Therapy of Venous Thromboembolism: Anticoagulant Treatment
Abstract
Résumé
Introduction
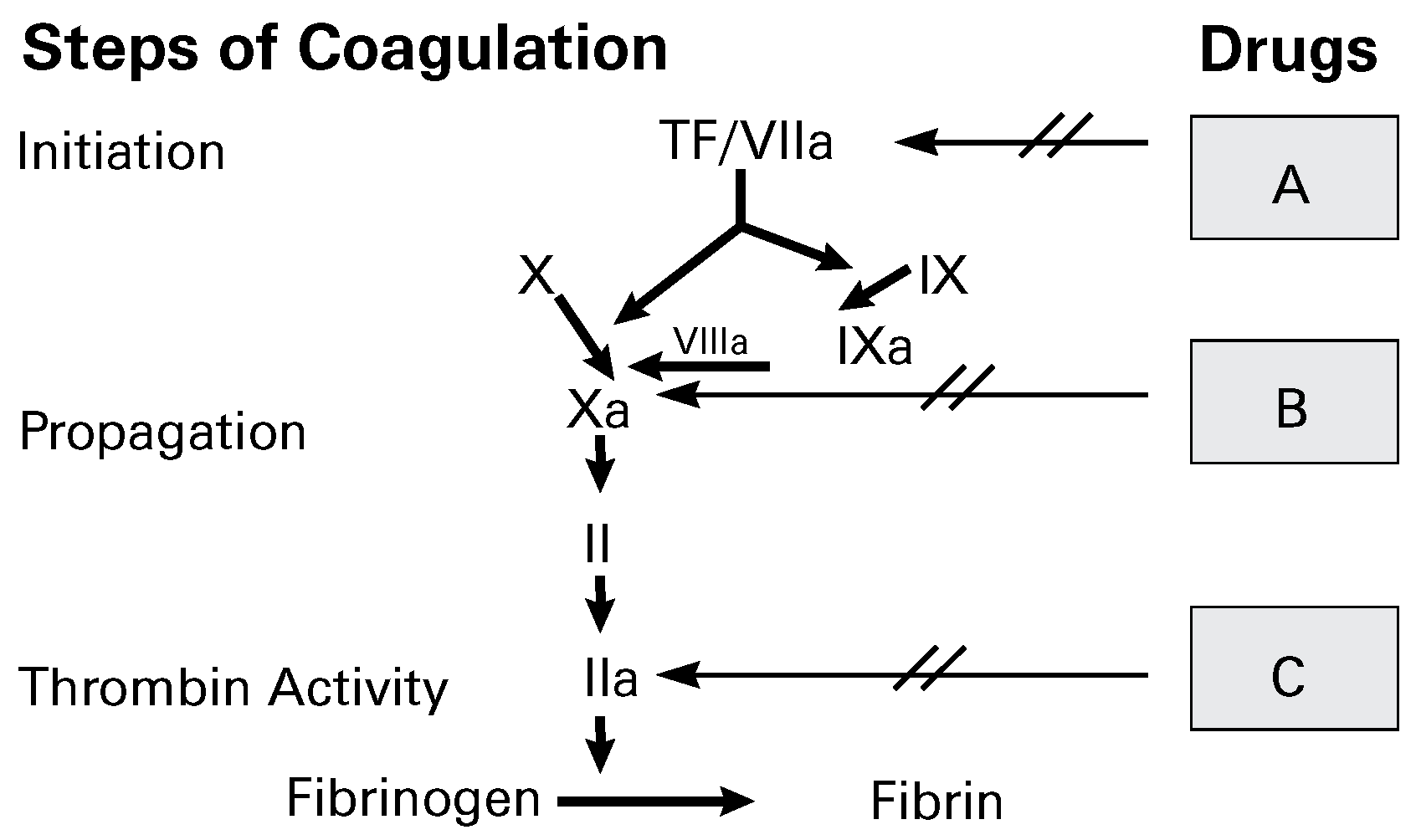
Anticoagulant drugs
