Inverse Association between Dietary Iron Intake and Gastric Cancer: A Pooled Analysis of Case-Control Studies of the Stop Consortium
Abstract
:1. Introduction
2. Methods
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Helicobacter and Cancer Collaborative Group. Gastric cancer and Helicobacter pylori: A combined analysis of 12 case control studies nested within prospective cohorts. Gut 2001, 49, 347–353. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peleteiro, B.; Bastos, A.; Ferro, A.; Lunet, N. Prevalence of Helicobacter pylori Infection Worldwide: A Systematic Review of Studies with National Coverage. Am. J. Dig. Dis. 2014, 59, 1698–1709. [Google Scholar] [CrossRef] [PubMed]
- Ladeiras-Lopes, R.; Pereira, A.K.; Nogueira, A.; Pinheiro-Torres, T.; Pinto, I.; Santos-Pereira, R.; Lunet, N. Smoking and gastric cancer: Systematic review and meta-analysis of cohort studies. Cancer Causes Control 2008, 19, 689–701. [Google Scholar] [CrossRef]
- World Cancer Research Fund International. Diet, Nutrition, Physical Activity and Stomach Cancer; World Cancer Research Fund International: London, UK, 2018. [Google Scholar]
- Vahid, F.; Davoodi, S.H. Nutritional Factors Involved in the Etiology of Gastric Cancer: A Systematic Review. Nutr. Cancer 2020, 73, 376–390. [Google Scholar] [CrossRef]
- Abbaspour, N.; Hurrell, R.; Kelishadi, R. Review on iron and its importance for human health. J. Res. Med. Sci. 2014, 19, 164–174. [Google Scholar]
- Cook, M.B.; Kamangar, F.; Weinstein, S.J.; Albanes, D.; Virtamo, J.; Taylor, P.R.; Abnet, C.C.; Wood, R.J.; Petty, G.; Cross, A.J.; et al. Iron in Relation to Gastric Cancer in the Alpha-Tocopherol, Beta-Carotene Cancer Prevention Study. Cancer Epidemiol. Biomark. Prev. 2012, 21, 2033–2042. [Google Scholar] [CrossRef] [Green Version]
- Fonseca-Nunes, A.; Jakszyn, P.; Agudo, A. Iron and cancer risk—A systematic review and meta-analysis of the epidemiological evidence’. Cancer Epidemiol. Biomark. Prev. 2014, 23, 12–31. [Google Scholar] [CrossRef] [Green Version]
- Noto, J.M.; Gaddy, J.A.; Lee, J.Y.; Piazuelo, M.B.; Friedman, D.B.; Colvin, D.C.; Romero-Gallo, J.; Suarez, G.; Loh, J.; Slaughter, J.; et al. Iron deficiency accelerates Helicobacter pylori–induced carcinogenesis in rodents and humans. J. Clin. Investig. 2012, 123, 479–492. [Google Scholar] [CrossRef] [Green Version]
- González, C.A.; Sala, N.; Rokkas, T. Gastric Cancer: Epidemiologic Aspects. Helicobacter 2013, 18, 34–38. [Google Scholar] [CrossRef]
- Ward, M.H.; Cross, A.J.; Abnet, C.; Sinha, R.; Markin, R.S.; Weisenburger, D.D. Heme iron from meat and risk of adenocarcinoma of the esophagus and stomach. Eur. J. Cancer Prev. 2012, 21, 134–138. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- International Agency for Research on Cancer. Red Meat and Processed Meat: IARC Monographs on the Evaluation of Carcinogenic Risks to Humans; IARC: Lyon, France, 2018; Volume 114. [Google Scholar]
- Pelucchi, C.; Lunet, N.; Boccia, S.; Zhang, Z.F.; Praud, D.; Boffetta, P.; Levi, F.; Matsuo, K.; Ito, H.; Hu, J.; et al. The stomach cancer pooling (StoP) project: Study design and presentation. Eur. J. Cancer Prev. 2015, 24, 16–23. [Google Scholar] [CrossRef] [PubMed]
- EFSA. Draft Scientific Opinion: Scientific Opinion on Dietary Reference Values for Iron. Available online: https://www.efsa.europa.eu/sites/default/files/consultation/150526.pdf (accessed on 31 March 2022).
