Experimental Study on the Mix Ratio of Restored Heritage Building Adobe
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experiment Material
2.1.1. Soil
2.1.2. Quicklime
2.1.3. Sodium Methyl Silicate
2.1.4. Straw
2.2. Experiment Methods
2.2.1. Sample Plan
2.2.2. Sample Preparation
- (1)
- When making the samples of the single-mixed quicklime group, mix 2 kg of the dried soil sample with a 5 mm sieve and quicklime evenly, add wheat straw and mix again. Then add plain water to control the moisture content of the prepared soil samples at 32% (the liquid limit of the soil). Considering that the reaction between quicklime and water consumes water and the reaction speed is fast, based on the reaction principle of CaO + H2O = Ca(OH)2, it has been concluded that 1 g of CaO will consume 0.32 g of water, so the water consumption of lime is also considered when adding water. When the temperature of the prepared soil samples drops to 25 °C, pour them into the mold, vibrate and compact them, demold immediately, and then carry out natural curing in the laboratory.
- (2)
- When making the samples of the single-doped sodium methyl silicate group, first mix 2 kg of the dried soil sample with a 5 mm sieve and the wheat straw, and then dissolve the sodium methyl silicate solution in the added water and stir evenly. Finally, the mixed solution and the mixed soil sample are fully mixed before sample preparation.
- (3)
- When making the sample of the compound blending group, first mix 2 kg of the dried soil sample with a 5 mm sieve and quicklime, and then fully mix it with the wheat straw, then dissolve the sodium methyl silicate in the added water and stir evenly. Finally, the mixed solution and the mixed soil sample are fully mixed, the sample is prepared, and the mold is released immediately. When the pure soil group was made, the moisture content was 32% of the dry soil, and the samples were prepared immediately.
2.2.3. Unconfined Compressive Strength Test
2.2.4. PH Test
2.2.5. Electrical Conductivity Test
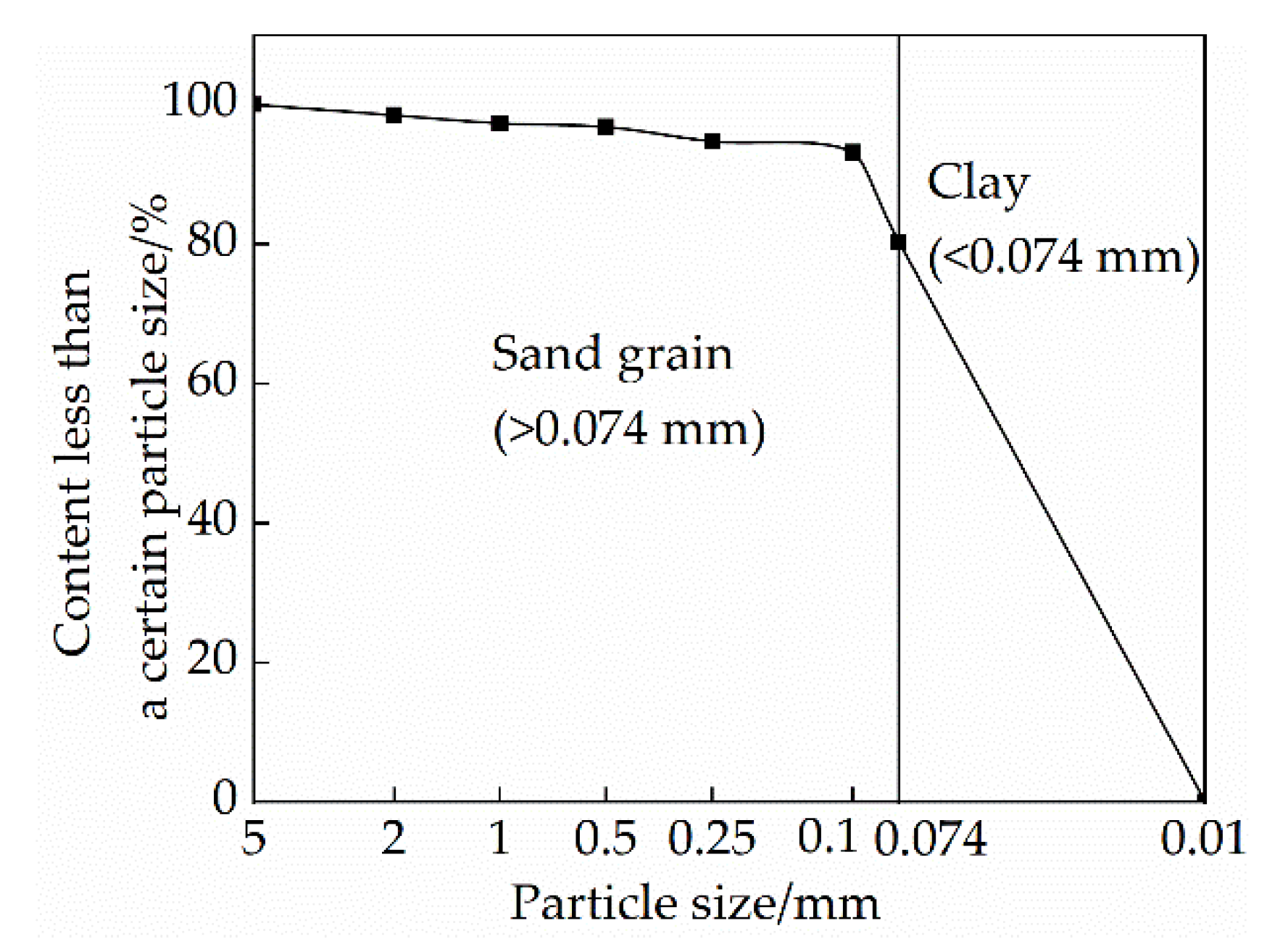
2.2.6. Particle Size Test
- MXn: fineness modulus of soil particles.
- n: curing age.
- A: cumulative sieve residue of particles of a certain size.
