Abstract
Dietary inflammatory potential has been proven to be correlated with the incidence of diabetes and cardiovascular diseases. However, the evidence regarding the impact of dietary inflammatory patterns on long-term mortality is scarce. This cohort study aims to investigate the dietary inflammatory pattern of the general US individuals by baseline glycemic status and to estimate its association with long-term mortality. A total of 20,762 general American adults with different glycemic statuses from the National Health and Nutrition Examination Survey were included. We extracted 24-h dietary information, and the dietary inflammatory index (DII) was calculated. The outcomes were defined as 5-year all-cause and cardiovascular mortality. Compared with the normoglycemia group, individuals with prediabetes and type 2 diabetes had higher DII scores (overall weighted p < 0.001). Compared with low DII scores, participants with high DII scores were at a higher risk of long-term all-cause mortality (HR: 1.597, 95% CI: 1.370, 1.861; p < 0.001) and cardiovascular mortality (HR: 2.036, 95% CI: 1.458, 2.844; p < 0.001). The results were stable after adjusting for potential confounders. Moreover, the prognostic value of DII for long-term all-cause mortality existed only in diabetic individuals but not in the normoglycemia or prediabetes group (p for interaction = 0.006). In conclusion, compared to the normoglycemia or prediabetes groups, participants with diabetes had a higher DII score, which indicates a greater pro-inflammatory potential. Diabetic individuals with higher DII scores were at a higher risk of long-term all-cause and cardiovascular mortality.
1. Introduction
Globally, the number of patients with diabetes and its devastating complications is increasing persistently in the past three decades, which is a major health threat to both developed and developing countries [1,2]. Due to the damage to the vascular smooth muscle cell and endothelial cell function [3], vascular diabetic complications cover almost all types of blood vessels and contribute to most of the mortality, hospitalization, and morbidity in patients with diabetes [4,5]. Obesity, decreased physical activity, population aging, and energy-dense diets are the primary causes of the rising diabetes rate [6]. Among those risk factors, the relationship between diabetes and nutrition or diet has received considerable attention [7,8,9,10].
Chronic inflammation plays a significant role in the etiology of diabetes [11,12]. Diet may interfere with the development of diabetes, which may be achieved through the influence of chronic inflammation. Many studies have demonstrated the correlation between pro-inflammatory food and diabetic risk [13,14]. A cross-sectional study of diabetes-free women revealed that red meat consumption was associated with elevated plasma inflammatory factors, fasting insulin, and glycated hemoglobin [13]. Moreover, an increasing number of studies have found that the Mediterranean diet, which was proven to have an anti-inflammatory effect [15,16,17], was associated with a lower diabetic risk [18,19,20,21].
The dietary inflammatory index (DII) was a dietary assessment tool developed based on the summary of published literature and aimed to estimate the inflammatory potential of an individual’s diet [22]. A high DII score, which was associated with elevated inflammatory markers, such as C-reactive protein (CRP), indicates a pro-inflammation diet and has been reported to be correlated with an increased risk of obesity, type 2 diabetes, and cardiovascular diseases [23,24,25,26,27]. Moreover, populations with higher DII scores were proven to have higher cardiovascular mortality [28,29]. However, currently, evidence about the relationship between DII and long-term mortality of subjects with different glycemic statuses is scarce. Therefore, our study aims to investigate the long-term prognostic value of DII among participants with normoglycemia, prediabetes, and type 2 diabetes, which may contribute to precise prognosis prediction and diabetes management.
2. Materials and Methods
2.1. Study Population
This cohort study was conducted following the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline for cohort studies [30]. The participants included in our analysis were extracted from the National Health and Nutrition Examination Survey (NHANES), a periodically conducted survey that obtains nationally-representative samples of the general Americans with a complex, multistage probability design [31]. In this study, we extracted participants from the 2007–2014 cycle. Adults with complete 24-h dietary data were included. The exclusion criteria included: (1) age <20 years old; (2) participants with pregnancy; and (3) participants without endpoint information.
2.2. Dietary Information
Dietary information was extracted from NHANES, which was collected through 24-h dietary recall interviews in the mobile examination center and was validated by the Nutrition Methodology Working Group [32]. Following the DII calculating protocol reported by N. Shivappa et al. [22], 28 food parameters in NHANES were used to calculate the DII, including carbohydrates, protein, total fat, alcohol, fiber, cholesterol, saturated fat, monounsaturated fatty acids (MUFAs), polyunsaturated fatty acids (PUFAs), niacin, vitamin A, thiamin, riboflavin, vitamin B6, vitamin B12, vitamin C, vitamin D, vitamin E, Fe, Mg, Zinc, Selenium, folic acid, β-carotene, caffeine, energy, n-3 fatty acids, and n-6 fatty acids. Previous studies have reported that DII calculated based on less than 30 food parameters kept its predictive ability [33,34].