- Lucenteforte, E.; Scita, V.; Bosetti, C.; Bertuccio, P.; Negri, E.; La Vecchia, C. Food Groups and Alcoholic Beverages and the Risk of Stomach Cancer: A Case-Control Study in Italy. Nutr. Cancer 2008, 60, 577–584. [Google Scholar] [CrossRef] [PubMed]
- Pakseresht, M.; Forman, D.; Malekzadeh, R.; Yazdanbod, A.; West, R.M.; Greenwood, D.C.; Crabtree, J.E.; Cade, J. Dietary habits and gastric cancer risk in north-west Iran. Cancer Causes Control 2011, 22, 725–736. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lunet, N.; Valbuena, C.; Lacerda Vieira, A.; Lopes, C.; David, L.; Carneiro, F.; Barros, H. Fruit and vegetable consumption and gastric cancer by location and histological type: Case-control and meta-analysis. Eur. J. Cancer Prev. 2007, 16, 312–327. [Google Scholar] [CrossRef]
- Castaño-Vinyals, G.; Aragones, N.; Pérez-Gómez, B.; Martín, V.; Llorca, J.; Moreno, E.; Altzibar, J.M.; Ardanaz, E.; de Sanjosé, S.; Jimenez-Moleon, J.J.; et al. Population-based multicase-control study in common tumors in Spain (MCC-Spain): Rationale and study design. Gac. Sanit. 2015, 29, 308–315. [Google Scholar] [CrossRef] [Green Version]
- Santibañez, M.; Alguacil, J.; de la Hera, M.G.; Navarrete-Muñoz, E.M.; Llorca, J.; Aragonés, N.; Kauppinen, T.; Vioque, J.; for the PANESOES Study Group. Occupational exposures and risk of stomach cancer by histological type. Occup. Environ. Med. 2011, 69, 268–275. [Google Scholar] [CrossRef]
- Hernández-Ramírez, R.U.; Galván-Portillo, M.V.; Ward, M.H.; Agudo, A.; González, C.A.; Oñate-Ocaña, L.F.; Herrera-Goepfert, R.; Palma-Coca, O.; López-Carrillo, L. Dietary intake of polyphenols, nitrate and nitrite and gastric cancer risk in Mexico City. Int. J. Cancer 2009, 125, 1424–1430. [Google Scholar] [CrossRef] [Green Version]
- Ward, M.H.; Bravo-Alvarado, J.; López-Carrillo, L.; López-Cervantes, M.; Ramírez-Espitia, A. Nutrient intake and gastric cancer in Mexico. Int. J. Cancer 1999, 83, 601–605. [Google Scholar] [CrossRef]
- López-Carrillo, L.; López-Cervantes, M.; Robles-Díaz, G.; Ramírez-Espitia, A.; Mohar-Betancourt, A.; Meneses-García, A.; López-Vidal, Y.; Blair, A. Capsaicin consumption, Helicobacter pylori positivity and gastric cancer in Mexico. Int. J. Cancer 2003, 106, 277–282. [Google Scholar] [CrossRef]