2.2.7. Capillary Water Absorption Test
2.2.8. Microstructure Testing
3. Results and Analysis
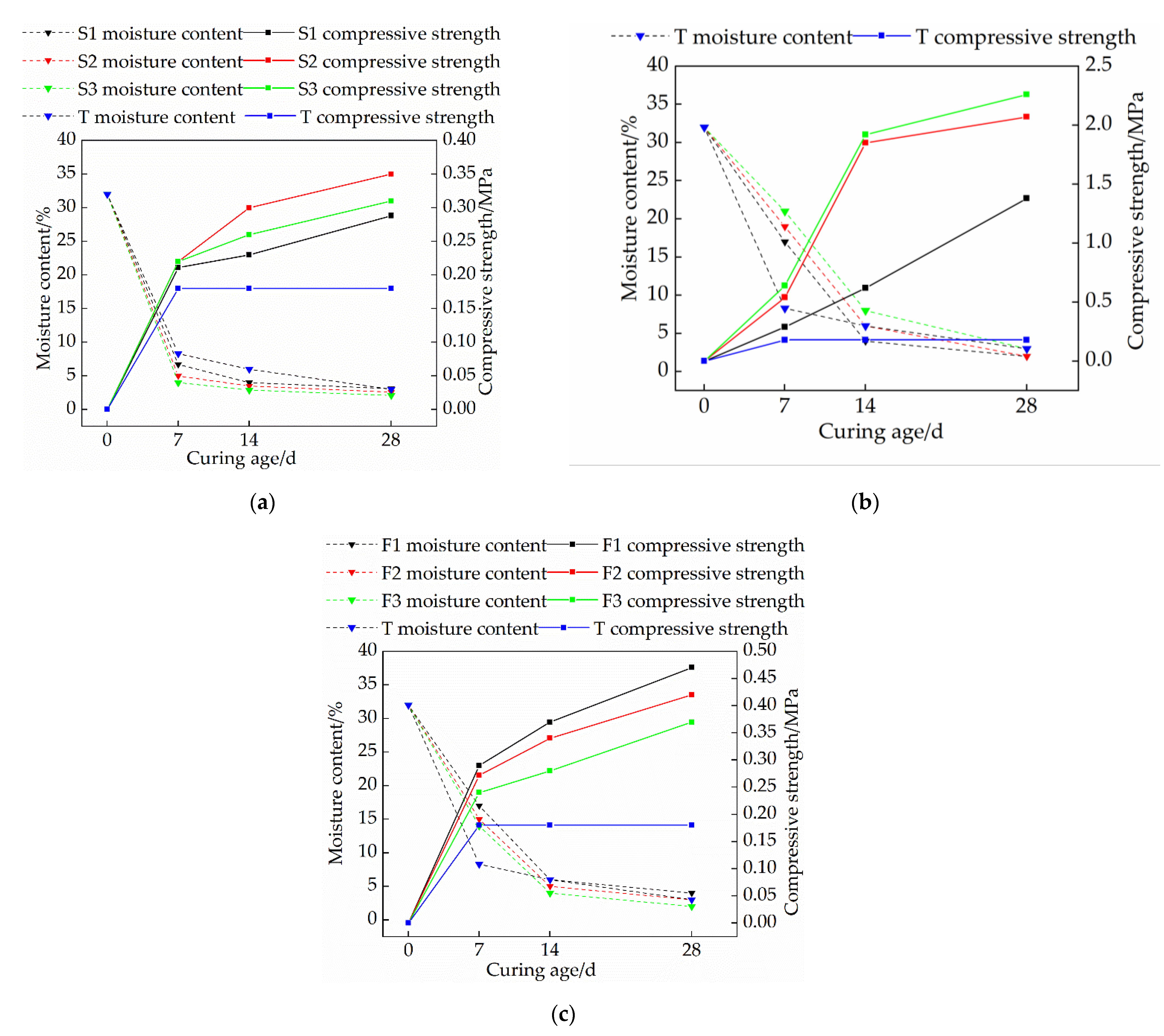
3.1. Effects of Mix Ratio and Curing Age on the Strength of Modified Raw Adobe
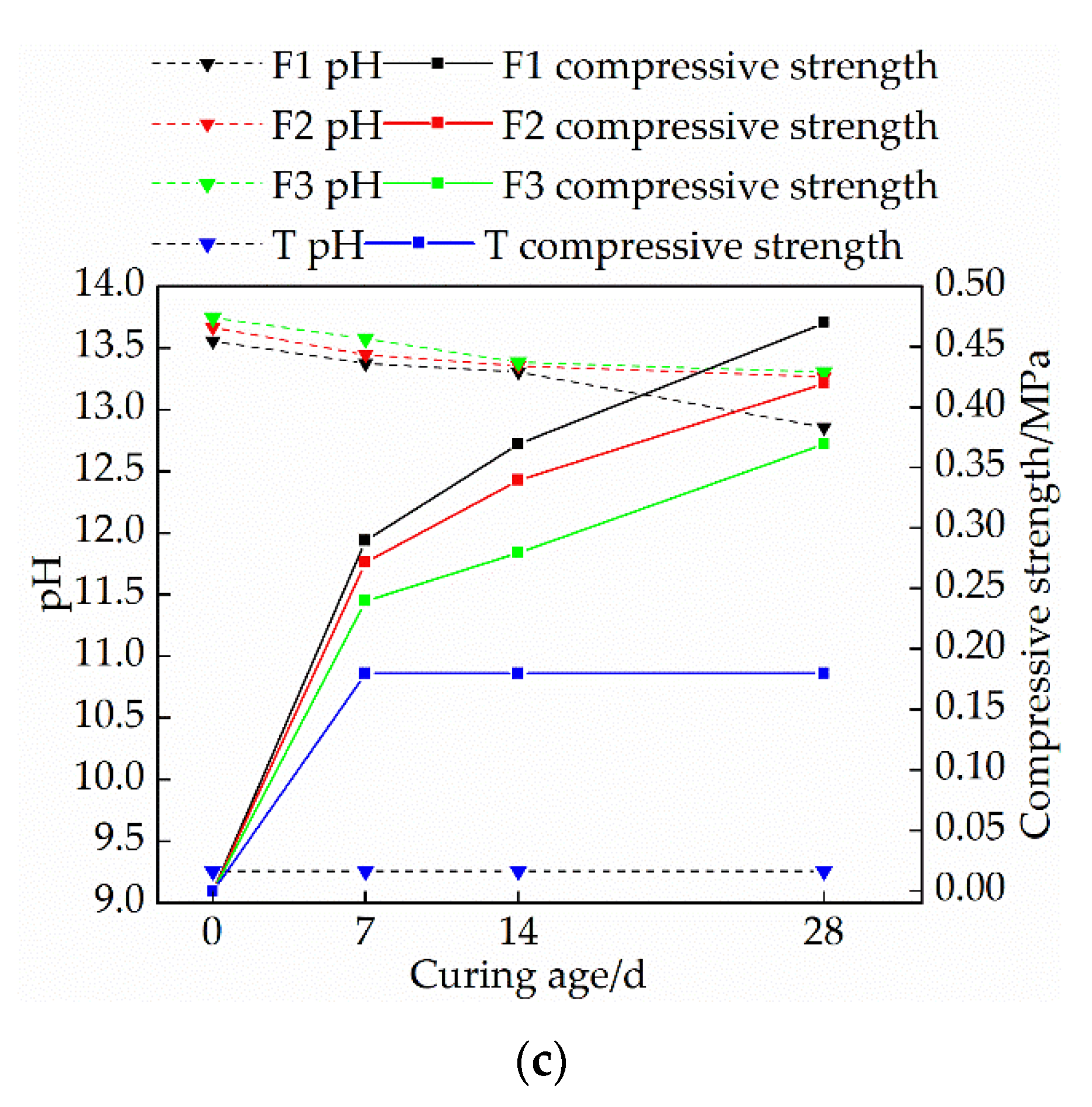
3.2. Effects of Mix Ratio and Curing Age on pH Value of Modified Raw Adobe
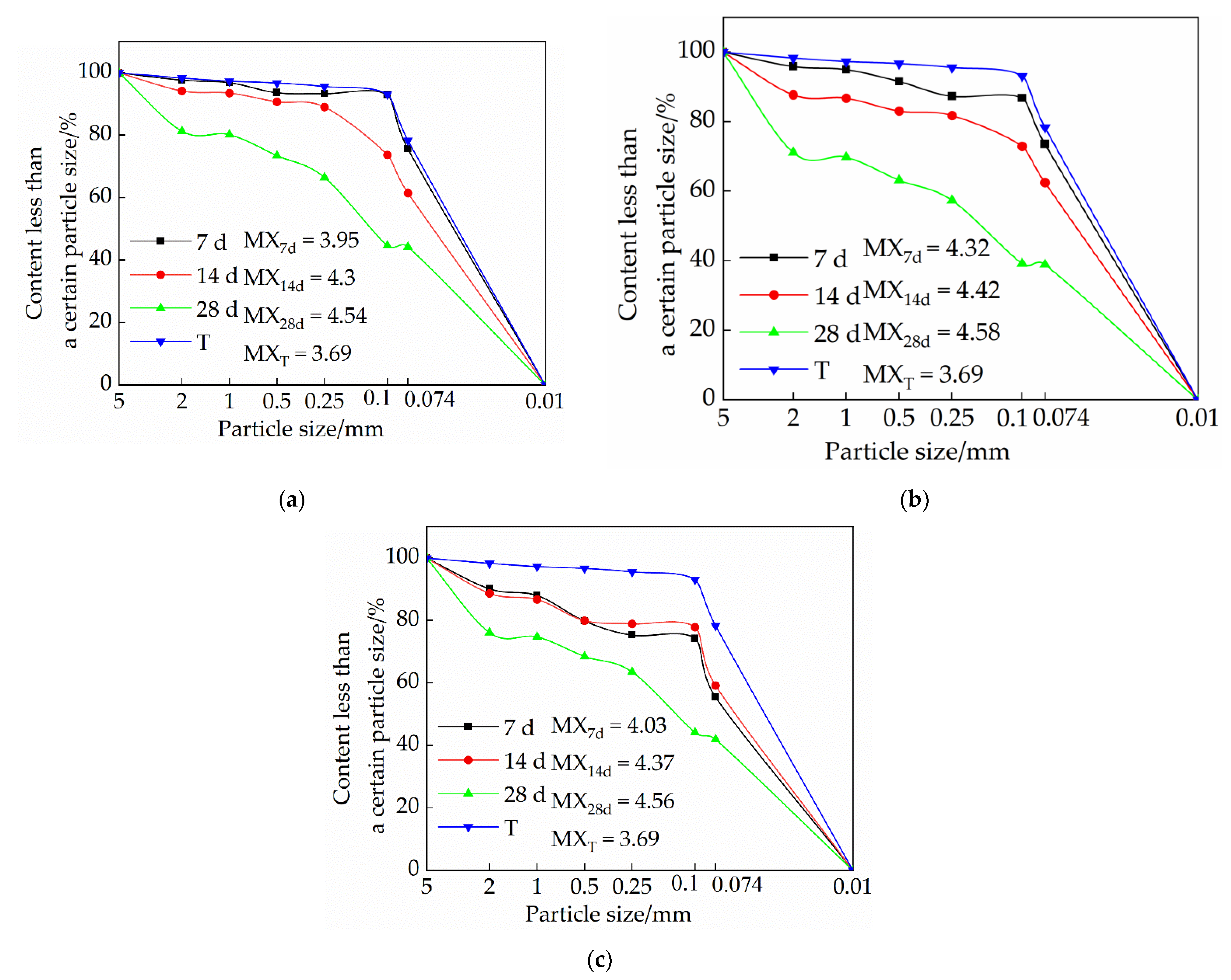
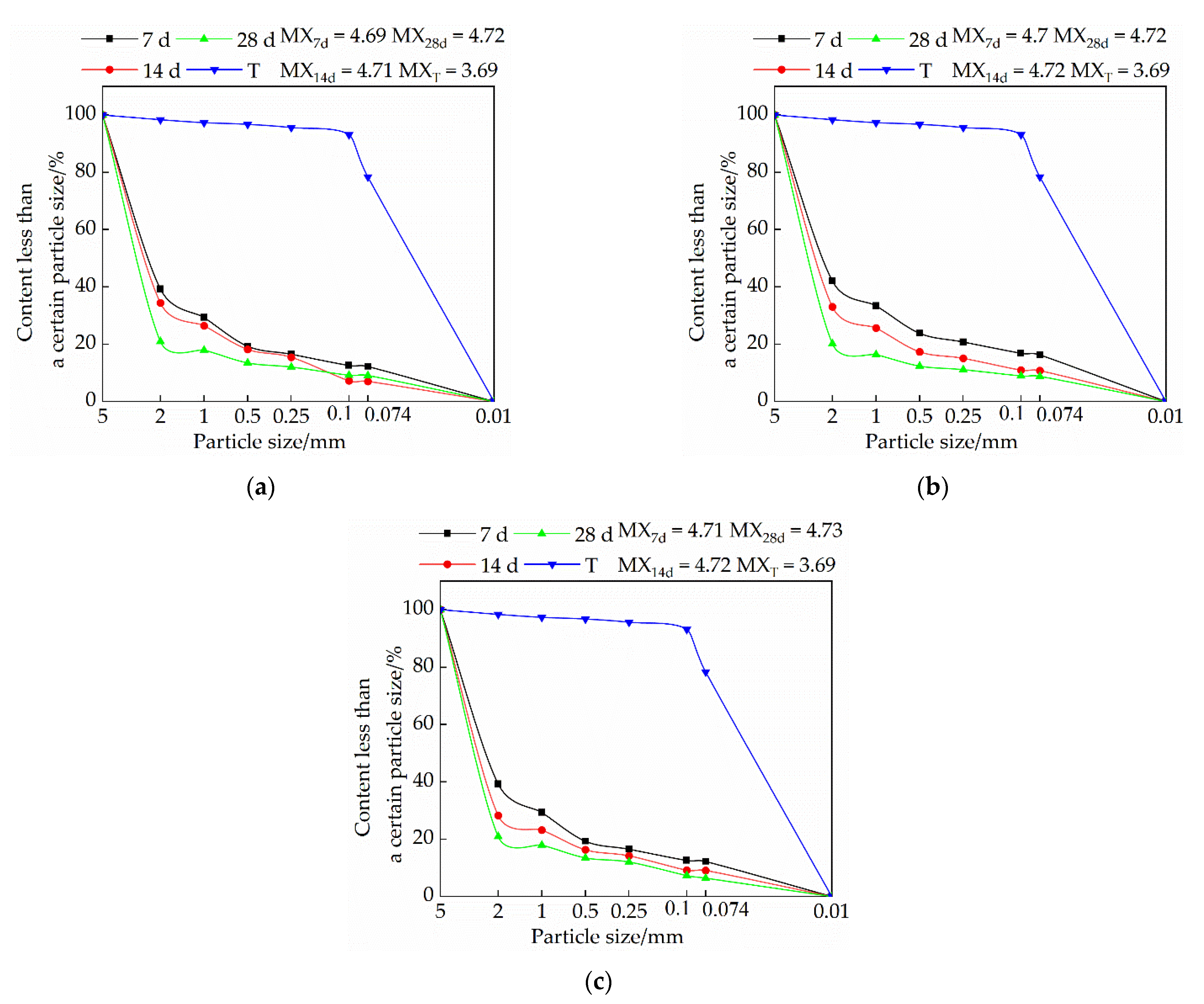
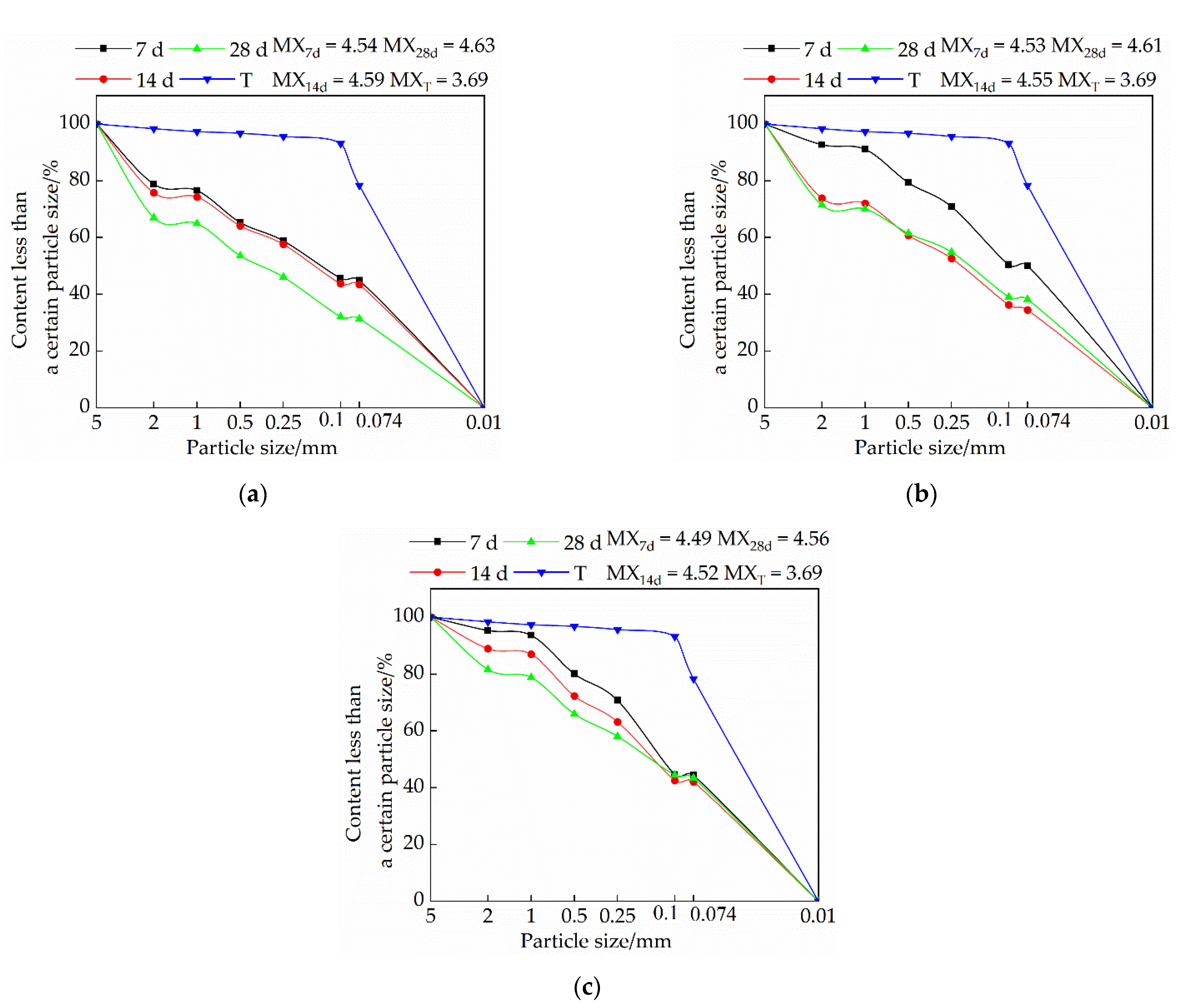
3.3. Effects of Mix Ratio and Age on Particle Gradation of Modified Raw Adobe
