An Outbreak of Human Systemic Anthrax, including One Case of Anthrax Meningitis, Occurred in Calabria Region (Italy): A Description of a Successful One Health Approach
Abstract
:1. Introduction
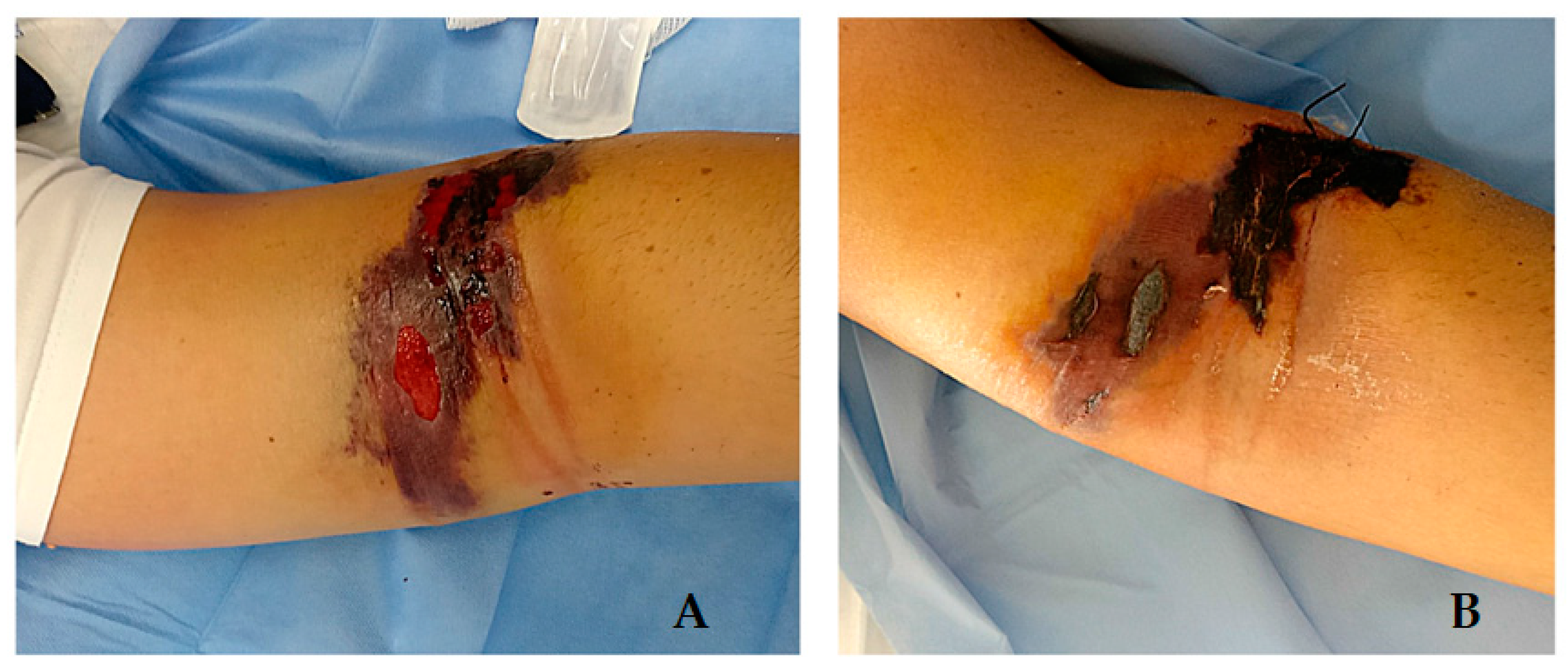
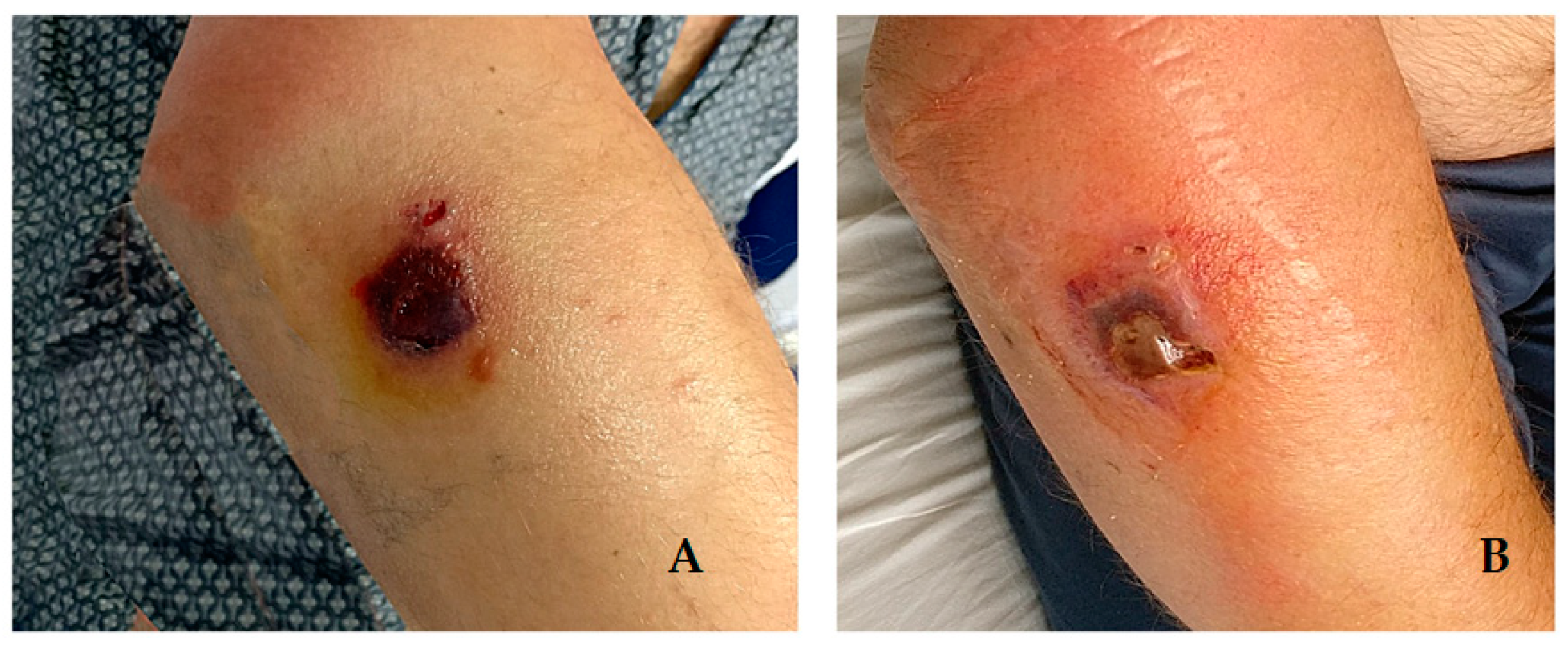
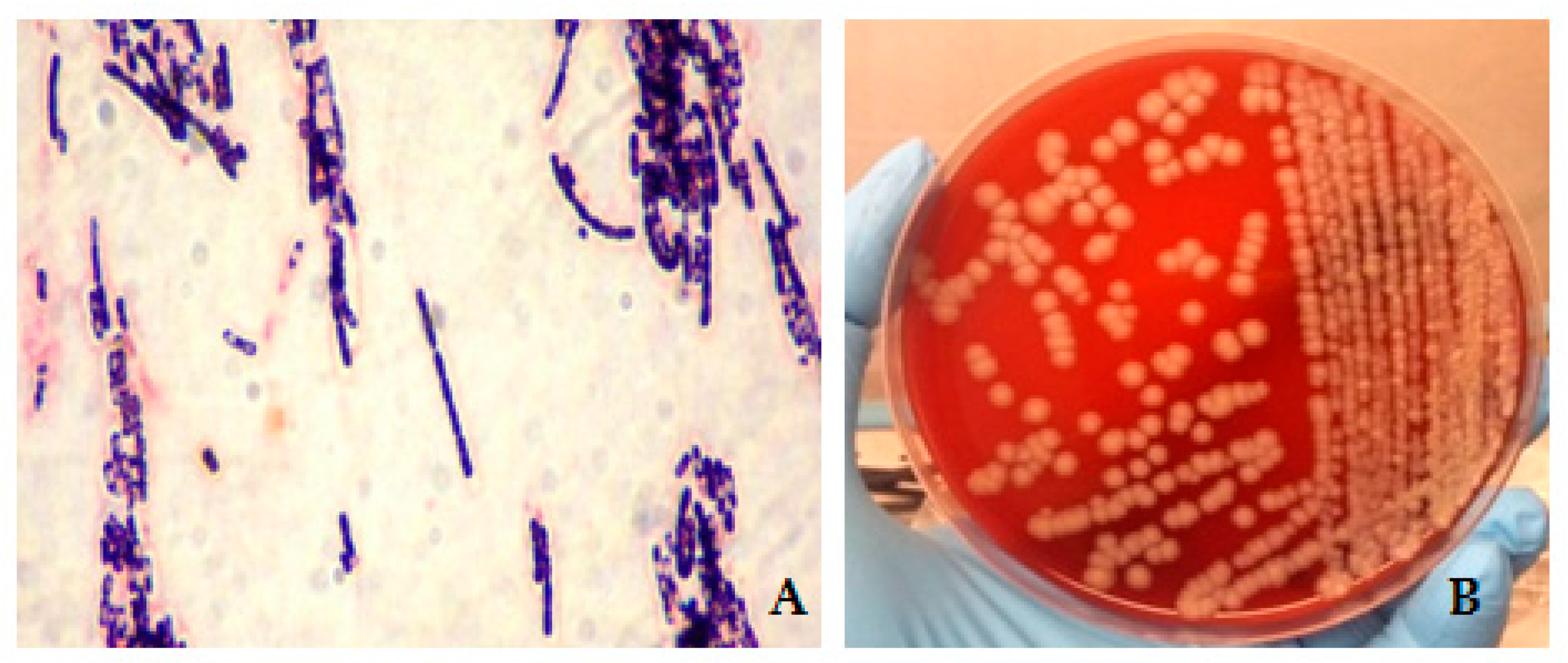
2. Cases Description
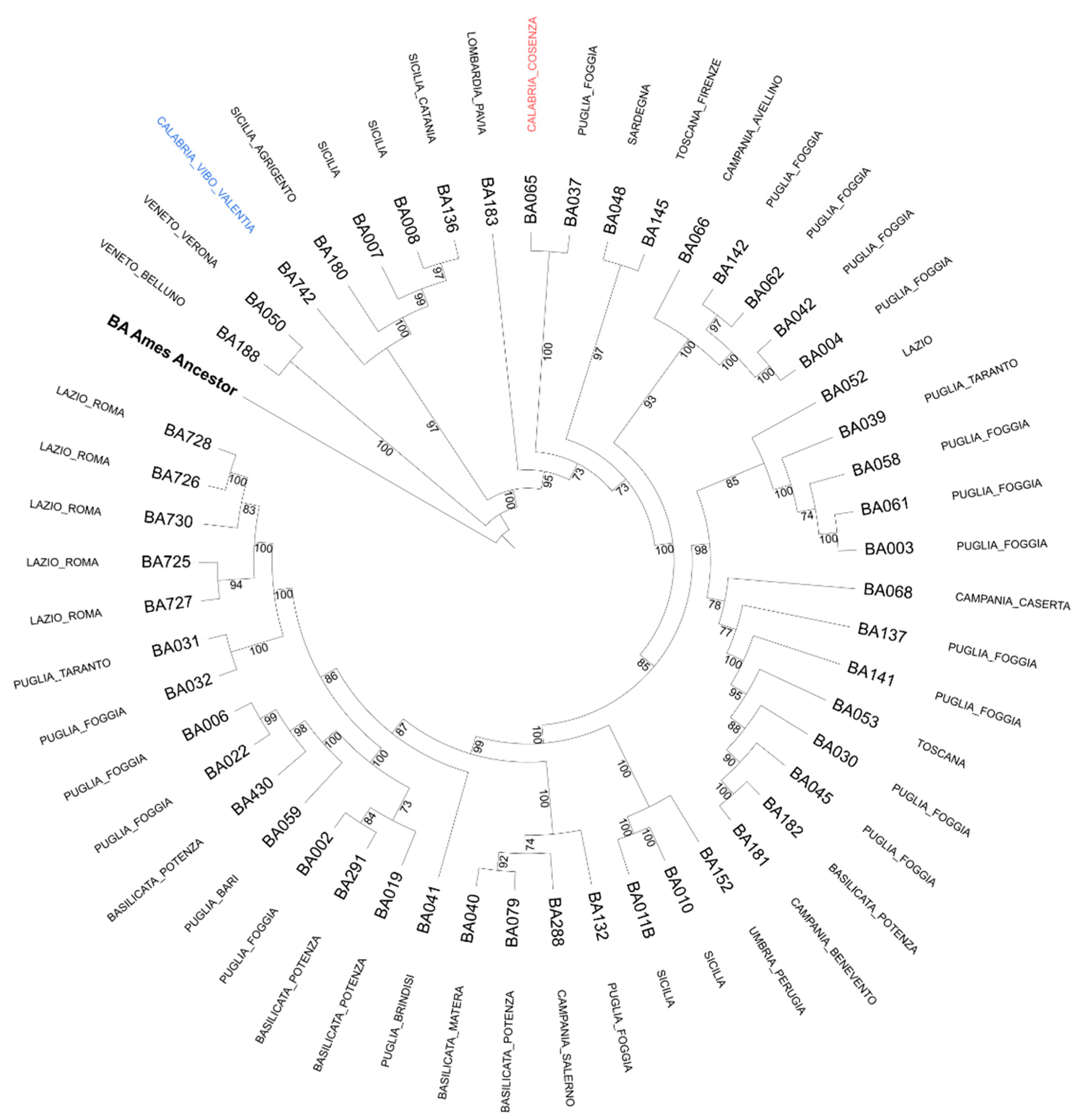
Epidemiological Analysis
3. Conclusions and Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Berger, T.; Kassirer, M.; Aran, A.A. Injectional anthrax—New presentation of an old disease. Eurosurveillance 2014, 19, 20877. [Google Scholar] [CrossRef] [Green Version]
- Hilmas, C.J.; Anderson, J. Chapter 29—Anthrax A2. In Handbook of Toxicology of Chemical Warfare Agents, 2nd ed.; Gupta, R.C., Ed.; Academic Press: Boston, MA, USA, 2015; pp. 387–410. [Google Scholar]
- Fasanella, A.; Garofolo, G.; Galella, M.; Troiano, P.; De Stefano, C.; Pace, L.; Aceti, A.; Serrecchia, L.; Adone, R. Suspect Vector Transmission of Human Cutaneous Anthrax During an Animal Outbreak in Southern Italy. Vector Borne Zoonotic Dis. 2013, 13, 769–771. [Google Scholar] [CrossRef]
- Nicastri, E.; Vairo, F.; Mencarini, P.; Battisti, A.; Agrati, C.; Cimini, E.; Carrara, S.; D’Arezzo, S.; Adone, R.; Vulcano, A.; et al. Unexpected human cases of cutaneous anthrax in Latium region, Italy, August 2017: Integrated human-animal investigation of epidemiological, clinical, microbiological, and ecological factors. Eurosurveillance 2019, 24, 1800685. [Google Scholar] [CrossRef] [PubMed]