- Machida-Montani, A.; Sasazuki, S.; Inoue, M.; Natsukawa, S.; Shaura, K.; Koizumi, Y.; Kasuga, Y.; Hanaoka, T.; Tsugane, S. Association of Helicobacter pylori infection and environmental factors in non-cardia gastric cancer in Japan. Gastric Cancer 2004, 7, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Ward, M.H.; Sinha, R.; Heineman, E.F.; Rothman, N.; Markin, R.; Weisenburger, E.E.; Correa, P.; Zahm, S.H. Risk of adenocarcinoma of the stomach and esophagus with meat cooking method and doneness preference. Int. J. Cancer 1997, 71, 14–19. [Google Scholar] [CrossRef]
- Schatzkin, A.; Subar, A.F.; Thompson, F.E.; Harlan, L.C.; Tangrea, J.; Hollenbeck, A.R.; Hurwitz, P.E.; Coyle, L.; Schussler, N.; Michaud, D.S.; et al. Design and serendipity in establishing a large cohort with wide dietary intake distributions: The National Institutes of Health-American Association of Retired Persons Diet and Health Study. Am. J. Epidemiol. 2001, 154, 1119–1125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vander Weele, T.J.; Knol, M.J. A Tutorial on Interaction. Epidemiol. Methods 2014, 3, 1. [Google Scholar]
- Der Simonian, R.; Laird, N. Meta-analysis in clinical trials. Control Clin. Trials 1986, 7, 177–188. [Google Scholar] [CrossRef]
- StataCorp. Stata Statistical Software: Release 16; StataCorp LLC.: College Station, TX, USA, 2019. [Google Scholar]
- Report of the Advisory Group to Recommend Priorities for IARC Monographs during 2015–2019. Available online: https://monographs.iarc.who.int/wp-content/uploads/2018/08/14-002.pdf (accessed on 10 December 2021).
- Jakszyn, P.; Bingham, S.; Pera, G.; Agudo, A.; Luben, R.; Welch, A.; Boeing, H.; del Giudice, G.; Palli, D.; Saieva, C.; et al. Endogenous versus exogenous exposure to N -nitroso compounds and gastric cancer risk in the European Prospective Investigation into Cancer and Nutrition (EPIC-EURGAST) study. Carcinogenesis 2006, 27, 1497–1501. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cross, A.J.; Freedman, N.D.; Ren, J.; Ward, M.H.; Hollenbeck, A.R.; Schatzkin, A.; Sinha, R.; Abnet, C. Meat Consumption and Risk of Esophageal and Gastric Cancer in a Large Prospective Study. Am. J. Gastroenterol. 2011, 106, 432–442. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pelucchi, C.; Tramacere, I.; Bertuccio, P.; Tavani, A.; Negri, E.; La Vecchia, C. Dietary intake of selected micronutrients and gastric cancer risk: An Italian case-control study. Ann. Oncol. 2008, 20, 160–165. [Google Scholar] [CrossRef]
