Relation of Dietary Patterns and Nutritional Profile to Hepatic Fibrosis in a Sample of Lebanese Non-Alcoholic Fatty Liver Disease Patients
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Sample Selection, Inclusion Criteria
2.3. Study Protocol
2.4. Measuring Tools
2.5. Validity and Reproducibility of the Food Frequency Questionnaire
2.6. Ethical Considerations
2.7. Analysis Plan and Statistical Tests
3. Results
3.1. Variation of Age, Sociodemographic, Clinical, and Biochemical Parameters of the NAFLD Patients between the NFS Group (<−1.45/≥−1.45)
3.2. Dietary Patterns
3.3. Association between Dietary Patterns and Sociodemographic, Environmental, and Clinical Characteristics in the Study Population
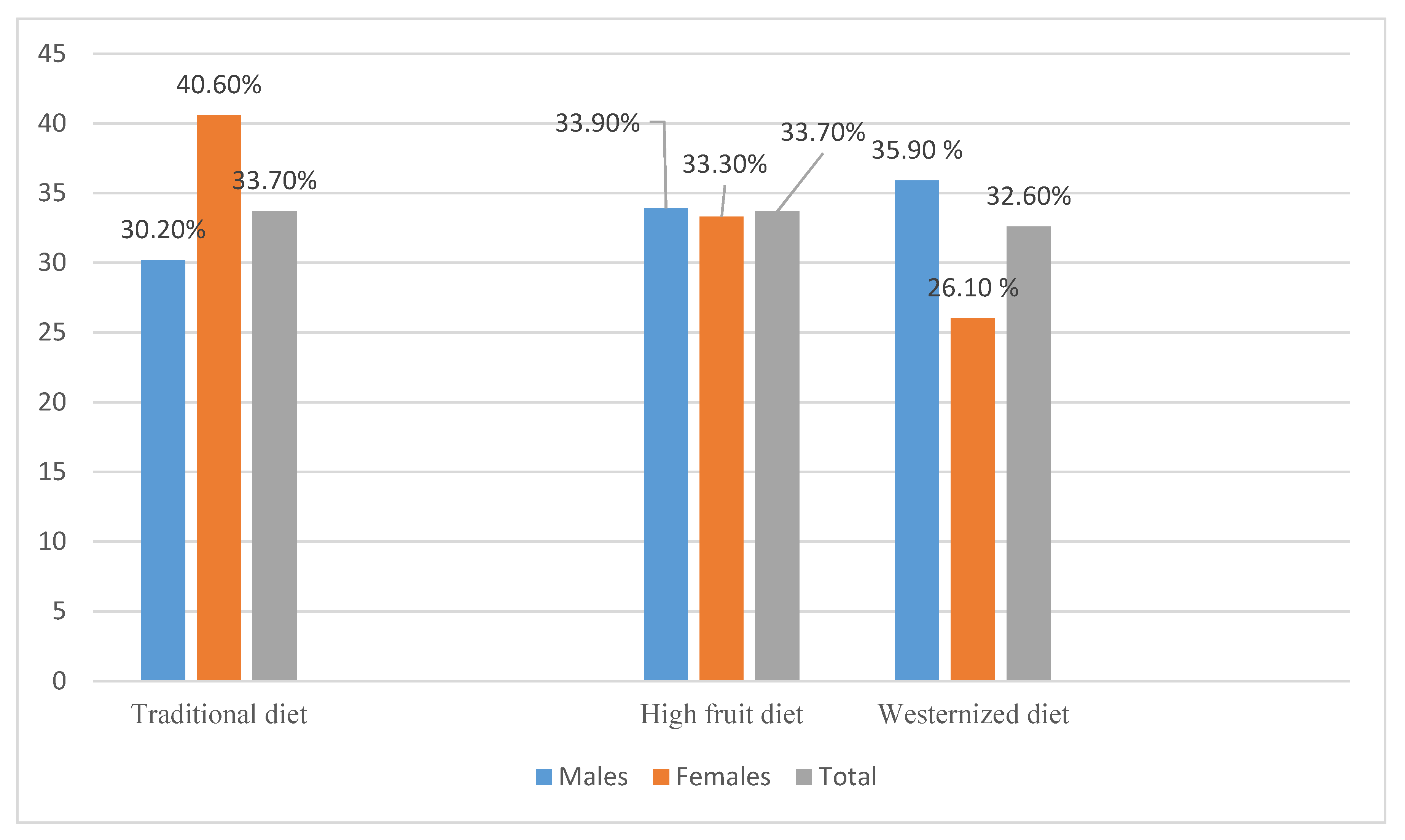
3.4. Hepatic Fibrosis across Tertiles (T) of Dietary Pattern Scores
3.5. Hepatic Fibrosis across Tertiles of Food Groups
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ong, J.P.; Younossi, Z.M. Epidemiology and Natural History of NAFLD and NASH. Clin. Liver Dis. 2007, 11, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Vernon, G.; Baranova, A.; Younossi, Z.M. Systematic review: The epidemiology and natural history of non-alcoholic fatty liver disease and non-alcoholic steatohepatitis in adults. Aliment. Pharmacol. Ther. 2011, 34, 274–285. [Google Scholar] [CrossRef] [PubMed]
- Ong, J.P.; Pitts, A.; Younossi, Z.M. Increased overall mortality and liver-related mortality in non-alcoholic fatty liver disease. J Hepatol. 2008, 49, 608–612. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, E.; Tokushige, K. Prevalence, gender, ethnic variations, and prognosis of NASH. J. Gastroenterol. 2011, 46, 63–69. [Google Scholar] [CrossRef]
- Rikhi, R.; Singh, T.; Esfeh, J.M. Work up of fatty liver by primary care physicians, review. Ann. Med. Surg. 2020, 50, 41–48. [Google Scholar] [CrossRef]
- Marchesini, G.; Brizi, M.; Morselli/labate, A.M.; Bianchi, G.; Bugianesi, E.; McCullough, A.J.; Forlani, G.; Melchionda, N. Association of nonalcoholic fatty liver disease with insulin resistance. Am. J. Med. 1999, 107, 450–455. [Google Scholar] [CrossRef]
- Adams, L.A.; Angulo, P. Role of Liver Biopsy and Serum Markers of Liver Fibrosis in Non-alcoholic Fatty Liver Disease. Clin. Liver Dis. 2007, 11, 25–35. [Google Scholar] [CrossRef]
