Diagnostic Strategies in Deep Venous Thrombosis
Abstract
Zusammenfassung
Introduction
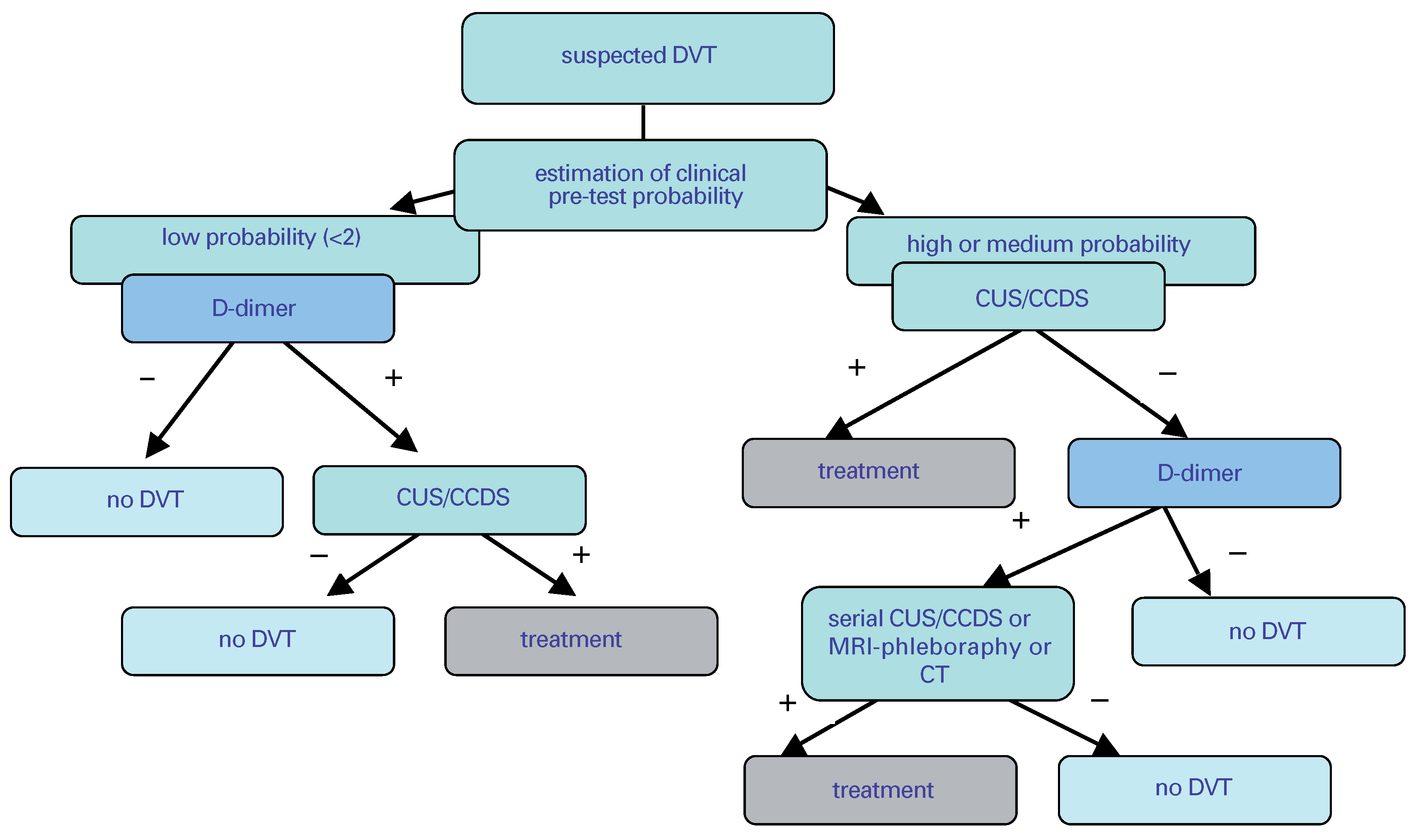
Clinical probability and risk factor assessment for the diagnosis of DVT
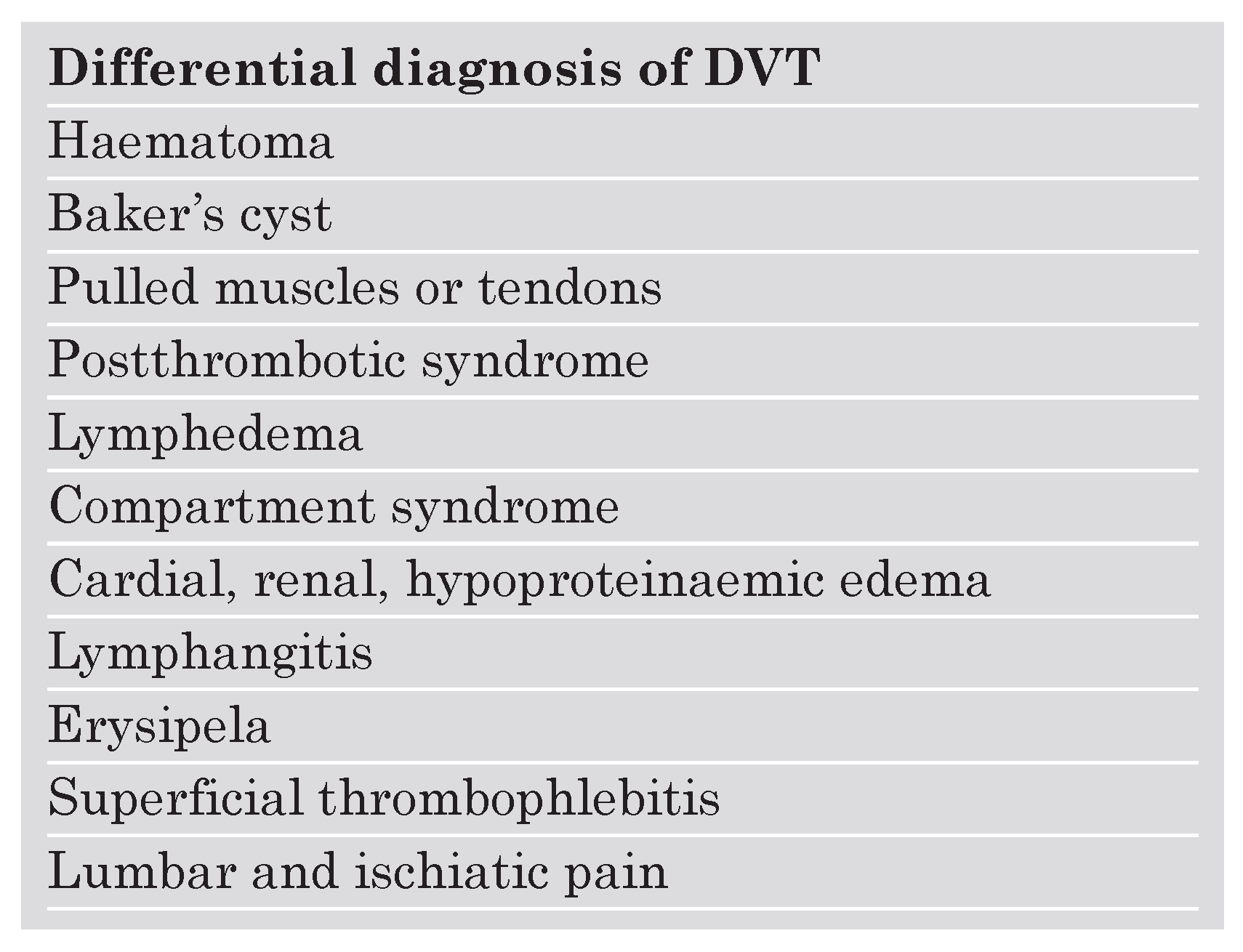
Acute symptomatic proximal DVT
Lower leg thrombosis
Asymptomatic DVT
Recurrent DVT
Upper extremity DVT
Objective testing methods
Imaging
Contrast venography
Real-time compression ultrasound and colour coded duplex sonography
MRI phlebography
Contrast-enhanced multislice chest computer tomography (CT)
Impendance plethysmography (IPG)
D-dimer assessment
References
- Nordstrom, M.; Lindblad, B.; Bergqvist, D.; Kjellstrom, T. A prospective study of the incidence of deep-vein thrombosis within a defined urban population. J Internal Med 1992, 232, 155–160. [Google Scholar] [CrossRef]
- Anderson, F.A., Jr.; Wheeler, H.B.; Goldberg, R.J.; Hosmer, D.W.; Patwardhan, N.A.; Jovanovic, B.; et al. A population-based perspective of the hospital incidence and case-fatality rates of deep vein thrombosis and pulmonary embolism. The Worcester DVT Study. Arch Intern Med 1991, 151, 933–938. [Google Scholar] [CrossRef]
- Prandoni, P.; Lensing, A.W.; Cogo, A.; Cuppini, S.; Villalta, S.; Carta, M.; et al. The long-term clinical course of acute deep venous thrombosis. Ann Intern Med 1996, 125, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Hansson, P.O.; Sörbo, J.; Eriksson, H. Recurrent venous thromboembolism after deep vein thrombosis: Incidence and risk factors. Arch Intern Med 2000, 160, 769–774. [Google Scholar] [CrossRef] [PubMed]
- Kearon, C. Natural history of venous thromboembolism. Circulation 2003, 107, I22–30. [Google Scholar] [CrossRef]