A lower negative DII score suggests an anti-inflammatory effect, while a higher positive DII score means a pro-inflammatory effect of diet. According to the methods of N. Shivappa et al. [22], the DII calculation should be standardized to a world database that contains standard mean and standard deviation for each food parameter. The database was constructed by examining the relationship between parameters, including food components, and inflammation, in 1943 published articles. A parameter with proof of anti-inflammation effect would obtain a score of “−1”, while a food parameter would receive a score of “+1” if it was reported to be correlated with a reduced level of anti-inflammatory cytokines or increased level of proinflammatory cytokines.
These values were further weighted according to the study design. For each included parameter, we first extracted the individualized consumption value and then subtracted it from the standard mean and divided this value by the standard deviation. To minimize the effect of right skewing, these values were converted to a centered percentile score. To achieve a symmetrical distribution with values centered around 0 and bounded between −1 and +1, each percentile score was doubled, and then we subtracted “1”. This centered percentile value for each food parameter was then multiplied by its corresponding inflammatory effect score to obtain the DII score for each food component. Finally, 28 food-specific DII scores were summed to create the overall DII score for each participant.
2.3. Diseases and Endpoint Definitions
Type 2 diabetes was defined as a self-reported physician diagnosis of diabetes, glycated hemoglobin A1c (HbA1c) ≥ 6.5%, fasting glucose ≥ 7.8 mmol/L, or use of insulin or oral hypoglycemic medication. Prediabetes was defined as HbA1c 5.7%–6.4% (39–46 mmol/mol) or impaired fasting glucose (IFG) [fasting plasma glucose (FPG): 110–125 mg/dL (6.1–6.9 mmol/L)], or impaired glucose tolerance (IGT) [Oral glucose tolerance test 2-h glucose value ≥ 140 mg/dL (7.8 mmol/L) but < 200 mg/dL (11.1 mmol/L) and FPG < 126 (7.0 mmol/L)]. Hypertension was diagnosed as a self-reported physician diagnosis of hypertension, use of antihypertensive drugs, or systolic blood pressure ≥ 140 mmHg and/or diastolic blood pressure ≥ 90 mmHg (at least three times).
Participants who met at least one of the following criteria were diagnosed with hyperlipidemia: (1) elevated total cholesterol ≥ 200 mg/dL (5.18 mmol/L); (2) high triglyceride level (≥150 mg/dL); (3) low density lipoprotein-cholesterol (LDL-c) ≥ 130 mg/dL (3.37 mmol/L); (4) high density lipoprotein-cholesterol (HDL-c) < 40 mg/dL (1.04 mmol/L) in men and 50 mg/dL (1.30 mmol/L) in women; and (5) use of cholesterol-lowering drugs. We set the time of follow-up time as 5 years. The primary endpoint of follow-up was all-cause death, which was extracted from the records of the National Death Index (NDI). The secondary endpoint was cardiovascular death, which was defined according to the International Classification of Diseases-10 codes (I00–I09, I11, I13, I20–I51) and was also extracted from NDI.
2.4. Statistics
To represent the overall US population, all analyses incorporated oversampling, clustering, and stratification as recommended by the NHANES data analysis guideline [31]. Continuous variables are listed as the weighted mean and 95% confidence interval (CI), while categorical variables are presented as weighted proportions. Basic characteristics are compared by baseline glycemic status using the adjusted Wald test for continuous variables and Rao-Scott χ2 test for categorical variables.
The weighted Cox proportional hazard regression models were adopted to assess the impact of DII on participants’ long-term mortality, which were adjusted for age, sex, educational level, BMI, smoke, hypertension, hyperlipidemia, glycemic status, recreational activity, and alcohol consumption. In addition to estimating DII as a continuous variable, we equally classified participants into three groups: low DII, medium DII, and high DII. Similar Cox regression models as well as weighted Kaplan-Meier curves were adopted to estimate the correlation between all-cause and cardiovascular mortality and different DII groups.
Furthermore, to test whether the impact of DII on prognosis is different across patients with different glycemic statuses, the weighted Cox regression model and interaction p value were used to estimate the relationship between DII (continuous/categorical variable) and participants’ long-term mortality in participants with normal glucose status, prediabetes, and type 2 diabetes. The regression model was adjusted for age, sex, educational level, BMI, smoke, hypertension, hyperlipidemia, recreational activity, and alcohol consumption.
All analyses were conducted by the R software (version 4.1.2, R Foundation for Statistical Computing, Vienna, Austria) and Stata (version 16.0, StataCorp, College Station, TX, USA). A two-sided p value < 0.05 was considered statistically significant.