- Lee, D.H.; Anderson, K.E.; Folsom, A.R.; Jacobs, D.R., Jr. Heme iron, zinc and upper digestive tract cancer: The Iowa Women’s Health Study. Int. J. Cancer. 2005, 117, 643–647. [Google Scholar] [CrossRef]
- Tran, T.; Gunathilake, M.; Lee, J.; Choi, I.; Kim, Y.-I.; Kim, J. The Associations of Dietary Iron Intake and the Transferrin Receptor (TFRC) rs9846149 Polymorphism with the Risk of Gastric Cancer: A Case–Control Study Conducted in Korea. Nutrients 2021, 13, 2600. [Google Scholar] [CrossRef]
- Cornée, J.; Pobel, D.; Riboli, E.; Guyader, M.; Hémon, B. A case-control study of gastric cancer and nutritional factors in Marseille, France. Eur. J. Epidemiol. 1995, 11, 55–65. [Google Scholar] [CrossRef] [PubMed]
- Crownover, B.K.; Covey, C.J. Hereditary hemochromatosis. Am. Fam. Physician 2013, 87, 183–190. [Google Scholar] [PubMed]
- Mikhailova, S.V.; Babenko, V.; Ivanoshchuk, D.E.; Gubina, M.A.; Maksimov, V.N.; Solovjova, I.G.; Voevoda, M.I. Haplotype analysis of the HFE gene among populations of Northern Eurasia, in patients with metabolic disorders or stomach cancer, and in long-lived people. BMC Genet. 2016, 17, 83. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lagergren, K.; Wahlin, K.; Mattsson, F.; Alderson, D.; Lagergren, J. Haemochromatosis and gastrointestinal cancer. Int. J. Cancer 2016, 139, 1740–1743. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prá, D.; Rech Franke, S.I.; Pegas Henriques, J.A.; Fenech, M. A possible link between iron deficiency and gastrointestinal carcino-genesis. Nutr. Cancer 2009, 61, 415–426. [Google Scholar] [CrossRef]
- Broitman, S.A.; Velez, H.; Vitale, J.J. A Possible Role of Iron Deficiency in Gastric Cancer in Colombia. Adv. Exp. Med. Biol. 1981, 135, 155–181. [Google Scholar] [CrossRef]
- Hung, N.; Shen, C.-C.; Hu, Y.-W.; Hu, L.-Y.; Yeh, C.-M.; Teng, C.-J.; Kuan, A.-S.; Chen, S.-C.; Chen, T.-J.; Liu, C.-J. Risk of Cancer in Patients with Iron Deficiency Anemia: A Nationwide Population-Based Study. PLoS ONE 2015, 10, e0119647. [Google Scholar] [CrossRef] [Green Version]
- Hudak, L.; Jaraisy, A.; Haj, S.; Muhsen, K. An updated systematic review and meta-analysis on the association between Helico-bacter pylori infection and iron deficiency anemia. Helicobacter 2017, 22, e12330. [Google Scholar] [CrossRef]