- Nakano, M.; Murohisa, T.; Imai, Y.; Hiraishi, H. Validity of the NAFLD fibrosis score in a Japanese population. Nihon Shokakibyo Gakkai Zasshi 2012, 109, 751–759. [Google Scholar]
- Koch, M.; Nöthlings, U.; Lieb, W. Dietary patterns and fatty liver disease. Curr. Opin. Lipidol. 2015, 26, 35–41. [Google Scholar] [CrossRef]
- Landrivon, G.; Delahaye, F. La Recherche Clinique de l’idée à la Publication; Elsevier-Masson: Paris, France, 1997; p. 296. [Google Scholar]
- Hu, F.B.; Satija, A.; Rimm, E.B.; Spiegelman, D.; Sampson, L.; Rosner, B.; Camargo, C.A.; Stampfer, M.; Willett, W.C. Diet Assessment Methods in the Nurses’ Health Studies and Contribution to Evidence-Based Nutritional Policies and Guidelines. Am. J. Public Health 2016, 106, 1567–1572. [Google Scholar] [CrossRef]
- Alberti, K.G.M.M.; Eckel, R.H.; Grundy, S.M.; Zimmet, P.Z.; Cleeman, J.I.; Donato, K.A.; Fruchart, J.C.; James, W.P.T.; Loria, C.M.; Smith, S.C., Jr. Harmonizing the metabolic syndrome: A joint interim statement of the international diabetes federation task force on epidemiology and prevention; National heart, lung, and blood institute; American heart association; World heart federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation 2009, 120, 1640–1645. [Google Scholar] [CrossRef] [PubMed]
- Ainsworth, B.E.; Haskell, W.L.; Leon, A.S.; Jacobs, D.R., Jr.; Montoye, H.J.; Sallis, J.F.; Paffenbarger, R.S., Jr. Compendium of Physical Activities: Classification of energy costs of human physical activities. Med. Sci. Sports Exerc. 1993, 25, 71–80. [Google Scholar] [CrossRef] [PubMed]
- International Physical Activity Questionnaires. (May 2001); IPAQ: Short Last 7 Days Self-Administered Format. Available online: www.ipaq.ki.se (accessed on 16 December 2021).
- Nelson, M.; Atkinson, M.; Darbyshire, S. Food photography II: Use of food photographs for estimating portion size and the nutrient content of meals. Br. J. Nutr. 1996, 76, 31–49. [Google Scholar] [CrossRef] [PubMed]
- Food Composition | Food and Nutrition Information Center | NAL | USDA. 2020. Available online: https://www.nal.usda.gov/fnic/food-composition (accessed on 10 December 2021).