3. Results
3.1. Basic Characteristics by Baseline Glycemic Status
Following the pre-specified inclusion and exclusion criteria, a total of 20,762 participants were included in our study, among which 3859 were diagnosed with type 2 diabetes, 5489 with prediabetes, and 11,417 with normal glucose status (Figure 1). Table 1 lists the comparison of basic characteristics by glycemic status. Many variables showed an increasing relationship among patients with normoglycemia, prediabetes, and type 2 diabetes, such as age, BMI, waist, systolic blood pressure, HbA1c, and triglycerides, which may indicate a worse health status in patients with prediabetes or type 2 diabetes. Similarly, we also found that patients with abnormal glucose metabolism were more likely to have a combination of hypertension or hyperlipidemia.
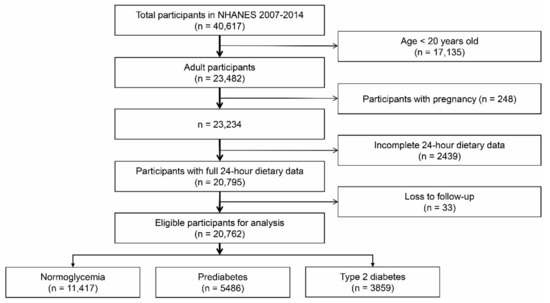
Figure 1.
Flowchart of participant selection from NHANES database. NHANES: National Health and Nutrition Examination Survey.

Table 1.
Basic characteristics of participants by baseline glycemic status.
Interestingly, compared with the normoglycemia group [113.94 (112.40,115.48) mg/dL], patients with prediabetes had a high level of LDL-c [122.04 (119.84, 124.24) mg/dL], while patients with type 2 diabetes had a better control of LDL-c [106.52 (103.97, 109.06) mg/dL]. As for the living habits, the percentage of former smokers was higher while the percentage of current smokers was lower in the diabetic population. The proportion of moderate or heavy drinking was also lower in participants with prediabetes or type 2 diabetes. Moreover, participants with type 2 diabetes were less likely to participate in recreational activity.
3.2. Comparison of DII Score by Baseline Glycemic Status
Compared with the normoglycemia group (0.883, 95% CI: 0.793, 0.973), participants with prediabetes (1.081, 95% CI: 0.981, 1.181) and type 2 diabetes (1.249, 95% CI: 1.151, 1.346) had higher DII scores (overall weighted p < 0.001). Figure 2 presents the distribution of DII scores among three groups. The proportion of high DII scores was higher in participants with prediabetes or type 2 diabetes. Moreover, we compared the component of DII scores among the three groups to find the main cause of the difference.
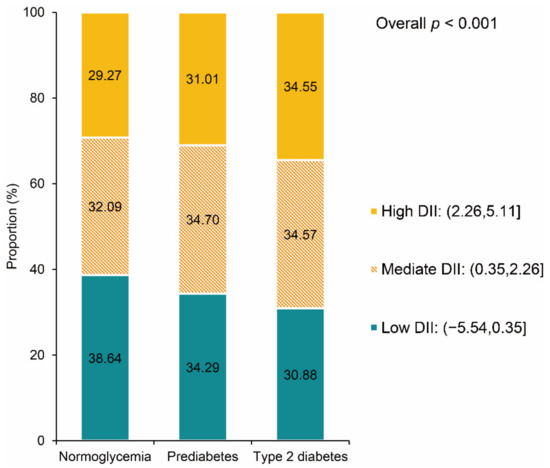
Figure 2.
DII distribution by baseline glycemic status. DII: dietary inflammatory index.
Participants with type 2 diabetes had higher scores in alcohol, fiber, MUFA, PUFA, niacin, thiamin, riboflavin, vitamin B6, vitamin C, vitamin E, Mg, Zinc, Selenium, folic acid, N-3 fatty acids, and N-6 fatty acids (Figure 3, Table 2). We also noticed lower scores of participants with type 2 diabetes in certain components, such as carbohydrates, protein, total fat, saturated fat, vitamin B12, Fe, and energy. When compared to the normoglycemia group, the DII component scores remained consistent between participants with prediabetes and type 2 diabetes but to a lesser extent in the former.
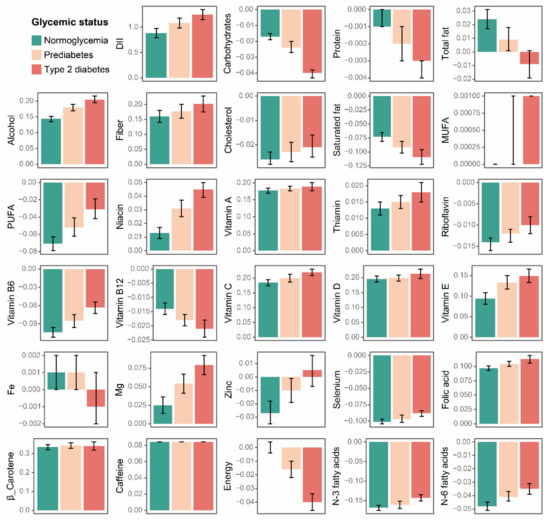
Figure 3.