- Katharios-Lanwermeyer, S.; Holty, J.-E.; Person, M.; Sejvar, J.; Haberling, D.; Tubbs, H.; Meaney-Delman, D.; Pillai, S.K.; Hupert, N.; Bower, W.A.; et al. Identifying Meningitis During an Anthrax Mass Casualty Incident: Systematic Review of Systemic Anthrax Since 1880. Clin. Infect. Dis. 2016, 62, 1537–1545. [Google Scholar] [CrossRef] [Green Version]
- Schmid, G.; Kaufmann, A. Anthrax in Europe: Its epidemiology, clinical characteristics, and role in bioterrorism. Clin. Microbiol. Infect. 2002, 8, 479–488. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fasanella, A.; Garofolo, G.; Galante, D.; Quaranta, V.; Palazzo, L.; Lista, F.; Adone, R.; Jones, M.H. Severe anthrax outbreaks in Italy in 2004: Considerations on factors involved in the spread of infection. New Microbiol. 2010, 33, 83–86. [Google Scholar] [PubMed]
- Derzelle, S.; Thierry, S. Genetic diversity of Bacillus anthracis in Europe: Genotyping methods in forensic and epidemiologic investigations. Biosecur Bioterror. 2013, 11 (Suppl. S1), S166–S176. [Google Scholar] [CrossRef]
- Rondinone, V.; Serrecchia, L.; Parisi, A.; Fasanella, A.; Manzulli, V.; Cipolletta, D.; Galante, D. Genetic characterization of Bacillus anthracis strains circulating in Italy from 1972 to 2018. PLoS ONE 2020, 15, e0227875. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Walsh, M.G.; de Smalen, A.W.; Mor, S.M. Climatic influence on anthrax suitability in warming northern latitudes. Sci. Rep. 2018, 8, 9269. [Google Scholar] [CrossRef] [Green Version]