3.3.1. Influence of Lime on Particle Size Distribution of Samples
3.3.2. Influence of Sodium Methyl Silicate on Particle Size Distribution of Samples
3.3.3. The Effect of Sodium Methyl Silicate and Quicklime on the Particle Size Distribution of Samples
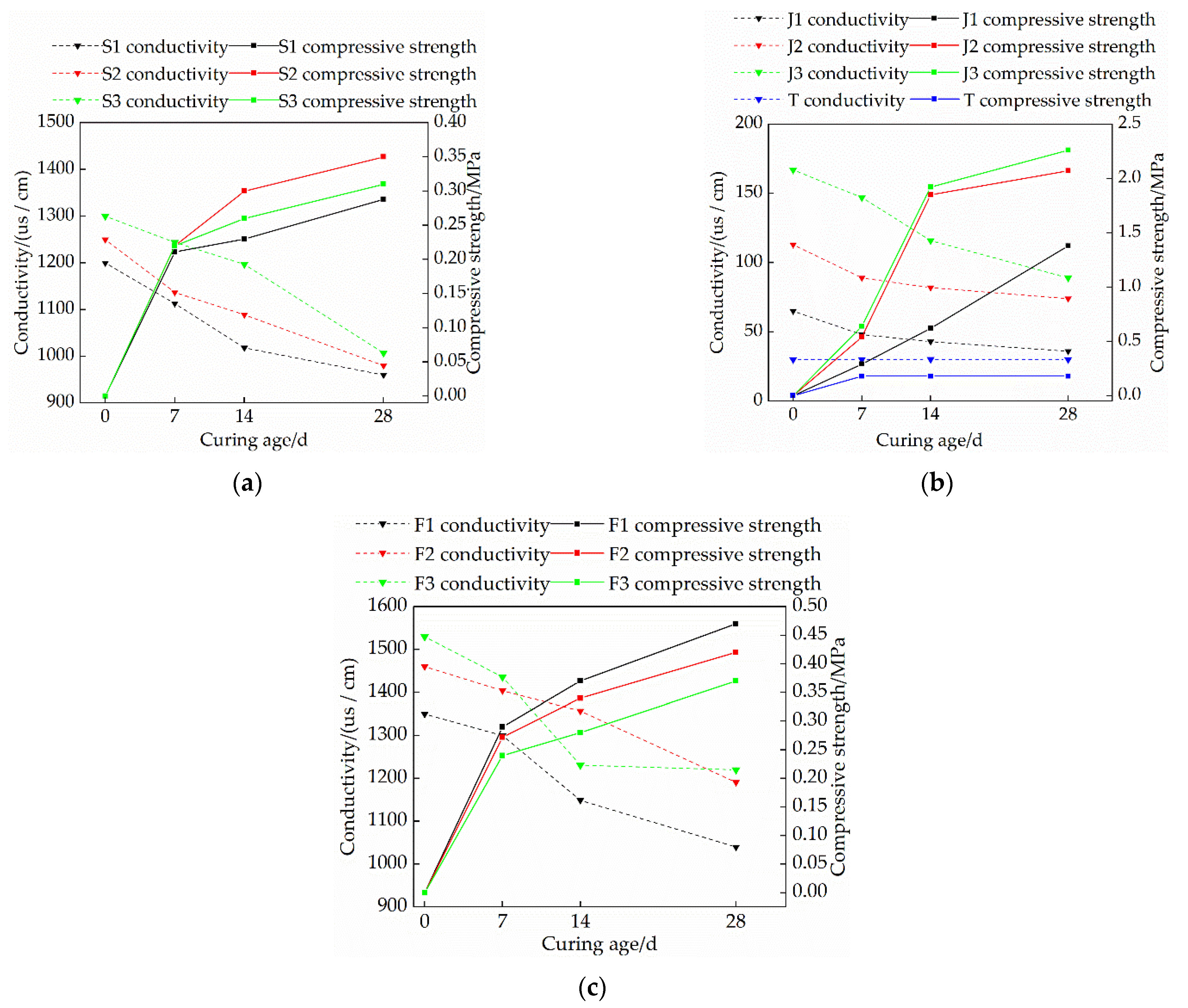
3.4. Effects of Mix Ratio and Curing Age on the Electrical Conductivity of Modified Raw Adobe
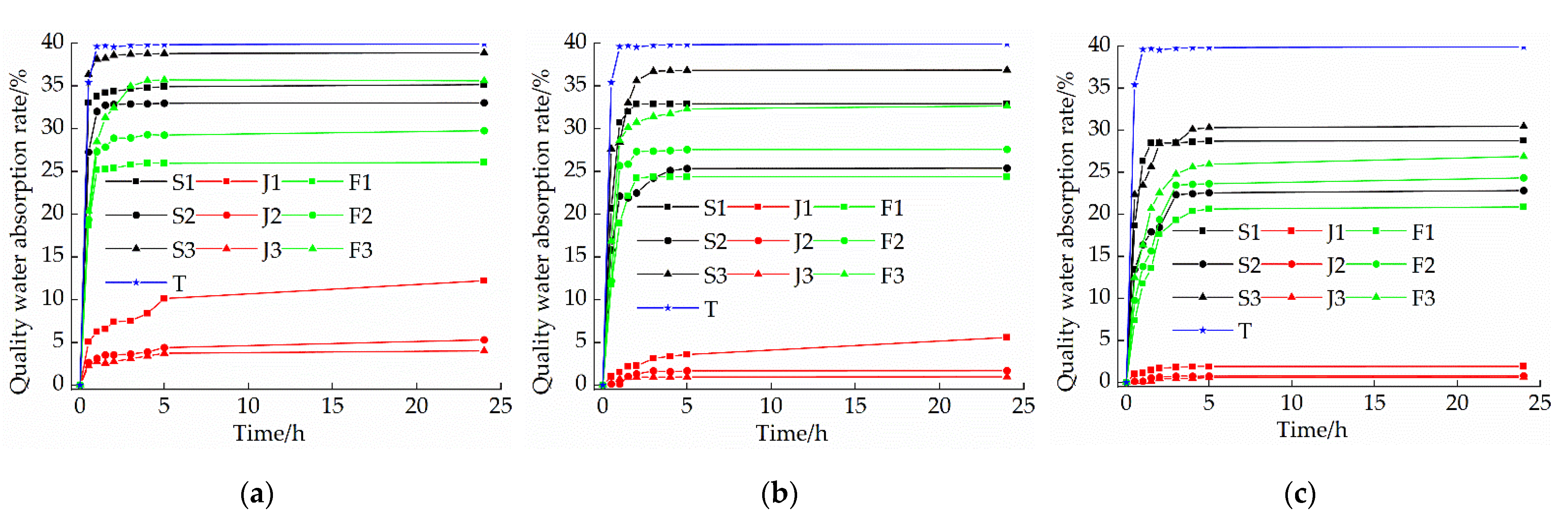
3.5. Capillary Water Absorption Test