Comparison of the DII component scores by baseline glycemic status. Data are presented as the weighted mean value and 95%CI. DII: dietary inflammatory index; MUFA: monounsaturated fatty acids; and PUFA: polyunsaturated fatty acids.

Table 2.
Comparison of the components of DII by baseline glycemic status.
3.3. Association between Dietary Inflammation and Long-Term Mortality
The overall weighted 5-year all-cause mortality was 4.56%, and the weighted 5-year cardiovascular mortality was 1.17%. The Cox regression models revealed that higher DII scores were associated with higher long-term all-cause mortality (HR per 1 score increase: 1.105, 95% CI: 1.065, 1.147; p < 0.001) and cardiovascular mortality (HR per 1 score increase: 1.172, 95% CI: 1.092, 1.258; p < 0.001) (Table S1 in the Supplementary Materials).
The association was stable after adjusting for age, sex, educational level, BMI, smoke, hypertension, hyperlipidemia, glycemic status, recreational activity, and alcohol consumption. Compared with participants with low DII scores, participants with mediate or high DII scores had higher risk of all-cause death (Mediate DII: adjusted HR: 1.181, 95% CI: 1.009, 1.381; p = 0.038; high DII: adjusted HR: 1.240, 95% CI: 1.053, 1.459; p = 0.010) and cardiovascular death (adjusted HR: 1.442, 95% CI: 1.051, 1.979; p = 0.023; high DII: adjusted HR: 1.423, 95% CI: 1.006, 2.013; p = 0.046) (Figure 4, Table S1 in the Supplementary Materials).
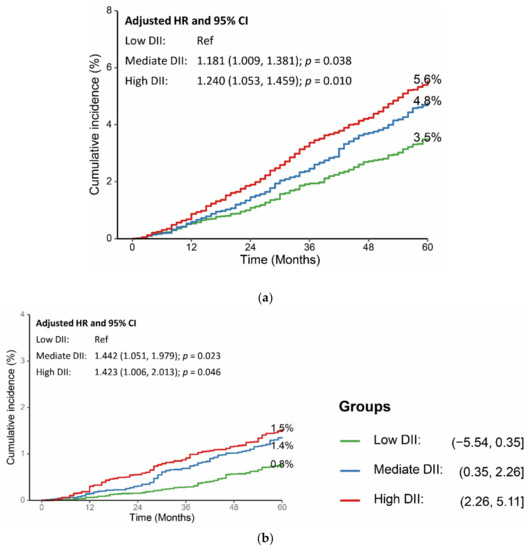
Figure 4.
Association between DII scores and long-term (a) all-cause mortality and (b) cardiovascular mortality. HR was adjusted for age, sex, educational level, BMI, smoke, hypertension, hyperlipidemia, glycemic status, recreational activity, and alcohol consumption. CI: confidence interval; DII: dietary inflammatory index; HR: hazard ratio; and Ref: reference.
3.4. Dietary inflammation and Long-Term Mortality across Participants with Different Glycemic STATUSES
To estimate the impact of baseline glycemic status on the long-term prognostic value of DII, we performed adjusted Cox regression models in three groups: normoglycemia, prediabetes, and type 2 diabetes groups. As shown in Table 3, the association between DII scores and 5-year all-cause mortality was only significant in participants with type 2 diabetes (adjusted HR per 1 score increase 1.083, 95% CI: 1.014, 1.156; p = 0.017). DII was a better long-term all-cause mortality indicator in the type 2 diabetes group than was the normoglycemia or prediabetes group (p for interaction = 0.030).

Table 3.
Association between DII and the long-term mortality of participants by baseline glycemic status.
When treated as a categorical variable, a high DII score of participants with type 2 diabetes was associated with higher 5-year all-cause (adjusted HR 1.626, 95% CI: 1.208, 2.188; p = 0.001) and cardiovascular mortality (adjusted HR 1.980, 95% CI: 1.043, 3.761; p = 0.037) compared with low DII score. Participants with mediate DII scores in the type 2 diabetes group had a similar risk of long-term mortality. However, there was no significant correlation between DII and long-term mortality in the normoglycemia and prediabetes groups. The superiority of DII’s prognostic value for long-term all-cause mortality in the type 2 diabetes group over the normoglycemia or prediabetes group was robust. (Continuous DII: p for interaction = 0.030; categorical DII: p for interaction = 0.006)
4. Discussion
Our study included a total of 20,762 participants, which represented 218,988,071 of the general US population, and we found that prediabetic or diabetic participants had a more pro-inflammatory diet compared with the normoglycemia group. Participants with mediate or higher DII scores were at higher risk of long-term all-cause and cardiovascular mortality. The prognostic effect of DII was only significant in diabetic participants and not in the prediabetic or normoglycemia group.