- Berthier, M.; Fauchère, J.-L.; Perrin, J.; Grignon, B.; Oriot, D. Fulminant meningitis due to Bacillus anthracis in 11-year-old girl during Ramadan. Lancet 1996, 347, 828. [Google Scholar] [CrossRef]
- Leblebicioglu, H.; Turan, D.; Eroglu, C.; Esen, S.; Sunbul, M.; Bostanci, F. A cluster of anthrax cases including meningitis. Trop. Dr. 2006, 36, 51–53. [Google Scholar] [CrossRef] [PubMed]
- Anaraki, S.; Addiman, S.; Nixon, G.; Krahe, D.; Ghosh, R.; Brooks, T.; Lloyd, G.; Spencer, R.; Walsh, A.; McCloskey, B.; et al. Investigations and control measures following a case of inhalation anthrax in East London in a drum maker and drummer, October 2008. Eurosurveillance 2008, 13, 19076. [Google Scholar] [CrossRef] [PubMed]
- Cinquetti, G.; Banal, F.; Dupuy, A.L.; Girault, P.Y.; Couderc, A.; Guyot, P.; Alauzet, C.; Oddoux, O.; Ragot, C.; Puyhardy, J.M.; et al. Three related cases of cutaneous anthrax in France: Clinical and laboratory as-pects. Medicine 2009, 88, 371–375. [Google Scholar] [CrossRef] [PubMed]
- Popescu, R.; Pistol, A.; Miltaru, L.; Caplan, D.; Cucuiu, R.; Popovici, F. Two cases of infection with Bacillus anthracis, Romania, October 2011. Eurosurveillance 2011, 16, 20008. [Google Scholar] [CrossRef]
- Stefos, A.; Gatselis, N.K.; Goudelas, A.; Mpakarosi, M.; Papaparaskevas, J.; Dalekos, G.N.; Petinaki, E. Cutaneous infection caused by Bacillus anthracis in Larissa, Thessaly, Central Greece, July 2012. Eurosurveillance 2012, 17, 20245. [Google Scholar] [CrossRef] [Green Version]