- International Agency for Research on Cancer. Infection with Helicobacter pylori: IARC Monographs on the Evaluation of Carcinogenic Risks to Humans, Vol. 61, Schistosomes, Liver Flukes and Helicobacter pylori; IARC: Lyon, France, 1994; pp. 177–240. [Google Scholar]
- Bielik, V.; Kolisek, M. Bioaccessibility and Bioavailability of Minerals in Relation to a Healthy Gut Microbiome. Int. J. Mol. Sci. 2021, 22, 6803. [Google Scholar] [CrossRef]
- Epplein, M.; Zheng, W.; Li, H.; Peek, R.M., Jr.; Correa, P.; Gao, J.; Michel, A.; Pawlita, M.; Cai, Q.; Xiang, Y.-B.; et al. Diet, Helicobacter pylori strain-specific infection, and gastric cancer risk among Chinese men. Nutr. Cancer. 2014, 66, 550–557. [Google Scholar] [CrossRef] [Green Version]
- Ferro, A.; Morais, S.; Rota, M.; Pelluchi, C.; Bertuccio, P.; Bonzi, R.; Galeone, C.; Zhang, F.-Z.; Matsuo, K.; Ito, H.; et al. Alcohol intake and gastric cancer: Meta-analyses of published data versus individual par-ticipant data pooled analyses (StoP Project). Cancer Epidemiol. 2018, 54, 125–132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buiatti, E.; Palli, D.; Bianchi, S.; Decarli, A.; Amadori, D.; Avellini, C.; Cipriani, F.; Cocco, P.; Giacosa, A.; Lorenzini, L.; et al. A case-control study of gastric cancer and diet in Italy. III. Risk patterns by histologic type. Int. J. Cancer 1991, 48, 369–374. [Google Scholar] [CrossRef] [PubMed]
- Buckland, G.; Agudo, A.; Luján, L.; Jakszyn, P.; Bueno-de-Mesquita, H.B.; Palli, D.; Boeing, H.; Carneiro, F.; Krogh, V.; Sacerdote, C.; et al. Adherence to a Mediterranean diet and risk of gastric adenocarcinoma within the Eu-ropean Prospective Investigation into Cancer and Nutrition (EPIC) cohort study. Am. J. Clin. Nutr. 2010, 91, 381–390. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harrison, L.E.; Zhang, Z.F.; Karpeh, M.S.; Sun, M.; Kurtz, R.C. The role of dietary factors in the intestinal and diffuse histologic subtypes of gastric adenocarcinoma: A case-control study in the U.S. Cancer 1997, 80, 1021–1028. [Google Scholar] [CrossRef]
- Machlowska, J.; Baj, J.; Sitarz, M.; Maciejewski, R.; Sitarz, R. Gastric Cancer: Epidemiology, Risk Factors, Classification, Genomic Characteristics and Treatment Strategies. Int. J. Mol. Sci. 2020, 21, 4012. [Google Scholar] [CrossRef] [PubMed]
- Rosania, R.; Chiapponi, C.; Malfertheiner, P.; Venerito, M. Nutrition in Patients with Gastric Cancer: An Update. Gastrointest. Tumors 2016, 2, 178–187. [Google Scholar] [CrossRef]