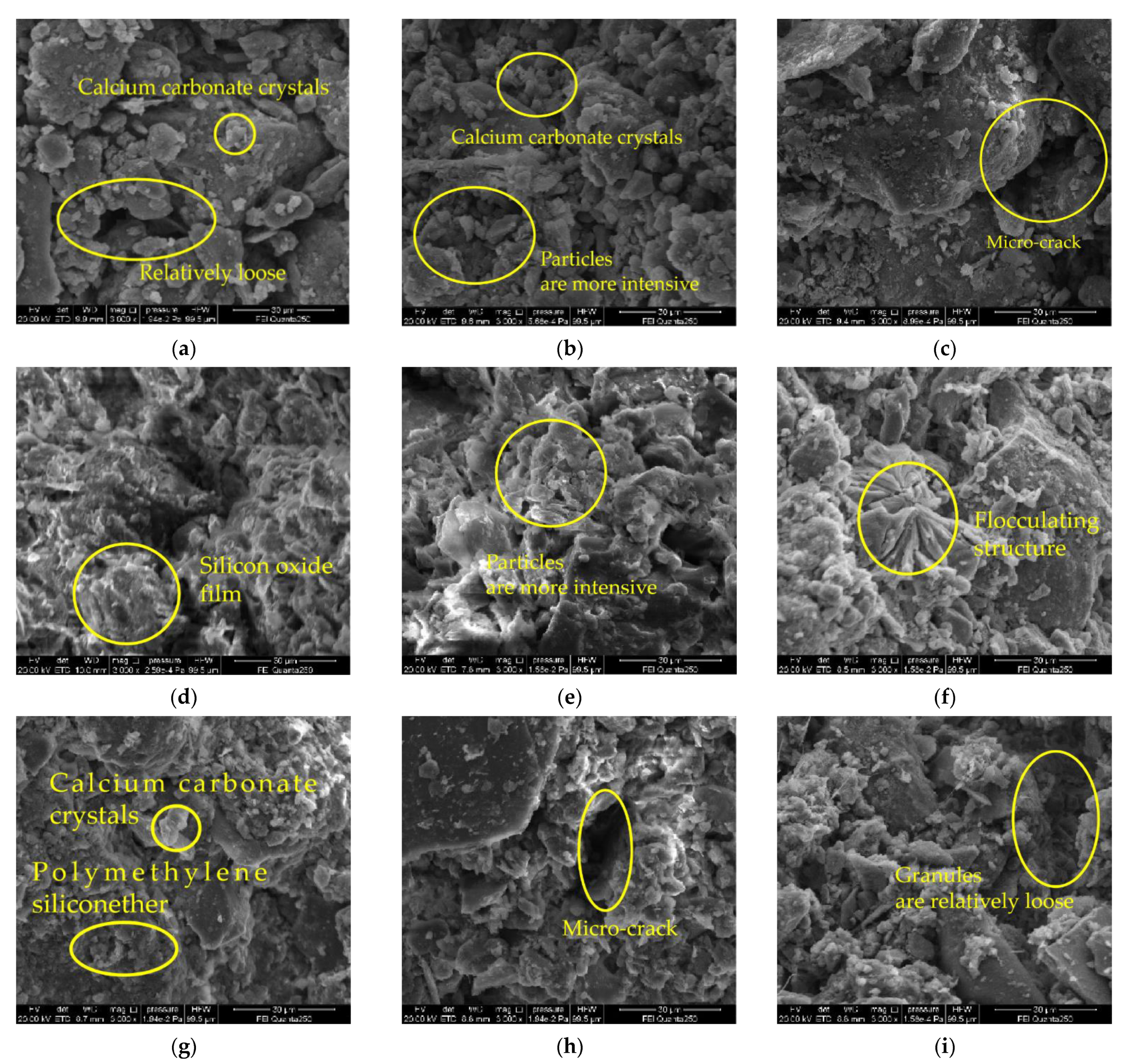
3.6. Micro Structure
4. Discussion
5. Conclusions
- (1)
- The compressive strength of each group of modified raw adobe increased with the increase of the curing age. The compressive strengths of the samples of the single-mixed quicklime group, the single-mixed sodium methyl silicate sample, and the composite-mixed sample for 28 days were 1.94 times, 12.6 times, and 2.61 times higher than those of the plain soil samples, respectively. In addition, in the 0–14-day interval, the intensity growth rate is faster, and in the 14–28-day interval, the intensity growth rate slows down.
- (2)
- The changing speed of pH and conductivity can express the degree of the carbonization reaction of the quicklime and sodium methylsilicate in the sample from the side. The pH and electrical conductivity of modified green adobe decreased with the increase of the curing age, decreased with the increase of the compressive strength, and the decrease range was larger from 0 d to 14 d and was smaller from 14 d to 28 d. Among them, the pH value of the 1.5% sodium methylsilicate sample decreased by 0.95 and the conductivity decreased by 31 (us/cm) within 0–14 days. For comparison, the pH value decreased by 0.32 and the conductivity decreased by 8 (us/cm) within 14–28 days. It is evident that the carbonization reaction speed of the improved sample is faster in 0–14 d, and the carbonization reaction speed is relatively slow in 14–28 d.