- Weir, C.B.; Jan, A. BMI Classification Percentile and Cut off Points. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Stern, S.E.; Williams, K.; Ferrannini, E.; DeFronzo, R.A.; Bogardus, C.; Stern, M.P. Identification of Individuals With Insulin Resistance Using Routine Clinical Measurements. Diabetes 2005, 54, 333–339. [Google Scholar] [CrossRef] [PubMed]
- Fakhoury-Sayegh, N.; Younes, H.; Heraoui, G.N.H.A.; Sayegh, R. Nutritional Profile and Dietary Patterns of Lebanese Non-Alcoholic Fatty Liver Disease Patients: A Case-Control Study. Nutrients 2017, 14, 1245. [Google Scholar] [CrossRef] [PubMed]
- Distefano, C.; Zhu, M.; Mindrilla, D. Understanding and using factor scores: Considerations for the applied researcher. Pract. Assess. Res. Eval. 2009, 14, 1–11. [Google Scholar] [CrossRef]
- Bedogni, G.; Miglioli, L.; Masutti, F.; Tiribelli, C.; Marchesini, G.; Bellentani, S. Prevalence of and risk factors for nonalcoholic fatty liver disease: The Dionysos nutrition and liver study. Hepatology 2005, 42, 44–52. [Google Scholar] [CrossRef]
- Shen, L.; Fan, J.-G.; Shao, Y.; Zeng, M.-D.; Wang, J.-R.; Luo, G.-H.; Li, J.-Q.; Chen, S.-Y. Prevalence of nonalcoholic fatty liver among administrative officers in Shanghai: An epidemiological survey. World J. Gastroenterol. 2003, 9, 1106–1110. [Google Scholar] [CrossRef]
- Sibai, A.-M.; Obeid, O.; Batal, M.; Adra, N.; Khoury, D.E.; Hwalla, N. Prevalence and correlates of metabolic syndrome in an adult Lebanese population. Glob. Heart 2008, 3, 83–90. [Google Scholar] [CrossRef]
- Dowman, J.K.; Tomlinson, J.; Newsome, P. Pathogenesis of non-alcoholic fatty liver disease. QJM Int. J. Med. 2009, 103, 71–83. [Google Scholar] [CrossRef]
- Ricci, G.; Canducci, E.; Pasini, V.; Rossi, A.; Bersani, G.; Ricci, E.; Alvisi, V. Nutrient intake in Italian obese patients: Relationships with insulin resistance and markers of non-alcoholic fatty liver disease. Nutrition 2011, 27, 672–676. [Google Scholar] [CrossRef] [PubMed]
- WHO | Diet, Nutrition and the Prevention of Chronic Diseases. WHO. 2020. Available online: https://www.who.int/dietphysicalactivity/publications/trs916/en/ (accessed on 10 November 2021).
- Illouz, F.; Roulier, V.; Rod, A.; Gallois, Y.; Pellé, C.-P.; Aubé, C.; Rohmer, V.; Ritz, P.; Ducluzeau, P. Distribution of adipose tissue: Quantification and relationship with hepatic steatosis and vascular profiles of type 2 diabetic patients with metabolic syndrome. Diabetes Metab. 2008, 34, 68–74. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ghamar-Chehreh, M.E.; Khedmat, H.; Amini, M.; Taheri, S. Predictive value of having positive family history of cardio-vascular disorders, diabetes mellitus, dyslipidemia, and hypertension in non-alcoholic fatty liver disease patients. Acta. Med. Iran 2013, 51, 307–313. [Google Scholar]