- Collatuzzo, G.; Pelucchi, C.; Negri, E.; López-Carrillo, L.; Tsugane, S.; Hidaka, A.; Hamada, G.S.; Hernández-Ramírez, R.U.; López-Cervantes, M.; Malekzadeh, R.; et al. Exploring the interactions between Helicobacter pylori (Hp) infection and other risk factors of gastric cancer: A pooled analysis in the Stomach cancer Pooling (StoP) Project. Int. J. Cancer 2021, 149, 1228–1238. [Google Scholar] [CrossRef]
- Rothman, K.J.; Vander Weele, T.J.; Lash, T.L. Cohort Studies. In Modern Epidemiology, 4th ed.; Lash, T.L., Vander Weele, T.J., Haneuse, S., Rothman, K.J., Eds.; Wolters Kluwer: Philadelphia, PA, USA, 2021; pp. 143–160. [Google Scholar]
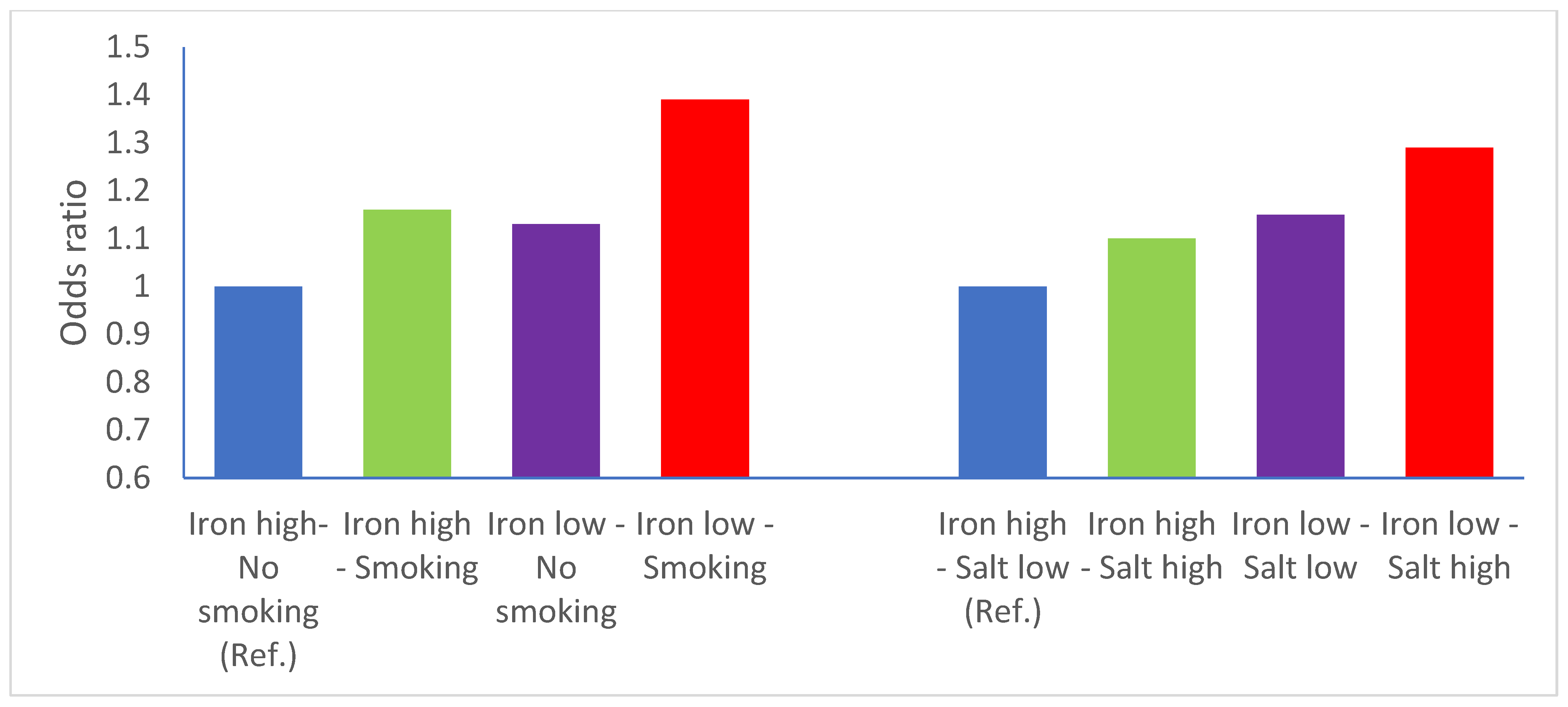

| Cases N (%) | Controls N (%) | |
|---|---|---|
| Total | 4658 (100.0) | 12,247 (100.0) |
| Sex | ||
| Male | 3138 (67.4) | 7343 (60.0) |
| Female | 1520 (32.6) | 4904 (40.0) |
| Age (years) | ||
| ≤55 | 871 (18.7) | 2776 (22.7) |
| 56–65 | 1048 (22.5) | 3025 (24.7) |
| 66–75 | 1819 (39.1) | 4392 (35.9) |
| ≥76 | 920 (19.8) | 2050 (16.7) |
| Cigarette smoking | ||
| Never | 1876 (41.6) | 5444(45,6) |
| Former | 1710 (37.9) | 4187 (35.1) |
| Current | 927 (20.5) | 2311 (19.4) |
| Alcohol drinking | ||
| Never | 1205 (28.7) | 3144 (28.4) |
| Low | 1464 (34.8) | 4412 (39.8) |
| Intermediate | 958 (22.8) | 2533 (22.9) |
| High | 576 (13.7) | 985 (8.9) |
| Socio-economic status | ||
| Low | 1823 (40.3) | 4142 (34.4) |
| Intermediate | 1726 (38.1) | 4555 (37.8) |
| High | 976 (21.6) | 3342 (27.8) |
| Salt consumption | ||
| Low | 2046 (45.4) | 5080 (45.1) |
| Intermediate | 1168 (25.9) | 3149 (27.9) |
| High | 1289 (28.6) | 3046 (27.0) |
| Meat intake | ||
| Q1 | 986 (24.2) | 2357 (25.4) |
| Q2 | 900 (22.1) | 2439 (26.3) |
| Q3 | 988 (24.2) | 2359 (25.4) |
| Q4 | 1202 (29.5) | 2131 (23.0) |
| Vegetables and fruit intake | ||
| Low | 1552 (36.5) | 3406 (30.8) |
| Intermediate | 1410 (33.2) | 3773 (34.2) |
| High | 1286 (30.3) | 3869 (35.0) |
| Total caloric intake | ||
| Q1 | 965 (21.8) | 2853 (24.5) |
| Q2 | 1162 (26.3) | 3303 (28.4) |
| Q3 | 1127 (25.5) | 3030 (26.0) |
| Q4 | 1171 (26.5) | 2456 (21.1) |
| Dietary iron intake | ||
| Q1 | 969 (21.6) | 2874 (24.5) |
| Q2 | 1083 (24.1) | 3171 (27.0) |
| Q3 | 1160 (25.9) | 2908 (24.8) |
| Q4 | 1274 (28.4) | 2780 (23.7) |
| Family history of GC | ||
| No | 1170 (84.0) | 5693 (93.0) |
| Yes | 355 (16.0) | 426 (7.0) |
| BMI | ||
| 18.5–24.9 | 1557 (40.6) | 3608 (36.6) |