- (3)
- The particle size of each group of samples increased with the increase of the curing age. The samples of the single-doped sodium methyl silicate group were the densest, and the particles larger than 2 mm were the densest. The particle size of the samples in the single-doped CaO group first increased and then decreased with the increase of the lime content. The particle size of the samples in the single-doped sodium methyl silicate group increased with the increase of the sodium methyl silicate content. The particle size of the samples in the compound blending group decreased with the increase of the lime content.
- (4)
- It is evident from the SEM pictures that the internal pores of the samples in the modified group are all smaller, the particle structure of the sample doped with quicklime is relatively loose, and the particle structure of the sample doped with sodium methyl silicate is the densest. However, there are still micropores in the composite samples, resulting in the improvement of the strength and water resistance as “one plus one less than two”.
- (5)
- With the addition of quicklime and sodium methyl silicate, the water-resistance of the samples was improved. Among them, the water-resistance of the sample mixed with 1.5% sodium methyl silicate alone was the best, and the capillary water absorption was only 0.79%. However, when the content of sodium methyl silicate exceeds 1.5%, the water-resistance of modified raw adobe is improved to a lesser degree.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Giuffrida, G.; Caponetto, R.; Nocera, F.; Cuomo, M. Prototyping of a novel rammed earth technology. Sustainability 2021, 13, 11948. [Google Scholar] [CrossRef]
- Yue, J.; Lin, J.; Wang, Y.; Liu, T.; Zhang, Y.; Wang, S.; Wang, W. Improvement of soil and hydrological properties of Kaifeng imitation site. Eng. Sci. Technol. 2020, 52, 46–55. [Google Scholar] [CrossRef]
- Larionova, N.A. Influence of the chemical-mineral composition of active ashes on the processes of ash and ash-soil mixture hardening. Eng. Geol. World 2018, 13, 74–85. [Google Scholar] [CrossRef]
- Savary, M.; Saradj, M.; Ma Nesh, S.; Tahmasebiboldaji, N.; Kazemi, A.S. Improving the adobe material properties by laser material processing. Constr. Build. Mater. 2020, 249, 118591. [Google Scholar] [CrossRef]
- Cuccurullo, A.; Gallipoli, D.; Bruno, A.W.; Augarde, C.; Hughes, P.; Borderie, C.L. Influence of particle grading on the hydromechanical properties of hypercompacted earth. J. Build. Pathol. Rehabil. 2019, 5, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Zakrevskaya, L.; Gavrilenko, A.; Andreeva, K.; Lubin, P.; Udin, I. Man-made soils and mining wastes as raw materials for building composites. IOP Conf. Ser. Mater. Sci. Eng. 2020, 896, 012077. [Google Scholar] [CrossRef]
- Liu, J.; Shi, J.; Lin, F.; Sun, W. The effects of admixtures on the adobe wall materials’ durability. Appl. Mech. Mater. 2012, 174–177, 1306–1311. [Google Scholar] [CrossRef]
- Chen, W.; Zhang, K.; Guo, Z.; Fu, X.; Yang, P. Experimental study on engineering properties of SH-C serous calculi. Chin. J. Undergr. Space Eng. 2017, 13, 518–523. [Google Scholar]
- Liu, S.; Yu, J.; Han, L.; Cai, Y.; Tu, B.; Zhou, J. Experimental study on water-resistance of calcium carbonate precipitation induced by microbes on the surface of trihex soil. Chin. J. Rock Mech. Eng. 2019, 38, 11. [Google Scholar] [CrossRef]
- Niu, B.; Liao, H.; Lv, J.; Wu, J.; Dong, Y. Study on disintegration characteristics of improved ancient site soil by sticky rice solution. IOP Conf. Ser. Earth Environ. Sci. 2020, 455, 012120. [Google Scholar] [CrossRef]
- Kong, D.; Chen, J.; Wan, R.; Liu, H. Study on restoration materials for historical silty earthen sites based on lime and starch ether. Adv. Mater. Sci. Eng. 2020, 2020, 1–16. [Google Scholar] [CrossRef]
- Qian, J.; Wang, Q.; Jia, X.; Bie, A. Study on the use of desulfurization waste from coal-fired power plants to modify raw soil materials. New Build. Mater. 2009, 36, 4. [Google Scholar] [CrossRef]
- Zhou, T.; Liu, W. Experimental study on basic mechanical properties of machine-made adobe brick masonry. J. Huazhong Univ. Sci. Technol. Nat. Sci. Ed. 2020, 7, 93–98. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, S.; Rong, H.; Zhang, L.; Zhang, Y.; Xu, R.; Wang, X.; Yang, J. Study on the Effect and Mechanism of Lime-based Materials on Soil Modification. Funct. Mater. 2019, 50, 4067–4073. [Google Scholar] [CrossRef]
- Guo, Z.; Zhu, Q.; Liu, C.; Xing, Z. Preparation of ca-al-fe deicing salt and modified with sodium methyl silicate for reducing the influence of concrete structure. Constr. Build. Mater. 2018, 172, 263–271. [Google Scholar] [CrossRef]
- Ma, Q.; Liu, S. Effect on silt capillary water absorption upon addition of sodium methyl silicate (SMS) and microscopic mechanism analysis. Coatings 2020, 10, 724. [Google Scholar] [CrossRef]
- Vali, H.; Akenjiang, T.; Hasiyet, H.; Kamil, V. Experimental study on compressive resistance of single-block adobe of plant fiber. Build. Sci. 2012, 28, 61–64. [Google Scholar] [CrossRef]
- Wang, D.; He, F. Investigation on performance and mechanism of CO2 carbonated slag/fly ash solidified soils. Chin. J. Rock Mech. Eng. 2020, 7, 1493–1502. [Google Scholar] [CrossRef]
