean J. Radiol. 2019, 20, 5–17. [Google Scholar] [CrossRef]


| Risk Factor | Dose | Notes | |||
|---|---|---|---|---|---|
| 150 mCi (5.55 GBq) | 125 mCi (4.62 GBq) | 100 mCi (3.7 GBq) | 75 mCi (2.78 GBq) | ||
| Age (years) | <65 | 65–75 | >75 | If more than 2 risk factors identified. | - |
| GFR * | ≥65 corrected | 56–65 corrected | 50–55 corrected | <50 corrected: Tx not offered. | |
| Liver disease involvement (CT/MRI/177Lu scan) and LFT ** | ≤70% of liver involved approximately | >70% of liver involved | Outside 3 × normal limits: Therapy not offered. | ||
| Platelets | ≥100 × 109/L | ≥100 × 109/L | ≥100 × 109/L | <100 × 109/L (<95 × 109/L for subsequent Tx). | |
| WBC | ≥3 × 109/L | ≥3 × 109/L | ≥3 × 109/L | ANC < 1.5 × 109/L: Tx not offered. | |
| Previous nephron- and/or marrow-toxic therapies | None | 1 course of chemotherapy or ≤4 RIT with dose < 800 mCi. | >1 course of chemotherapy or >4 RIT with dose > 800 mCi. | ||
| Weight(kg) | ≥60 | 50–59 | <50 | Dose to be not >2.5 mCi/kg (0.07 GBq/kg) | |
| Bone and marrow disease involvement of axial skeleton | ≤5 bone metastases | 6–10 bone metastases | >10 bone metastases | Diffuse bone marrow involvement | |
| Baseline Characteristics | Number of Patients, n = 47 (% of Total) |
|---|---|
| Sex (M, F) | 28, 19 (59.6%, 40.4%) |
| Mean age at enrollment (Mean ±SD) | 62 ± 11 |
| Mean age at diagnosis (Mean ± SD) | 55.77 ± 10.23 |
| Primary tumor | |
| Midgut NET(MNET) | 20 (42.5%) |
| Pancreatic NET(PNET) | 13 (27.7%) |
| Other | 14 (29.8%) |
| Lung NET | 3 |
| Rectal NET | 2 |
| Ovarian NET | 1 |
| Pheochromocytoma | 1 |
| Renal NET | 1 |
| Eustachian tube NET | 1 |
| Thymic NET | 1 |
| Ampullary NET | 1 |
| Unknown primary | 3 |
| Grade | |
| Grade 1 (Ki-67 ≤ 2) | 15 (31.9%) |
| Grade 2 (Ki-67 3–20) | 32 (68.1%) |
| Chromogranin A | |
| ≤110 | 14 (30%) |
| >110 | 33 (70%) |
| ECOG performance | |
| 0 | 23 (48.9%) |
| 1 | 24 (51.1%) |
| 2 | 0 (0%) |
| Previous treatments | |
| Surgery | 38 (80.9%) |
| Non-surgical treatments: | |
| chemotherapy | 30 (63.8%) |
| radiation | 12 (25.3%) |
| liver targeted therapy | 8 (17%) |
| Total | 14 (29.8%) |
| Site of metastases | |
| Liver | 37 (78.7%) |
| Lymph node | 24 (51%) |
| Peritoneum or mesentery | 9 (19.1%) |
| Bone | 15 (31.9%) |
| Lung | 4 (8.5%) |
| Other | 7 (14.9%) |
| Baseline NET status | |
| Progressive NET | 46 (97.9%) |
| Inoperable recurrence | 1 (2.2%) |
| Disease Status | RECIST 1.1, n = 47(%) | Choi Criteria, n = 47(%) |
|---|---|---|
| Complete Response (CR) | 2 (4.3%) | 2 (4.3%) |
| Partial Response (PR) | 13 (27.7%) | 19 (40.4%) |
| Stable Disease (SD) | 25 (53.1%) | 18 (38.3%) |
| Progressive Disease (PD) | 7 (14.9%) | 8 (17%) |
| Primary Site of Tumor | RECIST 1.1 | ||||
|---|---|---|---|---|---|
| CR | PR | SD | PD | Total | |
| Midgut NET | 0 | 1 (5%) | 16 (80%) | 3 (15%) | 20 |
| PNET | 0 | 9 (69.2%) | 1 (7.7%) | 3 (23.1%) | 13 |
| Others | 2 (14.3%) | 3 (21.4%) | 8 (57.2%) | 1 (7.1%) | 14 |
| Total | 2 (4.3%) | 13(27.7%) | 25 (53.1%) | 7 (14.9%) | 47 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sistani, G.; Sutherland, D.E.K.; Mujoomdar, A.; Wiseman, D.P.; Khatami, A.; Tsvetkova, E.; Reid, R.H.; Laidley, D.T. Efficacy of 177Lu-Dotatate Induction and Maintenance Therapy of Various Types of Neuroendocrine Tumors: A Phase II Registry Study. Curr. Oncol. 2021, 28, 115-127. https://doi.org/10.3390/curroncol28010015
Sistani G, Sutherland DEK, Mujoomdar A, Wiseman DP, Khatami A, Tsvetkova E, Reid RH, Laidley DT. Efficacy of 177Lu-Dotatate Induction and Maintenance Therapy of Various Types of Neuroendocrine Tumors: A Phase II Registry Study. Current Oncology. 2021; 28(1):115-127. https://doi.org/10.3390/curroncol28010015
Chicago/Turabian StyleSistani, Golmehr, Duncan E. K. Sutherland, Amol Mujoomdar, Daniele P. Wiseman, Alireza Khatami, Elena Tsvetkova, Robert H. Reid, and David T. Laidley. 2021. "Efficacy of 177Lu-Dotatate Induction and Maintenance Therapy of Various Types of Neuroendocrine Tumors: A Phase II Registry Study" Current Oncology 28, no. 1: 115-127. https://doi.org/10.3390/curroncol28010015
APA StyleSistani, G., Sutherland, D. E. K., Mujoomdar, A., Wiseman, D. P., Khatami, A., Tsvetkova, E., Reid, R. H., & Laidley, D. T. (2021). Efficacy of 177Lu-Dotatate Induction and Maintenance Therapy of Various Types of Neuroendocrine Tumors: A Phase II Registry Study. Current Oncology, 28(1), 115-127. https://doi.org/10.3390/curroncol28010015