| 25–29.9 | 1539 (40.1) | 4297 (43.6) |
| 30–34.9 | 585 (15.2) | 1519 (15.4) |
| 35–50 | 157 (4.1) | 438 (4.4) |
| Anatomical site of GC | NA | |
| Cardia | 982 (30.3) | |
| Non-cardia | 2258 (69.7) | |
| Histological type of GC | NA | |
| Intestinal | 1119 (59.6) | |
| Diffuse | 791 (41.4) |
| Covariate | Adjusted OR | 95% CI |
|---|---|---|
| Dietary iron (quartiles) | ||
| Q1 | Ref | |
| Q2 | 0.88 | 0.78–0.99 |
| Q3 | 0.82 | 0.72–0.95 |
| Q4 | 0.66 | 0.56–0.78 |
| Dietary iron (one quartile increase) | 0.88 | 0.83–0.93 |
| Tobacco smoking | ||
| Never | Ref | |
| Current | 1.17 | 1.07–1.29 |
| Former | 1.22 | 1.09–1.36 |
| Socioeconomic status | ||
| Low | Ref | |
| Intermediate | 0.65 | 0.59–0.72 |
| High | 0.52 | 0.46–0.58 |
| Calories (quartiles) | ||
| Q1 | Ref | |
| Q2 | 1.20 | 1.06–1.36 |
| Q3 | 1.28 | 1.11–1.49 |
| Q4 | 1.57 | 1.33–1.87 |
| Salt intake | ||
| Low | Ref | |
| Medium | 1.12 | 1.01–1.24 |
| High | 1.15 | 1.03–1.29 |
| Meat intake (quartiles) | ||
| Q1 | Ref | |
| Q2 | 1.20 | 1.03–1.40 |
| Q3 | 1.23 | 1.03–1.48 |
| Q4 | 1.28 | 1.05–1.56 |
| BMI (kg/m2) | ||
| 18.5–24.9 | Ref | |
| 25–29.9 | 0.77 | 0.70–0.84 |
| 30–34.9 | 0.84 | 0.74–0.95 |
| 35–50 | 0.85 | 0.69–1.05 |
| Alcohol drinking | ||
| Never | Ref | |
| Low | 0.83 | 0.75–0.91 |
| Intermediate | 0.90 | 0.80–1.00 |
| High | 1.24 | 1.08–1.43 |
| Family history of GC | ||
| Negative | Ref | |
| Positive | 2.37 | 2.01–2.79 |
| Vegetable and fruit intake | ||
| Low | Ref. | |
| Medium | 0.80 | 0.73–0.88 |
| High | 0.69 | 0.61–0.76 |
| Dietary Iron Intake | Anatomical Site | Histological Type | ||
|---|---|---|---|---|
| Cardia OR (95%CI) | Non Cardia OR (95%CI) | Diffuse OR (95%CI) | Intestinal OR (95%CI) | |
| Quartiles | ||||
| Q1 | Ref | Ref | Ref | Ref |
| Q2 | 1.09 (0.86–1.39) | 0.81 (0.69–0.96) | 0.68 (0.51–0.91) | 0.82 (0.64–1.04) |
| Q3 | 0.96 (0.73–1.25) | 0.74 (0.61–0.90) | 0.58 (0.42–0.81) | 0.74 (0.56–0.98) |
| Q4 | 0.63 (0.47–0.86) | 0.64 (0.51–0.81) | 0.46 (0.31–0.68) | 0.65 (0.47–0.92) |
| One quartile increase | 0.85 (0.77–0.94) | 0.87 (0.81–0.94) | 0.79 (0.69–0.89) | 0.88 (0.79–0.98) |
| Reference | Iron Exposure | Comparison | OR | 95% CI | Design |
|---|---|---|---|---|---|
| [31] | Dietary iron intake (heme) | Top vs. bottom quartile | 1.67 | 1.20–2.34 | Cohort |
| [32] | Dietary iron intake (heme) | Top vs. bottom quintile | 0.83 cardia 0.72 non-cardia | 0.53–1.30 0.48–1.08 | Cohort |