- Durić, P.; Cosić, G.; Rajčević, S.; Petrovic, V.; Tomković, M.; Subić, Z.; Dimitrić, M. Three probable cases of cutaneous anthrax in autonomous province of Vojvodina, Serbia, June 2011. Eurosurveillance 2012, 17, 20050. [Google Scholar] [CrossRef]
- Wielinga, P.R.; Hamidjaja, R.; Ågren, J.; Knutsson, R.; Segerman, B.; Fricker, M.; Ehling-Schulz, M.; de Groot, A.; Burton, J.; Brooks, T.; et al. A multiplex real-time PCR for identifying and differentiating B. anthracis virulent types. Int. J. Food Microbiol. 2011, 145, S137–S144. [Google Scholar] [CrossRef]
- Clinical and Laboratory Standards Institute (CLSI). Methods for Antimicrobial Dilution and Disk Susceptibility Testing of Infrequently Isolated or Fastidious Bacteria, 3rd ed.; CLSI Guideline M45; CLSI: Wayne, PA, USA, 2016. [Google Scholar]
- Clinical and Laboratory Standards Institute (CLSI). Performance Standards for Antimicrobial Susceptibility Testing, 27th ed.; CLSI Supplement M100; CLSI: Wayne, PA, USA, 2017. [Google Scholar]
- Marston, C.K.; Allen, C.A.; Beaudry, J.; Price, E.P.; Wolken, S.R.; Pearson, T.; Keim, P.; Hoffmaster, A.R. Molecular Epidemiology of Anthrax Cases Associated with Recreational Use of Animal Hides and Yarn in the United States. PLoS ONE 2011, 6, e28274. [Google Scholar] [CrossRef] [Green Version]
- Girault, G.; Thierry, S.; Cherchame, E.; Derzelle, S. Application of High-Throughput Sequencing: Discovery of Informative SNPs to Subtype Bacillus anthracis. Adv. Biosci. Biotechnol. 2014, 5, 669–677. [Google Scholar] [CrossRef] [Green Version]