Many studies have shown that certain diet patterns, such as advanced glycation end products (AGEs), antioxidant diet, and the Mediterranean diet, can affect the low-level inflammation or body composition, and thus influence the incidence and development of some chronic diseases [15,35,36]. Previous research has suggested that dietary patterns may influence the incidence of diabetes. An analysis of 200,727 US participants from three prospective cohort studies conducted over 20 years revealed that eating more healthy plant foods and eating fewer animal foods was associated with a 20% reduction in diabetic risk [37].
A 20-year prospective cohort of 70,991 women discovered that a higher anti-inflammatory diet (as measured by DII) was linked to a reduced risk of type 2 diabetes [26]. Our study confirmed this relationship and found a sequentially increasing DII score across the normoglycemia, prediabetes, and type 2 diabetes groups. Moreover, component analysis in our results revealed that participants with prediabetes or type 2 diabetes had higher scores in alcohol, fiber, MUFA, PUFA, niacin, thiamin, riboflavin, vitamin B6, vitamin C, vitamin E, Mg, Zinc, Selenium, folic acid, N-3 fatty acids, and N-6 fatty acids compared with participants with normoglycemia.
Interestingly, diabetic participants had lower scores in some key nutritional indicators, such as carbohydrates, protein, total fat, saturated fat, and energy. This dietary pattern may come from the active adjustment after the diagnosis of prediabetes or diabetes. Another study based on the NHANES database discovered that participants with diagnosed prediabetes or diabetes were more likely to be concerned about nutrition fact labels when making daily food purchases [38].
However, rather than simple calorie calculations, we should be concerned about the complex and long-term influences of different foods on health [39]. Nutrition science found that overall dietary patterns and specific foods, instead of single isolated nutrients were more important for cardiometabolic health [40,41]. In participants with prediabetes or diabetes, a shortage of vitamins, critical micronutrients, and unsaturated fatty acids, as shown in our study, may lead to poor health and disease progression, which requires attention in diabetic care.
Dietary patterns are linked not only to the occurrence of chronic diseases but also to disease prognosis. A meta-analysis of 14 research articles found that individuals in the highest DII group had a higher risk of cardiovascular disease incidence as well as cardiovascular mortality [42]. Park et al. estimated the relationship between dietary inflammatory potential and prognosis in participants with different metabolic phenotypes [34]. They included 3733 adults from the NHANES III database (1988–1994) and revealed that the DII score was correlated with elevated all-cause and cardiovascular mortality in individuals with metabolically unhealthy obesity, which has not been observed in metabolically healthy obese individuals.
The target population of our study consists of 20,762 participants who participated in the NHANES project in the near twenty years (2007–2014). Similarly, our results demonstrated that a higher DII score was associated with higher long-term all-cause and cardiovascular mortality in participants with type 2 diabetes. The correlation was not identified in the prediabetes or normoglycemia group. Our findings imply that dietary inflammatory potential has a major influence on the long-term prognosis of diabetic patients, a topic that requires further attention in diabetes management.
To our knowledge, this is the first work that compares the long-term prognostic value of DII in the general American participants by baseline glycemic status. There are several limitations to our study. First, DII was calculated from self-reported dietary data, and recall bias was inevitable. Secondary, we extracted the 24 h dietary information to represent the daily pattern, which may change over time. Second, the DII used in our study was calculated from 28 food parameters due to the limitation of dietary data in the NHANES database. Previous studies have reported that DII calculated based on less than 30 food parameters kept its predictive ability [33,34].
Thirdly, we discovered that participants with prediabetes had higher LDL-c levels than the normoglycemia group, whereas patients with type 2 diabetes had better LDL-c control. This phenomenon could be explained by the fact that people with diabetes are more likely to visit the hospital and undergo laboratory tests, allowing their complications, such as hyperlipidemia, to be better managed.
However, this is our hypothesis, and because therapy data is limited, a specific reason should be investigated in future research. Finally, although we adjusted the potential risk factors including age, sex, body mass index, smoke, hypertension, educational level, hyperlipidemia, glycemic status, recreational activity, and moderate or heavy drinker in the multivariable Cox regression models, cardiovascular pathology and medication therapy were not involved due to the limitation of database, which may have an important impact on the cardiovascular mortality.
5. Conclusions
Our study identified a more pro-inflammatory diet in the diabetic participants compared with the general Americans. Participants with a higher DII score were at higher risk of 5-year all-cause and cardiovascular mortality. The prognostic value of DII existed only in individuals with type 2 diabetes but not in the normoglycemia or prediabetes group. The result calls for a comprehensive assessment of the dietary inflammatory potential in diabetic patients. Moreover, whether an anti-inflammatory dietary adjustment can improve the long-term prognosis of diabetes should be assessed in future trials.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/nu14132556/s1, Table S1: Survival analysis of the relationship between DII scores and long-term mortality.