- Gerber, L.; Otgonsuren, M.; Mishra, A.; Escheik, C.; Birerdinc, A.; Stepanova, M.; Younossi, Z.M. Non-alcoholic fatty liver disease (NAFLD) is associated with low level of physical activity: A population-based study. Aliment. Pharmacol. Ther. 2012, 36, 772–781. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.A.; Greenson, J.K.; Chao, C.; Anderson, L.; Peterman, D.; Jacobson, J.; Emick, D.; Lok, A.S.; Conjeevaram, H.S. One-Year Intense Nutritional Counseling Results in Histological Improvement in Patients with Nonalcoholic Steatohepatitis: A Pilot Study. Am. J. Gastroenterol. 2005, 100, 1072–1081. [Google Scholar] [CrossRef]
- Suzuki, A.; Lindor, K.; Saver, J.S.; Lymp, J.; Mendes, F.; Muto, A.; Okada, T.; Angulo, P. Effect of changes on body weight and lifestyle in nonalcoholic fatty liver disease. J. Hepatol. 2005, 43, 1060–1066. [Google Scholar] [CrossRef]
- Sibai, A.M.; Obeid, O.; Batal, M.; Adra, N.; El Khoury, D.; Hwalla, N. Non-Communicable Diseases and Behavioral Risk Factor Survey; World Health Organization: Beirut, Lebanon, 2010. [Google Scholar]
- De Castro, P.C.S.; Alberton, H.C.P.; Pedroso, M.L.A.; Morsoletto, D.B.G.; Junior, A.P.; Ivantes, C.A.P. Evaluation of progression of hepatic fibrosis in a group of patients with non-alcoholic fatty liver disease accompanied for 10 years. Arq. Gastroenterol. 2019, 56, 256–260. [Google Scholar] [CrossRef]
- Åberg, F.; Danford, C.J.; Thiele, M.; Talbäck, M.; Rasmussen, D.N.; Jiang, Z.G.; Hammar, N.; Nasr, P.; Ekstedt, M.; But, A.; et al. A Dynamic Aspartate-to-Alanine Aminotransferase Ratio Provides Valid Predictions of Incident Severe Liver Disease. Hepatol. Commun. 2021, 5, 1021–1035. [Google Scholar] [CrossRef]
- Bugianesi, E.; Zannoni, C.; Vanni, E.; Marzocchi, R.; Marchesini, G. Non-alcoholic fatty liver and insulin resistance: A cause–effect relationship? Dig. Liver Dis. 2004, 36, 165–173. [Google Scholar] [CrossRef]
- Abdelmalek, M.F.; Suzuki, A.; Guy, C.; Unalp-Arida, A.; Colvin, R.; Johnson, R.J.; Diehl, A.M.; Nonalcoholic Steatohepatitis Clinical Research Network. Increased fructose consumption is associated with fibrosis severity in patients with nonalcoholic fatty liver disease. Hepatology 2010, 51, 1961–1971. [Google Scholar] [CrossRef]
- Hiza, H.A.; Casavale, K.O.; Guenther, P.; Davis, C.A. Diet Quality of Americans Differs by Age, Sex, Race/Ethnicity, Income, and Education Level. J. Acad. Nutr. Diet. 2013, 113, 297–306. [Google Scholar] [CrossRef] [PubMed]
- Rippin, H.L.; Hutchinson, J.; Greenwood, D.C.; Jewell, J.; Breda, J.J.; Martin, A.; Rippin, D.M.; Schindler, K.; Rust, P.; Fagt, S.; et al. Inequalities in education and national income are associated with poorer diet: Pooled analysis of individual participant data across 12 European countries. PLoS ONE 2020, 15, e0232447. [Google Scholar] [CrossRef]
- Gutiérrez-Pliego, L.E.; Camarillo-Romero, E.D.S.; Montenegro-Morales, L.P.; Garduño-García, J.D.J. Dietary patterns associated with body mass index (BMI) and lifestyle in Mexican adolescents. BMC Public Health 2016, 16, 850. [Google Scholar] [CrossRef] [PubMed]
- Sofi, F.; Casini, A. Mediterranean diet and non-alcoholic fatty liver disease: New therapeutic option around the corner? World J. Gastroenterol. 2014, 20, 7339–7346. [Google Scholar] [CrossRef] [PubMed]