- Abdel-Glil, M.Y.; Chiaverini, A.; Garofolo, G.; Fasanella, A.; Parisi, A.; Harmsen, D.; Jolley, K.A.; Elschner, M.C.; Tomaso, H.; Linde, J.; et al. A Whole-Genome-Based Gene-by-Gene Typing System for Standardized High-Resolution Strain Typing of Bacillus anthracis. J. Clin. Microbiol. 2021, 59, e0288920. [Google Scholar] [CrossRef]
- Prjibelski, A.; Antipov, D.; Meleshko, D.; Lapidus, A.; Korobeynikov, A. Using SPAdes De Novo Assembler. Curr Protoc Bioinformatics 2020, 70, e102. [Google Scholar] [CrossRef] [PubMed]
- Seemann, T. Prokka: Rapid prokaryotic genome annotation. Bioinformatics 2014, 15, 2068–2069. [Google Scholar] [CrossRef] [PubMed]
- Page, A.J.; Cummins, C.A.; Hunt, M.; Wong, V.K.; Reuter, S.; Holden, M.T.; Fookes, M.; Falush, D.; Keane, J.A.; Parkhill, J. Roary: Rapid large-scale prokaryote pan genome analysis. Bioinformatics 2015, 15, 3691–3693. [Google Scholar] [CrossRef]
- Price, M.N.; Dehal, P.S.; Arkin, A.P. FastTree 2-approximately maximum-likelihood trees for large alignments. PLoS ONE 2010, 10, e9490. [Google Scholar] [CrossRef] [PubMed]
- Letunic, I.; Bork, P. Interactive Tree Of Life (iTOL) v5: An online tool for phylogenetic tree display and annotation. Nucleic Acids Res. 2021, 49, W293–W296. [Google Scholar] [CrossRef] [PubMed]
- Moayeri, M. The roles of anthrax toxin in pathogenesis. Curr. Opin. Microbiol. 2004, 7, 19–24. [Google Scholar] [CrossRef]