| 100 μg increase | 0.95 cardia 0.96 non-cardia | 0.86–1.05 0.87–1.06 | |||
| [33] | Dietary iron intake | Top vs. bottom quartile | 0.56 | 0.27–1.15 | Case-control |
| [34] | Dietary iron intake (heme) | Top vs. bottom quintile (p for trend = 0.18) | 4.83 | NA | Cohort |
| [35] | Dietary iron intake | Top vs. bottom tertile (p for trend = 0.018) | 0.65 total 0.81 heme 0.64 non heme | 0.45–0.94 0.56–1.17 0.44–0.92 | Case-control |
| [8] | Dietary iron intake (calorie adjusted) | Top vs. bottom quartile | 1.05 | 0.60–1.85 | Cohort |
| [12] | Dietary iron intake | Top vs. bottom quartile | 1.71 total 1.99 heme | 0.75–3.18 1.00–3.95 | Case-control |
| [36] | Dietary ironintake | Top vs. bottom tertile (p for trend = 0.02) | 0.41 | 0.19–0.89 | Case-control |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Collatuzzo, G.; Teglia, F.; Pelucchi, C.; Negri, E.; Rabkin, C.S.; Liao, L.M.; Sinha, R.; López-Carrillo, L.; Lunet, N.; Morais, S.; et al. Inverse Association between Dietary Iron Intake and Gastric Cancer: A Pooled Analysis of Case-Control Studies of the Stop Consortium. Nutrients 2022, 14, 2555. https://doi.org/10.3390/nu14122555
Collatuzzo G, Teglia F, Pelucchi C, Negri E, Rabkin CS, Liao LM, Sinha R, López-Carrillo L, Lunet N, Morais S, et al. Inverse Association between Dietary Iron Intake and Gastric Cancer: A Pooled Analysis of Case-Control Studies of the Stop Consortium. Nutrients. 2022; 14(12):2555. https://doi.org/10.3390/nu14122555
Chicago/Turabian StyleCollatuzzo, Giulia, Federica Teglia, Claudio Pelucchi, Eva Negri, Charles S. Rabkin, Linda M. Liao, Rashmi Sinha, Lizbeth López-Carrillo, Nuno Lunet, Samantha Morais, and et al. 2022. "Inverse Association between Dietary Iron Intake and Gastric Cancer: A Pooled Analysis of Case-Control Studies of the Stop Consortium" Nutrients 14, no. 12: 2555. https://doi.org/10.3390/nu14122555
APA StyleCollatuzzo, G., Teglia, F., Pelucchi, C., Negri, E., Rabkin, C. S., Liao, L. M., Sinha, R., López-Carrillo, L., Lunet, N., Morais, S., Aragonés, N., Moreno, V., Vioque, J., Garcia de la Hera, M., Ward, M. H., Malekzadeh, R., Pakseresht, M., Hernández-Ramírez, R. U., López-Cervantes, M., ... Boffetta, P. (2022). Inverse Association between Dietary Iron Intake and Gastric Cancer: A Pooled Analysis of Case-Control Studies of the Stop Consortium. Nutrients, 14(12), 2555. https://doi.org/10.3390/nu14122555