- Assy, N.; Nassar, F.; Nasser, G.; Grosovski, M. Olive oil consumption and non-alcoholic fatty liver disease. World J. Gastroenterol. 2009, 15, 1809–1815. [Google Scholar] [CrossRef]
- Oddy, W.H.; Herbison, C.E.; Jacoby, P.; Ambrosini, G.L.; O’Sullivan, T.A.; Ayonrinde, O.T.; Olynyk, J.K.; Black, L.J.; Beilin, L.J.; Mori, T.A.; et al. The Western Dietary Pattern Is Prospectively Associated With Nonalcoholic Fatty Liver Disease in Adolescence. Am. J. Gastroenterol. 2013, 108, 778–785. [Google Scholar] [CrossRef]
- Schwarz, J.M.; Neese, R.A.; Turner, S.; Dare, D.; Hellerstein, M.K. Short-term alterations in carbohydrate energy intake in humans. J. Clin. Investig. 1995, 96, 2735–2743. [Google Scholar] [CrossRef]
- Solga, S.; Alkhuraishe, A.R.; Clark, J.M.; Torbenson, M.; Greenwald, A.; Diehl, A.M.; Magnuson, T. Dietary Composition and Nonalcoholic Fatty Liver Disease. Dig. Dis. Sci. 2004, 49, 1578–1583. [Google Scholar] [CrossRef]
- Zivkovic, A.M.; German, J.B.; Sanyal, A.J. Comparative review of diets for the metabolic syndrome: Implications for nonalcoholic fatty liver disease. Am. J. Clin. Nutr. 2007, 86, 285–300. [Google Scholar] [CrossRef]
- Yoshikawa, T.; Shimano, H.; Yahagi, N.; Ide, T.; Amemiya-Kudo, M.; Matsuzaka, T.; Nakakuki, M.; Tomita, S.; Okazaki, H.; Tamura, Y.; et al. Polyunsaturated Fatty Acids Suppress Sterol Regulatory Element-binding Protein 1c Promoter Activity by Inhibition of Liver X Receptor (LXR) Binding to LXR Response Elements. J. Biol. Chem. 2002, 277, 1705–1711. [Google Scholar] [CrossRef]
- Nasreddine, L.; Hwalla, N.; Sibai, A.; Hamzé, M.; Parent-Massin, D. Food consumption patterns in an adult urban population in Beirut, Lebanon. Public Health Nutr. 2006, 9, 194–203. [Google Scholar] [CrossRef] [PubMed]
- Itoh, M.; Suganami, T.; Satoh, N.; Tanimoto-Koyama, K.; Yuan, X.; Tanaka, M.; Kawano, H.; Yano, T.; Aoe, S.; Takeya, M.; et al. Increased Adiponectin Secretion by Highly Purified Eicosapentaenoic Acid in Rodent Models of Obesity and Human Obese Subjects. Arter. Thromb. Vasc. Biol. 2007, 27, 1918–1925. [Google Scholar] [CrossRef] [PubMed]
- Sugano, M.; Hirahara, F. Polyunsaturated fatty acids in the food chain in Japan. Am. J. Clin. Nutr. 2000, 71, 189S–196S. [Google Scholar] [CrossRef]
- Keim, N.L.; Mares-Perlman, J.A. Development of Hepatic Steatosis and Essential Fatty Acid Deficiency in Rats with Hypercaloric, Fat-Free Parenteral Nutrition. J. Nutr. 1984, 114, 1807–1815. [Google Scholar] [CrossRef]
- Werner, A.; Havinga, R.; Bos, T.; Bloks, V.W.; Kuipers, F.; Verkade, H.J. Essential fatty acid deficiency in mice is associated with hepatic steatosis and secretion of large VLDL particles. Am. J. Physiol. Liver Physiol. 2005, 288, G1150–G1158. [Google Scholar] [CrossRef]
- Alwayn, I.P.; Javid, P.J.; Gura, K.M.; Nosé, V.; Ollero, M.; Puder, M. Do polyunsaturated fatty acids ameliorate hepatic steatosis in obese mice by SREPB-1 suppression or by correcting essential fatty acid deficiency. Hepatology 2004, 39, 1176–1177. [Google Scholar] [CrossRef] [PubMed]