From unfractionated (UFH) to low-molecular-weight (LMWH) heparin
Vitamin K antagonists
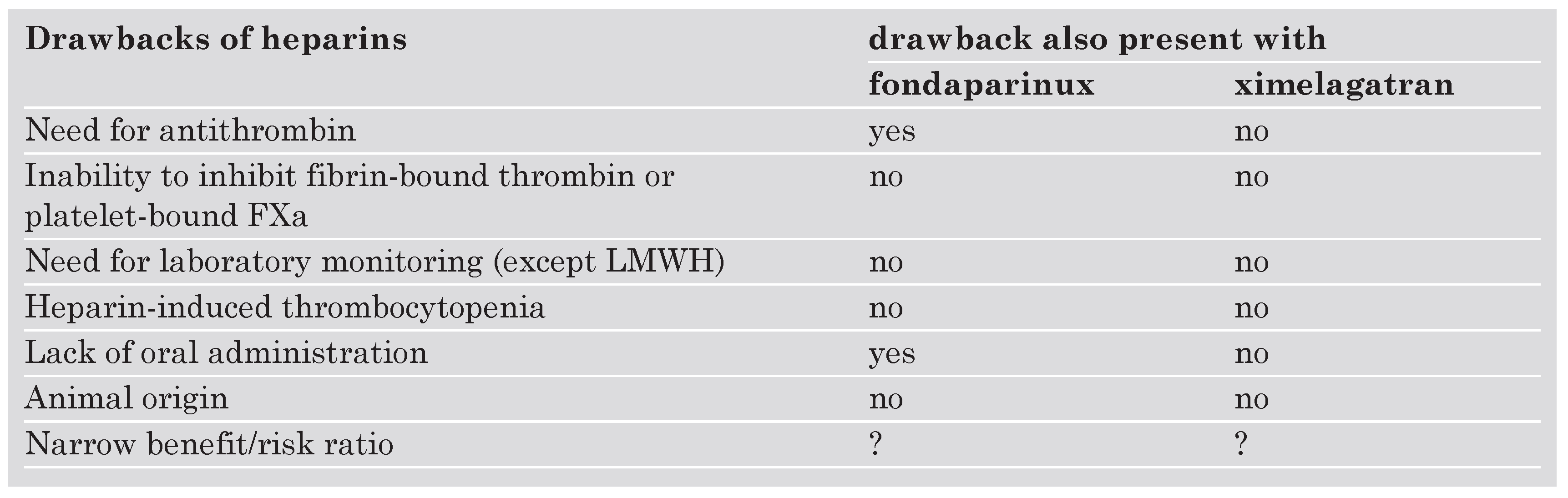
The upcoming anticoagulant drugs