- Pengo, V.; Lensing, A.W.; Prins, M.H.; Marchiori, A.; Davidson, B.L.; Tiozzo, F.; et al.; Thromboembolic Pulmonary Hypertension Study Group Incidence of chronic thromboembolic pulmonary hypertension after pulmonary embolism. N Engl J Med 2004, 350, 2257–2264. [Google Scholar] [CrossRef]
- Mclachlin, J.; Richards, T.; Paterson, J.C. An evaluation of clinical signs in the diagnosis of venous thrombosis. Arch Surg 1962, 85, 738–744. [Google Scholar] [CrossRef] [PubMed]
- Goodacre, S.; Sutton, A.J.; Sampson, F.C. Meta-analysis: The value of clinical assessment in the diagnosis of deep venous thrombosis. Ann Intern Med 2005, 143, 129–139. [Google Scholar] [CrossRef]
- Levi, M.; Hart, W.; Buller, H.R. Physical examination – the significance of Homan’s sign. Ned Tijdschr Geneeskd 1999, 143, 1861–1863. [Google Scholar]
- Kahn, S.R. The clinical diagnosis of deep venous thrombosis. Arch Intern Med 1998, 158, 2315–2323. [Google Scholar] [CrossRef]
- Wells, P.S.; Hirsh, J.; Anderson, D.R.; Lensing, A.W.; Foster, G.; Kearon, C.; et al. Accuracy of clinical assessment of deep-vein thrombosis. Lancet 1995, 345, 1326–1330. [Google Scholar] [CrossRef] [PubMed]
- Ahearn, G.S.; Bounameaux, H. The role of the D-dimer in the diagnosis of venous thromboembolism. Semin Respir Crit Care Med 2000, 21, 521–536. [Google Scholar] [CrossRef]
- Kraaijenhagen, R.A.; Piovella, F.; Bernardi, E.; Verlato, F.; Beckers, E.A.; Koopman, M.M.; et al. Simplification of the diagnostic management of suspected deep vein thrombosis. Arch Intern Med 2002, 162, 907–911. [Google Scholar] [CrossRef]
- Büller, H.R.; Agnelli, G.; Hull, R.D.; Hyers, T.M.; Prins, M.H.; Raskob, G.E. Antithrombotic therapy for venous thromboembolic disease: The Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy. Chest 2004, 126, 401–428S. [Google Scholar] [CrossRef]