- Cazeils, J.L.; Bouillier-Oudot, M.; Auvergne, A.; Candau, M.; Babile, R. Lipid composition of hepatocyte plasma membranes from geese overfed with corn. Lipids 1999, 34, 937–942. [Google Scholar] [CrossRef]
- Bouziane, M.; Prost, J.; Belleville, J. Dietary protein deficiency affects n−3 and n−6 polyunsaturated fatty acids hepatic storage and very low density lipoprotein transport in rats on different diets. Lipids 1994, 29, 265–272. [Google Scholar] [CrossRef]
- Byrne, C.D. Fatty liver: Role of inflammation and fatty acid nutrition. Prostaglandins Leukot. Essent. Fat. Acids 2010, 82, 265–271. [Google Scholar] [CrossRef]
- Schwenzer, N.F.; Springer, F.; Schraml, C.; Stefan, N.; Machann, J.; Schick, F. Non-invasive assessment and quantification of liver steatosis by ultrasound, computed tomography and magnetic resonance. J. Hepatol. 2009, 51, 433–445. [Google Scholar] [CrossRef]
- Abenavoli, L.; Milic, N.; Di Renzo, L.; Preveden, T.; Medić-Stojanoska, M.; De Lorenzo, A. Metabolic aspects of adult patients with nonalcoholic fatty liver disease. World J. Gastroenterol. 2016, 22, 7006–7016. [Google Scholar] [CrossRef] [PubMed]

| NFS Scores | p-Value | Total (n = 320) | ||
|---|---|---|---|---|
| <−1.45 n = 191 | ≥−1.45 n = 129 | |||
| Age (years), mean ± SD | 40.04 ± 0.11 | 47.65 ± 0.13 | 0.0001 | 43.34 ± 0.12 |
| Sex | ||||
| Male | 125 (65.5%) | 88 (67.9%) | 0.778 | 213 (66.5%) |
| Female | 66 (34.5%) | 41 (32.1%) | 107 (33.6%) | |
| Place of residence, n (%) | ||||
| Mount Lebanon | 57 (29.8%) | 54 (41.9%) | 0.314 | 111 (34.7%) |
| North | 23 (11.9%) | 6 (4.7%) | 29 (9.1%) | |
| South | 14 (7.4%) | 9 (6.9%) | 23 (7.2%) | |
| Beirut | 47 (24.4%) | 30 (23.6%) | 77 (24.1%) | |
| Bekaa | 22 (11.4%) | 11 (8.5%) | 33 (10.3%) | |
| Nabatieh | 17 (9.1%) | 13 (10.1%) | 30 (9.3%) | |
| Abroad | 11 (5.7%) | 6 (4.7%) | 17 (5.3%) | |
| Marital Status, n (%) | ||||
| Single | 42 (22.0%) | 24 (18.9%) | 0.853 | 66 (20.6%) |
| Married | 144 (75.4%) | 100 (77.5%) | 244 (75.9%) | |
| Divorced | 3 (1.6%) | 4 (2.8%) | 7 (2.10%) | |
| Widow/er | 2 (1.04%) | 1 (0.8%) | 3 (1.10%) | |
| Academic level, n (%) | ||||
| Illiterate | 1 (0.6%) | 2 (1.5%) | 0.329 | 3 (0.9%) |
| Elementary | 15 (8.0%) | 18 (13.9%) | 33 (10.3%) | |
| Intermediate, Secondary | 72 (37.4%) | 43 (33.3%) | 115 (35.9%) | |
| University | 103 (54.0%) | 66 (51.2%) | 169 (52.8%) | |
| Occupation, n (%) | ||||
| Self-employed | 85 (44.6%) | 53 (41%) | 0.001 | 138 (43.1%) |
| Employee | 60 (31.4%) | 40 (31.4%) | 100 (31.2%) | |
| Retired/unemployment | 36 (18.9%) | 32 (24.7%) | 68 (21.2%) | |
| Others | 10 (5.2%) | 4 (3.1%) | 14 (4.4%) | |
| Crowding index †, n (%) | ||||
| ≤1 | 144 (75.4%) | 103 (79.8%) | 0.53 | 247 (77.1%) |
| >1 | 47 (24.6%) | 26 (20.2%) | 73 (22.9%) | |
| Presence of metabolic syndrome, n (%) | 155 (81.2%) | 114 (88.5%) | 0.156 | 269 (83.9%) |
| Obesity (yes), n (%) | 103 (53.9%) | 101 (78.2%) | 0.001 | 204 (63.7%) |
| CVD (yes), n (%) | 8 (4.2%) | 21(16%) | 0.0001 | 29 (9.1%) |