Author Contributions
Conceptualization, K.D. and S.Y.; methodology, C.S. and S.Y.; software, S.Y. and J.H.; validation, R.Z. and J.H.; formal analysis, S.Y.; investigation, C.S.; resources, R.Z.; data curation, R.Z.; writing—original draft preparation, S.Y.; writing—review and editing, C.S. and K.D.; visualization, S.Y.; supervision, K.D.; project administration, K.D.; funding acquisition, K.D. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the CAMS Innovation Fund for Medical Sciences (CIFMS), grant number 2021-I2M-1-008 and the National Natural Science Foundation of China, grant number 81870277. The APC was funded by the CAMS Innovation Fund for Medical Sciences (CIFMS), grant number 2021-I2M-1-008.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data are available at NHANES website https://www.cdc.gov/nchs/nhanes/index.htm (accessed on 1 June 2022).
Acknowledgments
The NHANES protocol was approved by the NCHS Research Ethics Review Board.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Cole, J.B.; Florez, J.C. Genetics of Diabetes Mellitus and Diabetes Complications. Nat. Rev. Nephrol. 2020, 16, 377–390. [Google Scholar] [CrossRef]
- Zheng, Y.; Ley, S.H.; Hu, F.B. Global Aetiology and Epidemiology of Type 2 Diabetes Mellitus and Its Complications. Nat. Rev. Endocrinol. 2018, 14, 88–98. [Google Scholar] [CrossRef]
- Beckman, J.A.; Creager, M.A. Vascular Complications of Diabetes. Circ. Res. 2016, 118, 1771–1785. [Google Scholar] [CrossRef]
- Tseng, C.-H. Mortality and Causes of Death in a National Sample of Diabetic Patients in Taiwan. Diabetes Care 2004, 27, 1605–1609. [Google Scholar] [CrossRef]
- Morrish, N.J.; Wang, S.L.; Stevens, L.K.; Fuller, J.H.; Keen, H. Mortality and Causes of Death in the WHO Multinational Study of Vascular Disease in Diabetes. Diabetologia 2001, 44 (Suppl. S2), S14–S21. [Google Scholar] [CrossRef]
- Chatterjee, S.; Khunti, K.; Davies, M.J. Type 2 Diabetes. Lancet 2017, 389, 2239–2251. [Google Scholar] [CrossRef]
- Ley, S.H.; Hamdy, O.; Mohan, V.; Hu, F.B. Prevention and Management of Type 2 Diabetes: Dietary Components and Nutritional Strategies. Lancet 2014, 383, 1999–2007. [Google Scholar] [CrossRef]
- Wang, D.D.; Hu, F.B. Precision Nutrition for Prevention and Management of Type 2 Diabetes. Lancet Diabetes Endocrinol. 2018, 6, 416–426. [Google Scholar] [CrossRef]
- Forouhi, N.G.; Misra, A.; Mohan, V.; Taylor, R.; Yancy, W. Dietary and Nutritional Approaches for Prevention and Management of Type 2 Diabetes. BMJ 2018, 361, k2234. [Google Scholar] [CrossRef]
- Magkos, F.; Hjorth, M.F.; Astrup, A. Diet and Exercise in the Prevention and Treatment of Type 2 Diabetes Mellitus. Nat. Rev. Endocrinol. 2020, 16, 545–555. [Google Scholar] [CrossRef]
- Rohm, T.V.; Meier, D.T.; Olefsky, J.M.; Donath, M.Y. Inflammation in Obesity, Diabetes, and Related Disorders. Immunity 2022, 55, 31–55. [Google Scholar] [CrossRef]
- Furman, D.; Campisi, J.; Verdin, E.; Carrera-Bastos, P.; Targ, S.; Franceschi, C.; Ferrucci, L.; Gilroy, D.W.; Fasano, A.; Miller, G.W.; et al. Chronic Inflammation in the Etiology of Disease across the Life Span. Nat. Med. 2019, 25, 1822–1832. [Google Scholar] [CrossRef]
- Ley, S.H.; Sun, Q.; Willett, W.C.; Eliassen, A.H.; Wu, K.; Pan, A.; Grodstein, F.; Hu, F.B. Associations between Red Meat Intake and Biomarkers of Inflammation and Glucose Metabolism in Women. Am. J. Clin. Nutr. 2014, 99, 352–360. [Google Scholar] [CrossRef]
- Schulze, M.B.; Hoffmann, K.; Manson, J.E.; Willett, W.C.; Meigs, J.B.; Weikert, C.; Heidemann, C.; Colditz, G.A.; Hu, F.B. Dietary Pattern, Inflammation, and Incidence of Type 2 Diabetes in Women. Am. J. Clin. Nutr. 2005, 82, 675–684. [Google Scholar] [CrossRef]
- Mena, M.-P.; Sacanella, E.; Vazquez-Agell, M.; Morales, M.; Fitó, M.; Escoda, R.; Serrano-Martínez, M.; Salas-Salvadó, J.; Benages, N.; Casas, R.; et al. Inhibition of Circulating Immune Cell Activation: A Molecular Antiinflammatory Effect of the Mediterranean Diet. Am. J. Clin. Nutr. 2009, 89, 248–256. [Google Scholar] [CrossRef]
- Chrysohoou, C.; Panagiotakos, D.B.; Pitsavos, C.; Das, U.N.; Stefanadis, C. Adherence to the Mediterranean Diet Attenuates Inflammation and Coagulation Process in Healthy Adults: The ATTICA Study. J. Am. Coll. Cardiol. 2004, 44, 152–158. [Google Scholar] [CrossRef]