- Meissner, M.H.; Caps, M.T.; Bergelin, R.O.; Manzo, R.A.; Strandness, D.E., Jr. Early outcome after isolated calf vein thrombosis. Vasc Surg 1997, 26, 749–756. [Google Scholar] [CrossRef] [PubMed]
- Hansson, P.O.; Sörbo, J.; Eriksson, H. Recurrent venous thromboembolism after deep vein thrombosis. Incidence and risk factors. Arch Intern Med 2000, 160, 769–774. [Google Scholar] [CrossRef] [PubMed]
- Fraser, D.G.; Moody, A.R.; Morgan, P.S.; et al. Diagnosis of lower–limb deep venous thrombosis: A prospective blinded study of magnetic resonance direct thrombus imaging. Ann Intern Med 2002, 136, 89–98. [Google Scholar] [CrossRef]
- Baxter, G.M.; Kincaid, W.; Jeffrey, R.F.; Millar, G.M.; Porteous, C.; Morley, P. Comparison of colour Doppler ultrasound with venography in the diagnosis of axillary and subclavian vein thrombosis. Br J Radiol 1991, 6, 777–781. [Google Scholar] [CrossRef]
- Haire, W.D.; Lynch, T.G.; Lieberman, R.P.; Lund, G.B.; Edney, J.A. Utility of duplex ultrasound in the diagnosis of asymptomatic catheter-induced subclavian vein thrombosis. J Ultrasound Med 1991, 10, 493–496. [Google Scholar] [CrossRef]
- Prandoni, P.; Polistena, P.; Bernardi, E.; Cogo, A.; Casara, D.; Verlato, F.; et al. Upper-extremity deep vein thrombosis. Risk factors, diagnosis and complications. Arch Intern Med 1997, 157, 57–62. [Google Scholar] [CrossRef]
- Tapson, V.F.; Carroll, B.A.; Davidson, B.L.; Elliott, C.G.; Fedullo, P.F.; Hales, C.A.; et al. The diagnostic approach to acute venous thromboembolism. Clinical practice guideline. American Thoracic Society. Am J Respir Crit Care Med 1999, 160, 1043–1066. [Google Scholar] [PubMed]