| Family medical history (yes), n (%) | 167 (87.6%) | 104 (81.0%) | 0.182 | 271 (84.7%) |
| Smoking (yes), n (%) | 78 (40.8%) | 55(42.6%) | 0.187 | 133 (41.5%) |
| Physical Activities (Kcals)(yes) | 41 (21.46%) | 23 (17.82%) | 0.469 | 64 (20%) |
| Energy intake (kcal), (M), mean ± SD | 4525.8 ± 0.2 | 4127.6 ± 0.2 | 0.110 | 4162.9 ± 0.2 |
| Energy intake (kcal), (F), mean ± SD | 2829.4 ± 0.2 | 2731.5 ± 0.3 | 0.737 | 2747.3 ± 0.3 |
| Waist circumference (cm) (M)≥ 94 § (F) ≥80 § | 130 (68.2%) 180 (94.5%) | 107 (83.0%) 127 (98.7%) | 0.006 0.129 | 237 (74.2%) 307 (96.0%) |
| Waist/hip ratio (M) > 0.90 § (F) > 0.85 § | 144 (75.6%) 171 (89.8%) | 103 (79.6%) 124 (96.1%) | 0.438 0.058 | 247 (77.3%) 295 (92.2%) |
| Diabetes type 2, n (%) | 25 (13.0%) | 43 (33.0%) | 0.0001 | 68 (21.2%) |
| Homa > 3, n (%) | 92 (48.3%) | 66 (51.5%) | 0.697 | 158 (49.4%) |
| ASAT/ALAT ≥ 1 | 29 (15.3%) | 34 (26.4%) | 0.032 | 63 (19.7%) |
| Current dietary ** supplementation use, n (%) | 81 (42.4%) | 70 (54.3%) | 0.053 | 151 (47.2%) |
| Food Group | Pattern | ||
|---|---|---|---|
| Traditional Lebanese | High Fruits | Westernized | |
| Vegetables | 0.85 | ||
| Chickpeas, red beans, lentils, peas | 0.50 | ||
| Vegetable oil/olives | 0.33 | 0.20 | |
| Almonds, walnuts, hazelnuts, sesames | 0.27 | ||
| Fish | 0.21 | ||
| Sea Food | 0.36 | ||
| Red Wine | 0.21 | ||
| Fruits and fruit juices | 0.73 | ||
| Hamburger and fries | 0.63 | ||
| Beef meat | 0.53 | ||
| Chicken | 0.53 | ||
| Carbonated beverages | 0.52 | ||
| Pizza | 0.52 | ||
| Chips | 0.50 | ||
| Pork | 0.41 | ||
| Hot Dog | 0.45 | ||
| Ketchup | 0.50 | ||
| Mayonnaise or mustard | 0.41 | ||
| 1 chicken egg | 0.44 | ||
| Spaghetti or noodles | 0.41 | ||
| Cooked rice | 0.24 | 0.39 | |
| Pies or fatayer | 0.39 | ||
| Bread | 0.27 | ||
| Desserts, Arabic pastries | 0.30 | ||
| Milk chocolate | 0.39 | ||
| Laban/Lebanese yogurt | 0.25 | ||
| Energy drink | 0.22 | ||
| Beer | 0.21 | ||
| Pop Corn | 0.20 | ||
| Percent variance explained by each pattern | 18.22% | 9.84% | 7.22% |
| Traditional Lebanese | High Fruits | Westernized | ||||
|---|---|---|---|---|---|---|
| OR | CI | OR | CI | OR | CI | |
| Age (years) | 1.60 | 1.03–1.09 * | 1.01 | 0.98–1.03 | 0.92 | 0.89–0.95 * |
| BMI (kg/m2) | 0.90 | 0.91–1.02 | 0.95 | 0.89–1.01 | 1.11 | 1.04–1.18 * |
| Education ** (university level/others) | 2.90 | 1.50–5.76 * | 0.50 | 0.27–0.91 * | 0.76 | 0.39–1.48 |
| Dietary Pattern | T1 (Low Adherence) | T2 (Medium Adherence) | T3 (High Adherence) | p-Trend |
|---|---|---|---|---|
| Traditional Lebanese | ||||
| Crude Model | Ref * | 0.41 (0.15–1.12) | 0.36 (0.13–1.03) | 0.057 |
| Model 1 | Ref | 0.31 (0.99–1.18) | 0.21 (0.07–0.82) ‡ | 0.024 |
| Model 2 | Ref | 0.32 (0.07–1.21) | 0.18 (0.04–0.79) ‡ | 0.023 |
| Model 3 | Ref | 0.42 (0.11–1.96) | 0.18 (0.04–0.85) ‡ | 0.031 |
| High Fruits | ||||
| Crude Model | Ref | 1.22 (0.42–3.53) | 0.98 (0.32–3.02) | 0.969 |
| Model 1 | Ref | 1.68 (0.39–7.11) | 1.05 (0.24–4.69) | 0.943 |