- Lv, Q.; Gu, L.; Shan, X.; Liu, B.; Guo, L. Analysis of soluble salt content, electrical conductivity and grain size of coarse grained saline soil in Gansu Province. J. Lanzhou Univ. (Nat. Sci. Ed.) 2021, 4, 459–464. [Google Scholar] [CrossRef]
- Yusof, Z.M.; Aladhami, A.; Matore, M. Compressive Strength of Stabilised Granitic Residual Soil Using Mixture of Pineapple Fibre—Hydrated Lime. Sustainability 2022, 14, 3826. [Google Scholar] [CrossRef]
- Attoh-Okine, N.O. Lime Treatment of Laterites and Gravel Revisited. Constr. Build. Mater. 1995, 9, 283–287. [Google Scholar] [CrossRef]
- Bell, F.G. Lime Stabilization of Clay Minerals and Soils. Eng. Geol. 1996, 42, 223–237. [Google Scholar] [CrossRef]
- Maixent Loubouth, S.; Ahouet, L.; Elenga, R.; Okina, S.; Kimbembe, P. Improvement of the Geotechnical Properties of the Soil of Lime-Treated Cubitermes Mound Soil. Open J. Civ. Eng. 2020, 10, 22–31. [Google Scholar] [CrossRef] [Green Version]
- Millogo, Y.; Hajjaji, M.; Ouedraogo, R. Microstructure and Physical Properties of Lime-Clayey Adobe Bricks. Constr. Build. Mater. 2007, 22, 2386–2392. [Google Scholar] [CrossRef]
- Su, J.; Luo, Y.; Yong, Z. Molecular simulation of hydration mechanism of montmorillonite inhibited by potassium methyl silicate shale inhibitor. Chem. World 2019, 60, 45–53. [Google Scholar] [CrossRef]
- Eskisar, T. The role of carbide lime and fly ash blends on the geotechnical properties of clay soils. Bull. Eng. Geol. Environ. 2021, 80, 6343–6357. [Google Scholar] [CrossRef]
- Martínez, G.; Laguna, A.M.; Giráldez, J.V.; Vanderlinden, K. Concurrent variability of soil moisture and apparent electrical conductivity in the proximity of olive trees. Agric. Water Manag. 2021, 245, 106652. [Google Scholar] [CrossRef]
- Cai, G.; Liu, S.; Cao, J. Effect of Initial Moisture Content on Strength and Resistivity of MgO Carbide Silt. China J. Highw. Transp. 2017, 30, 18–26. [Google Scholar] [CrossRef]
- Bao, J.; Hu, W.; Zhang, P.; Li, Z.; Lei, F.; Zhao, T. Effect of Organic Silicon Hydrophobic Agent on Strength and Capillary Absorption of Concrete. J. Chin. Ceram. Soc. 2020, 48, 1644–1652. [Google Scholar] [CrossRef]
- Ramesh, H.N.; Manjunatha, B.V. Justification of strength properties of microstructural changes in the black cotton soil stabilized with rice husk ash and carbide lime in the presence of sodium salts. SN Appl. Sci. 2020, 2, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Zhang, S.; Yang, B.; Zhai, W.; Li, S.; Kong, G. Composition and properties of methyl silicate/silicate composite coatings. J. Mater. Eng. 2021, 49, 163–170. [Google Scholar]
- Zhang, S.; Li, H.; Chen, C. Effect of chemical admixture on properties of light desulfurized gypsum blocks. J.Xi’an Univ. Arch. Tech. (Nat. Sci. Ed.) 2021, 53, 9. [Google Scholar] [CrossRef]
- de Melo Ferreira, S.R.; Dantas de Araujo, A.G.; Silva Barbosa, F.A.; Rodrigues Silva, T.C.; de Lima Bezerra, I.M. Analysis of changes in volume and propagation of cracks in expansive soil due to changes in water content. Rev. Bras. De Cienc. Do Solo 2020, 44. [Google Scholar] [CrossRef]
- Yuan, Y.; Jia, M.; Li, W. Experimental study on methyl-sodium stabilized silty soil. China Sci. Technol. Pap. 2017, 12, 831–833, 844. [Google Scholar]
- Ciancio, D.; Beckett, C.T.S.; Carraro, J.A.H. Optimum lime content identification for lime-stabilised rammed earth. Constr. Build. Mater. 2014, 53, 59–65. [Google Scholar] [CrossRef] [Green Version]
- Xie, X.; Wang, L.; Deng, L.; Zhang, G. Study on the microscopic mechanism of the loess improved by quicklime. Coalf. Geol. Explor. 2021, 49, 7. [Google Scholar] [CrossRef]




| Soil | Liquid limit/% | Plastic limit/% | Plasticity Index | Maximum Dry Density/g/cm3 | Optimum Moisture Content/% | Natural Moisture Content/% |
|---|---|---|---|---|---|---|
| Silty clay | 32.72 | 20.28 | 12.46 | 1.73 | 17.1 | 17.97 |
| Trial Grouping | Test Number | Wheat Straw Content/% | Curing Age/d | Lime Content/% | Sodium Methyl Silicate Content/% |
|---|---|---|---|---|---|
| S1 | 0.5 | 7 | 0 | ||
| Single mixed quicklime group | S2 | 0.5 | 15 | 0 | |
| S3 | 0.5 | 23 | 0 | ||
| J1 | 0.5 | 0 | 0.5 | ||
| Mono-doped sodium methyl silicate group | J2 | 0.5 | 7, 14, 28 | 0 | 1.5 |
| J3 | 0.5 | 0 | 3 | ||
| F1 | 0.5 | 7 | 1.5 | ||
| Compounding group | F2 | 0.5 | 15 | 1.5 | |
| F3 | 0.5 | 23 | 1.5 | ||
| Pure soil | T | 0.5 | 28 | 0 | 0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yue, J.; Zhang, Y.; Li, P.; Zhang, J.; Huang, X.; Yue, Y.; Han, Z. Experimental Study on the Mix Ratio of Restored Heritage Building Adobe. Materials 2022, 15, 4034. https://doi.org/10.3390/ma15114034
Yue J, Zhang Y, Li P, Zhang J, Huang X, Yue Y, Han Z. Experimental Study on the Mix Ratio of Restored Heritage Building Adobe. Materials. 2022; 15(11):4034. https://doi.org/10.3390/ma15114034
Chicago/Turabian StyleYue, Jianwei, Yiang Zhang, Peng Li, Jing Zhang, Xuanjia Huang, Yang Yue, and Zhiguang Han. 2022. "Experimental Study on the Mix Ratio of Restored Heritage Building Adobe" Materials 15, no. 11: 4034. https://doi.org/10.3390/ma15114034
APA StyleYue, J., Zhang, Y., Li, P., Zhang, J., Huang, X., Yue, Y., & Han, Z. (2022). Experimental Study on the Mix Ratio of Restored Heritage Building Adobe. Materials, 15(11), 4034. https://doi.org/10.3390/ma15114034