- Estruch, R.; Martínez-González, M.A.; Corella, D.; Salas-Salvadó, J.; Ruiz-Gutiérrez, V.; Covas, M.I.; Fiol, M.; Gómez-Gracia, E.; López-Sabater, M.C.; Vinyoles, E.; et al. Effects of a Mediterranean-Style Diet on Cardiovascular Risk Factors: A Randomized Trial. Ann. Intern. Med. 2006, 145, 1–11. [Google Scholar] [CrossRef]
- Martín-Peláez, S.; Fito, M.; Castaner, O. Mediterranean Diet Effects on Type 2 Diabetes Prevention, Disease Progression, and Related Mechanisms: A Review. Nutrients 2020, 12, 2236. [Google Scholar] [CrossRef]
- Salas-Salvadó, J.; Bulló, M.; Estruch, R.; Ros, E.; Covas, M.-I.; Ibarrola-Jurado, N.; Corella, D.; Arós, F.; Gómez-Gracia, E.; Ruiz-Gutiérrez, V.; et al. Prevention of Diabetes with Mediterranean Diets: A Subgroup Analysis of a Randomized Trial. Ann. Intern. Med. 2014, 160, 1–10. [Google Scholar] [CrossRef]
- InterAct Consortium; Romaguera, D.; Guevara, M.; Norat, T.; Langenberg, C.; Forouhi, N.G.; Sharp, S.; Slimani, N.; Schulze, M.B.; Buijsse, B.; et al. Mediterranean Diet and Type 2 Diabetes Risk in the European Prospective Investigation into Cancer and Nutrition (EPIC) Study: The InterAct Project. Diabetes Care 2011, 34, 1913–1918. [Google Scholar] [CrossRef]
- Esposito, K.; Maiorino, M.I.; Ceriello, A.; Giugliano, D. Prevention and Control of Type 2 Diabetes by Mediterranean Diet: A Systematic Review. Diabetes Res. Clin. Pract. 2010, 89, 97–102. [Google Scholar] [CrossRef]
- Shivappa, N.; Steck, S.E.; Hurley, T.G.; Hussey, J.R.; Hébert, J.R. Designing and Developing a Literature-Derived, Population-Based Dietary Inflammatory Index. Public Health Nutr. 2014, 17, 1689–1696. [Google Scholar] [CrossRef]
- Hariharan, R.; Odjidja, E.N.; Scott, D.; Shivappa, N.; Hébert, J.R.; Hodge, A.; de Courten, B. The Dietary Inflammatory Index, Obesity, Type 2 Diabetes, and Cardiovascular Risk Factors and Diseases. Obes. Rev. 2022, 23, e13349. [Google Scholar] [CrossRef]
- Tyrovolas, S.; Koyanagi, A.; Kotsakis, G.A.; Panagiotakos, D.; Shivappa, N.; Wirth, M.D.; Hébert, J.R.; Haro, J.M. Dietary Inflammatory Potential Is Linked to Cardiovascular Disease Risk Burden in the US Adult Population. Int. J. Cardiol. 2017, 240, 409–413. [Google Scholar] [CrossRef]
- Sen, S.; Rifas-Shiman, S.L.; Shivappa, N.; Wirth, M.D.; Hebert, J.R.; Gold, D.R.; Gillman, M.W.; Oken, E. Associations of Prenatal and Early Life Dietary Inflammatory Potential with Childhood Adiposity and Cardiometabolic Risk in Project Viva. Pediatr. Obes. 2018, 13, 292–300. [Google Scholar] [CrossRef]
- Laouali, N.; Mancini, F.R.; Hajji-Louati, M.; El Fatouhi, D.; Balkau, B.; Boutron-Ruault, M.-C.; Bonnet, F.; Fagherazzi, G. Dietary Inflammatory Index and Type 2 Diabetes Risk in a Prospective Cohort of 70,991 Women Followed for 20 Years: The Mediating Role of BMI. Diabetologia 2019, 62, 2222–2232. [Google Scholar] [CrossRef]
- Wang, Y.; Armijos, R.X.; Xun, P.; Weigel, M.M. Dietary Inflammatory Index and Cardiometabolic Risk in Ecuadorian Women. Nutrients 2021, 13, 2640. [Google Scholar] [CrossRef]
- Okada, E.; Shirakawa, T.; Shivappa, N.; Wakai, K.; Suzuki, K.; Date, C.; Iso, H.; Hébert, J.R.; Tamakoshi, A. Dietary Inflammatory Index Is Associated with Risk of All-Cause and Cardiovascular Disease Mortality but Not with Cancer Mortality in Middle-Aged and Older Japanese Adults. J. Nutr. 2019, 149, 1451–1459. [Google Scholar] [CrossRef]
- Shivappa, N.; Steck, S.E.; Hussey, J.R.; Ma, Y.; Hebert, J.R. Inflammatory Potential of Diet and All-Cause, Cardiovascular, and Cancer Mortality in National Health and Nutrition Examination Survey III Study. Eur. J. Nutr. 2017, 56, 683–692. [Google Scholar] [CrossRef]
- Von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P. STROBE Initiative The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: Guidelines for Reporting Observational Studies. Ann. Intern. Med. 2007, 147, 573–577. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. National Center for Health Statistics NHANES Survey Methods and Analytic Guidelines. Available online: https://wwwn.cdc.gov/nchs/nhanes/analyticguidelines.aspx (accessed on 5 April 2022).