| Model 2 | Ref | 1.59 (0.32–7.93) | 1.06 (0.21–5.35) | 0.946 |
| Model 3 | Ref | 2.53 (0.43–14.97) | 1.78 (0.28–11.17) | 0.537 |
| Westernized | ||||
| Crude Model | Ref | 1.51 (0.47–4.82) | 1.03 (0.31–3.39) | 0.956 |
| Model 1 | Ref | 1.78 (0.53–6.01) | 1.41 (0.38–5.28) | 0.606 |
| Model 2 | Ref | 2.43 (0.59–9.99) | 1.19 (0.28–5.02) | 0.813 |
| Model 3 | Ref | 2.22 (0.51–9.61) | 1.04 (0.24–4.51) | 0.959 |
| Food Groups | Tertile 1 | Tertile 2 | Tertile 3 | p-Trend |
|---|---|---|---|---|
| Fructose (g/day) | Ref * | 1.07(0.52–2.20) | 1.15(0.54–2.45) | 0.718 |
| Fibres (g/day) | Ref | 0.67(0.32–1.39) | 1.24(0.61–2.52) | 0.554 |
| Monounsaturated Fatty acids (g/day) | Ref | 0.91(0.45–1.84) | 0.69(0.33–1.46) | 0.342 |
| Polyunsaturated Fatty acids (g/day) | Ref | 0.77 (0.39–1.52) | 0.65(0.31–1.36) | 0.253 |
| Saturated Fatty acids (g/day) | Ref | 1.50 (0.74–3.06) | 0.83(0.38–1.83) | 0.653 |
| ω-3 (g/day) | Ref | 1.12 (0.55–2.29) | 1.03(0.49–2.16) | 0.944 |
| ω-6 (g/day) | (1.20–13.70) Ref | (13.71–19.01) 2.24 (1.06–4.74) § | (19.02–52.70) 1.04(0.50–2.14) | 0.922 |
| Cholesterol (mg/day) | Ref | 0.79 (0.39–1.61) | 1.10(0.52–2.30) | 0.792 |
| Protein (g/day) | Ref | 0.91 (0.43–1.91) | 0.65(0.28–1.50) | 0.311 |
| Fat (g/day) | Ref | 1.06 (0.50–2.23) | 0.55(0.24–1.26) | 0.159 |
| Simple Carbohydrates (g/day) | (8.15–101.4) Ref | (101.50–164.69) 2.43 (1.12–5.26) § | (164.70–469.64) 2.06(0.87–4.88) | 0.099 |
| EPA (mg/day) | (0.1–2.0) Ref | (2.10–5.0) 0.35 (0.15–0.82) § | (5.1–160) 0.65(0.26–1.63) | 0.357 |
| DHA (mg/day) | (0.1–1.0) Ref | (1.1–9.0) 0.26 (0.09–0.73) § | (9.1–230) 0.53 (0.19–1.44) | 0.212 |
| Energy/day (Kcals), males | Ref | 0.63 (0.26–1.57) | 1.01 (0.38–2.64) | 0.994 |
| Energy/day (Kcals), females | Ref | 2.27 (0.48–10.80) | 1.53 (0.33–7.09) | 0.587 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sayegh, N.F.; Heraoui, G.N.H.A.; Younes, H.; Sayegh, L.N.; Boulos, C.; Sayegh, R. Relation of Dietary Patterns and Nutritional Profile to Hepatic Fibrosis in a Sample of Lebanese Non-Alcoholic Fatty Liver Disease Patients. Nutrients 2022, 14, 2554. https://doi.org/10.3390/nu14122554
Sayegh NF, Heraoui GNHA, Younes H, Sayegh LN, Boulos C, Sayegh R. Relation of Dietary Patterns and Nutritional Profile to Hepatic Fibrosis in a Sample of Lebanese Non-Alcoholic Fatty Liver Disease Patients. Nutrients. 2022; 14(12):2554. https://doi.org/10.3390/nu14122554
Chicago/Turabian StyleSayegh, Nicole Fakhoury, Gessica N. H. A. Heraoui, Hassan Younes, Lea Nicole Sayegh, Christa Boulos, and Raymond Sayegh. 2022. "Relation of Dietary Patterns and Nutritional Profile to Hepatic Fibrosis in a Sample of Lebanese Non-Alcoholic Fatty Liver Disease Patients" Nutrients 14, no. 12: 2554. https://doi.org/10.3390/nu14122554
APA StyleSayegh, N. F., Heraoui, G. N. H. A., Younes, H., Sayegh, L. N., Boulos, C., & Sayegh, R. (2022). Relation of Dietary Patterns and Nutritional Profile to Hepatic Fibrosis in a Sample of Lebanese Non-Alcoholic Fatty Liver Disease Patients. Nutrients, 14(12), 2554. https://doi.org/10.3390/nu14122554