- Lanska, D.J. Anthrax meningoencephalitis. Neurology 2002, 59, 327–334. [Google Scholar] [CrossRef]
- Popescu, C.P.; Zaharia, M.; Nica, M.; Stanciu, D.; Moroti, R.; Benea, S.; Melinte, V.; Vasile, T.; Ceausu, E.; Ruta, S.; et al. Anthrax meningoencephalitis complicated with brain abscess—A case report. Int. J. Infect. Dis. 2021, 108, 217–219. [Google Scholar] [CrossRef]
- Gainer, R.S.; Vergnaud, G.; Hugh-Jones, M.E. Review of Arguments for the Existence of Latent Infections of Bacillus anthracis, and Research Needed to Understand their Role in the Outbreaks of Anthrax. Microorganisms 2020, 8, 800. [Google Scholar] [CrossRef]

| Antimicrobial Agent | MIC Range (µg/mL) | MIC Breakpoints a (µg/mL) | MIC Value (µg/mL) | ||||
|---|---|---|---|---|---|---|---|
| S (≤) | I | R (≥) | S | I | R | ||
| Penicillin G | 0.001–32 | 0.5 | - | 1 | 0.25 | ||
| Amoxicillin | 0.004–0.5 | 0.12 | - | 0.25 | 0.125 | ||
| Clindamycin | 0.031–4 | 0.5 | 1–2 | 4 | 0.25 | ||
| Vancomycin | 0.25–32 | 2 | 4–8 | 16 | 2 | ||
| Linezolid | 0.125–8 | 4 | - | 8 | 1 | ||
| Tetracycline | 0.001–2 | 1 | - | - | 0.0625 | ||
| Ciprofloxacin | 0.008–1 | 0.25 | - | - | 0.125 | ||
| Levofloxacin | 0.008–1 | 0.25 | 0.125 | ||||
| Doxycycline | 0.001–2 | 1 | - | - | 0.031 | ||
| Rifampin | 0.031–4 | 1 | 2 | 4 | 0.5 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guastalegname, M.; Rondinone, V.; Lucifora, G.; Vallone, A.; D’Argenio, L.; Petracca, G.; Giordano, A.; Serrecchia, L.; Manzulli, V.; Pace, L.; et