- Plan and Operation of the Third National Health and Nutrition Examination Survey, 1988–1994. Series 1: Programs and Collection Procedures. Vital Health Stat. 1 1994, 32, 1–407.
- Shivappa, N.; Steck, S.E.; Hurley, T.G.; Hussey, J.R.; Ma, Y.; Ockene, I.S.; Tabung, F.; Hébert, J.R. A Population-Based Dietary Inflammatory Index Predicts Levels of C-Reactive Protein in the Seasonal Variation of Blood Cholesterol Study (SEASONS). Public Health Nutr. 2014, 17, 1825–1833. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.-M.M.; Choi, M.K.; Lee, S.-S.; Shivappa, N.; Han, K.; Steck, S.E.; Hébert, J.R.; Merchant, A.T.; Sandler, D.P. Dietary Inflammatory Potential and Risk of Mortality in Metabolically Healthy and Unhealthy Phenotypes among Overweight and Obese Adults. Clin. Nutr. 2019, 38, 682–688. [Google Scholar] [CrossRef] [PubMed]
- Garay-Sevilla, M.E.; Rojas, A.; Portero-Otin, M.; Uribarri, J. Dietary AGEs as Exogenous Boosters of Inflammation. Nutrients 2021, 13, 2802. [Google Scholar] [CrossRef] [PubMed]
- Rashidmayvan, M.; Sharifan, P.; Darroudi, S.; Saffar Soflaei, S.; Salaribaghoonabad, R.; Safari, N.; Yousefi, M.; Honari, M.; Ghazizadeh, H.; Ferns, G.; et al. Association between Dietary Patterns and Body Composition in Normal-Weight Subjects with Metabolic Syndrome. J. Diabetes Metab. Disord. 2022, 21, 735–741. [Google Scholar] [CrossRef]
- Satija, A.; Bhupathiraju, S.N.; Rimm, E.B.; Spiegelman, D.; Chiuve, S.E.; Borgi, L.; Willett, W.C.; Manson, J.E.; Sun, Q.; Hu, F.B. Plant-Based Dietary Patterns and Incidence of Type 2 Diabetes in US Men and Women: Results from Three Prospective Cohort Studies. PLoS Med. 2016, 13, e1002039. [Google Scholar] [CrossRef]
- An, R. Diabetes Diagnosis and Nutrition Facts Label Use among US Adults, 2005–2010. Public Health Nutr. 2016, 19, 2149–2156. [Google Scholar] [CrossRef]
- Mozaffarian, D. Dietary and Policy Priorities for Cardiovascular Disease, Diabetes, and Obesity: A Comprehensive Review. Circulation 2016, 133, 187–225. [Google Scholar] [CrossRef]
- Jacobs, D.R.; Tapsell, L.C. Food, Not Nutrients, Is the Fundamental Unit in Nutrition. Nutr. Rev. 2007, 65, 439–450. [Google Scholar] [CrossRef]
- Mozaffarian, D.; Ludwig, D.S. Dietary Guidelines in the 21st Century—A Time for Food. JAMA 2010, 304, 681–682. [Google Scholar] [CrossRef]
- Shivappa, N.; Godos, J.; Hébert, J.R.; Wirth, M.D.; Piuri, G.; Speciani, A.F.; Grosso, G. Dietary Inflammatory Index and Cardiovascular Risk and Mortality-A Meta-Analysis. Nutrients 2018, 10, E200. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).