- Stern, J.B.; Abehsera, M.; Grenet, D.; Friard, S.; Couderc, L.J.; Scherrer, A.; et al. Detection of pelvic vein thrombosis by magnetic resonance angiography in patients with acute pulmonary embolism and normal lower limb compression ultrasonography. Chest 2002, 122, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Taffoni, M.J.; Ravenel, J.G.; Ackerman, S.J. Prospective comparison of indirect CT venography versus venous sonography in ICU patients. Am J Roentgenol 2005, 185, 457–462. [Google Scholar] [CrossRef]
- Katz, D.S.; Loud, P.A.; Bruce, D.; Gittleman, A.M.; Mueller, R.; Klippenstein, D.L.; et al. Combined CT venography and pulmonary angiography: A comprehensive review. Radiographics 2002, S3–S19; discussion S20–S24. [Google Scholar] [CrossRef]
- Cham, M.D.; Yankelevitz, D.F.; Shaham, D.; Shah, A.A.; Sherman, L.; Lewis, A.; et al. Deep venous thrombosis: Detection by using indirect CT venography. The Pulmonary AngiographyIndirect CT Venography Cooperative Group. Radiology 2000, 216, 744–751. [Google Scholar] [CrossRef] [PubMed]

 |
 |
 |
© 2006 by the author. Attribution - Non-Commercial - NoDerivatives 4.0.
Share and Cite
Dörffler-Melly, J. Diagnostic Strategies in Deep Venous Thrombosis. Cardiovasc. Med. 2006, 9, 110. https://doi.org/10.4414/cvm.2006.01159
Dörffler-Melly J. Diagnostic Strategies in Deep Venous Thrombosis. Cardiovascular Medicine. 2006; 9(3):110. https://doi.org/10.4414/cvm.2006.01159
Chicago/Turabian StyleDörffler-Melly, Janine. 2006. "Diagnostic Strategies in Deep Venous Thrombosis" Cardiovascular Medicine 9, no. 3: 110. https://doi.org/10.4414/cvm.2006.01159
APA StyleDörffler-Melly, J. (2006). Diagnostic Strategies in Deep Venous Thrombosis. Cardiovascular Medicine, 9(3), 110. https://doi.org/10.4414/cvm.2006.01159