al. An Outbreak of Human Systemic Anthrax, including One Case of Anthrax Meningitis, Occurred in Calabria Region (Italy): A Description of a Successful One Health Approach. Life 2022, 12, 909. https://doi.org/10.3390/life12060909
Guastalegname M, Rondinone V, Lucifora G, Vallone A, D’Argenio L, Petracca G, Giordano A, Serrecchia L, Manzulli V, Pace L, et al. An Outbreak of Human Systemic Anthrax, including One Case of Anthrax Meningitis, Occurred in Calabria Region (Italy): A Description of a Successful One Health Approach. Life. 2022; 12(6):909. https://doi.org/10.3390/life12060909
Chicago/Turabian StyleGuastalegname, Maurizio, Valeria Rondinone, Giuseppe Lucifora, Alfredo Vallone, Laura D’Argenio, Giovanni Petracca, Antonia Giordano, Luigina Serrecchia, Viviana Manzulli, Lorenzo Pace, and et al. 2022. "An Outbreak of Human Systemic Anthrax, including One Case of Anthrax Meningitis, Occurred in Calabria Region (Italy): A Description of a Successful One Health Approach" Life 12, no. 6: 909. https://doi.org/10.3390/life12060909
APA StyleGuastalegname, M., Rondinone, V., Lucifora, G., Vallone, A., D’Argenio, L., Petracca, G., Giordano, A., Serrecchia, L., Manzulli, V., Pace, L., Fasanella, A., Simone, D., Cipolletta, D., & Galante, D. (2022). An Outbreak of Human Systemic Anthrax, including One Case of Anthrax Meningitis, Occurred in Calabria Region (Italy): A Description of a Successful One Health Approach. Life, 12(6), 909. https://doi.org/10.3390/life12060909