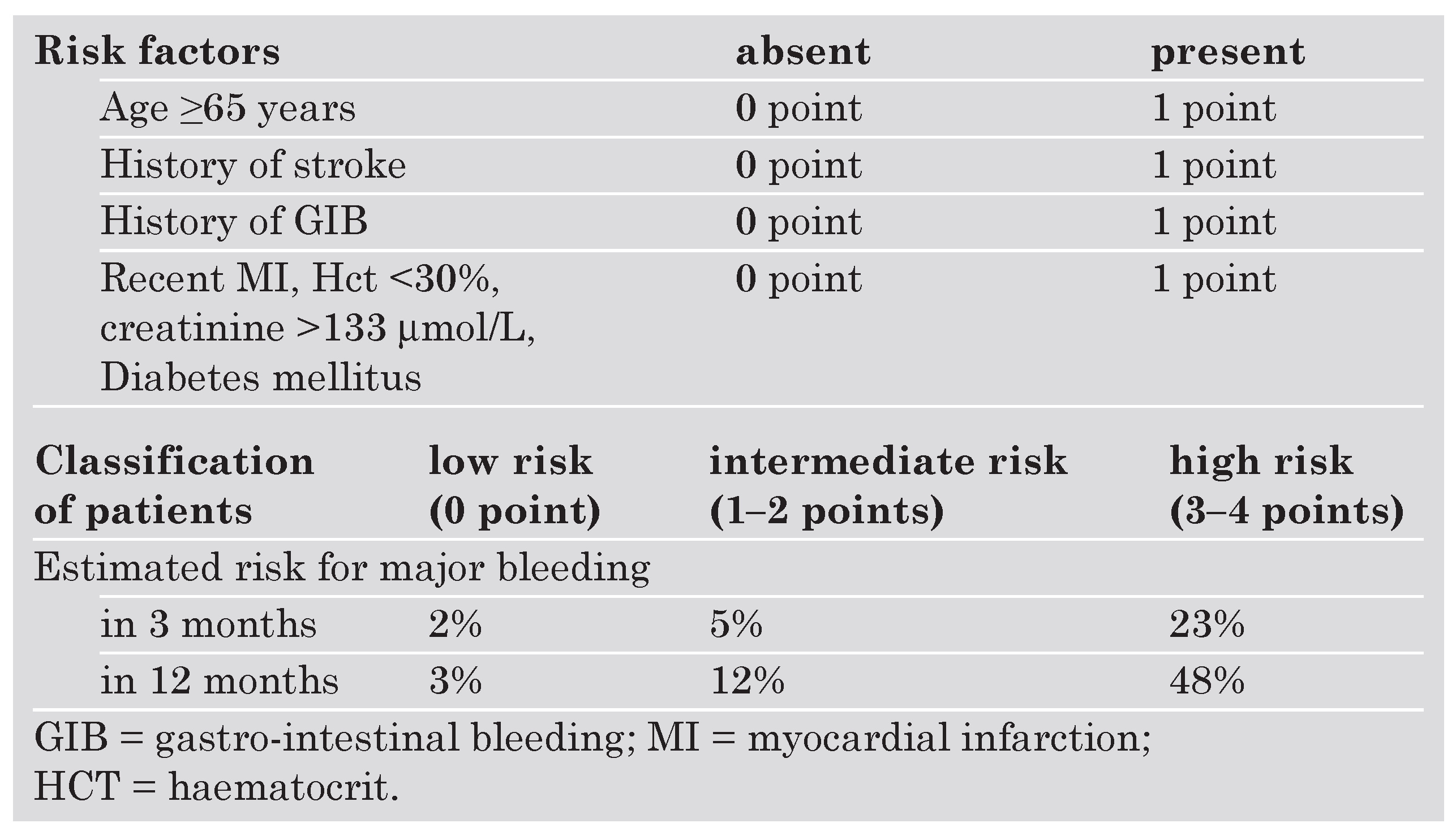
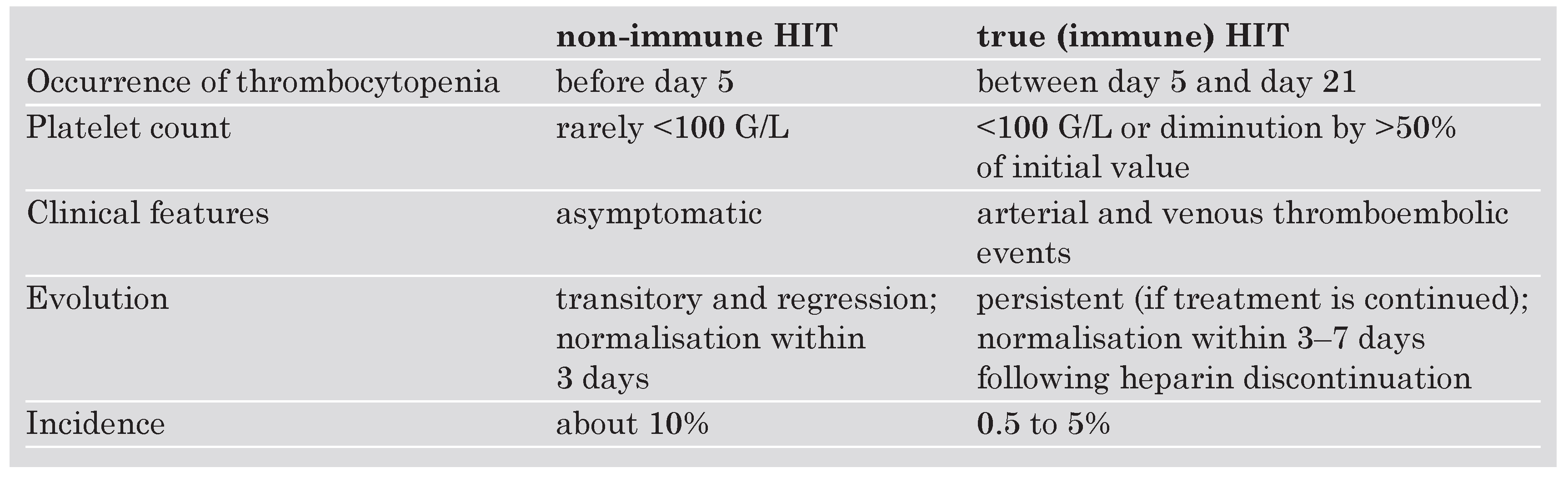
Adverse effects of anticoagulant drugs
Intensity of oral anticoagulant treatment
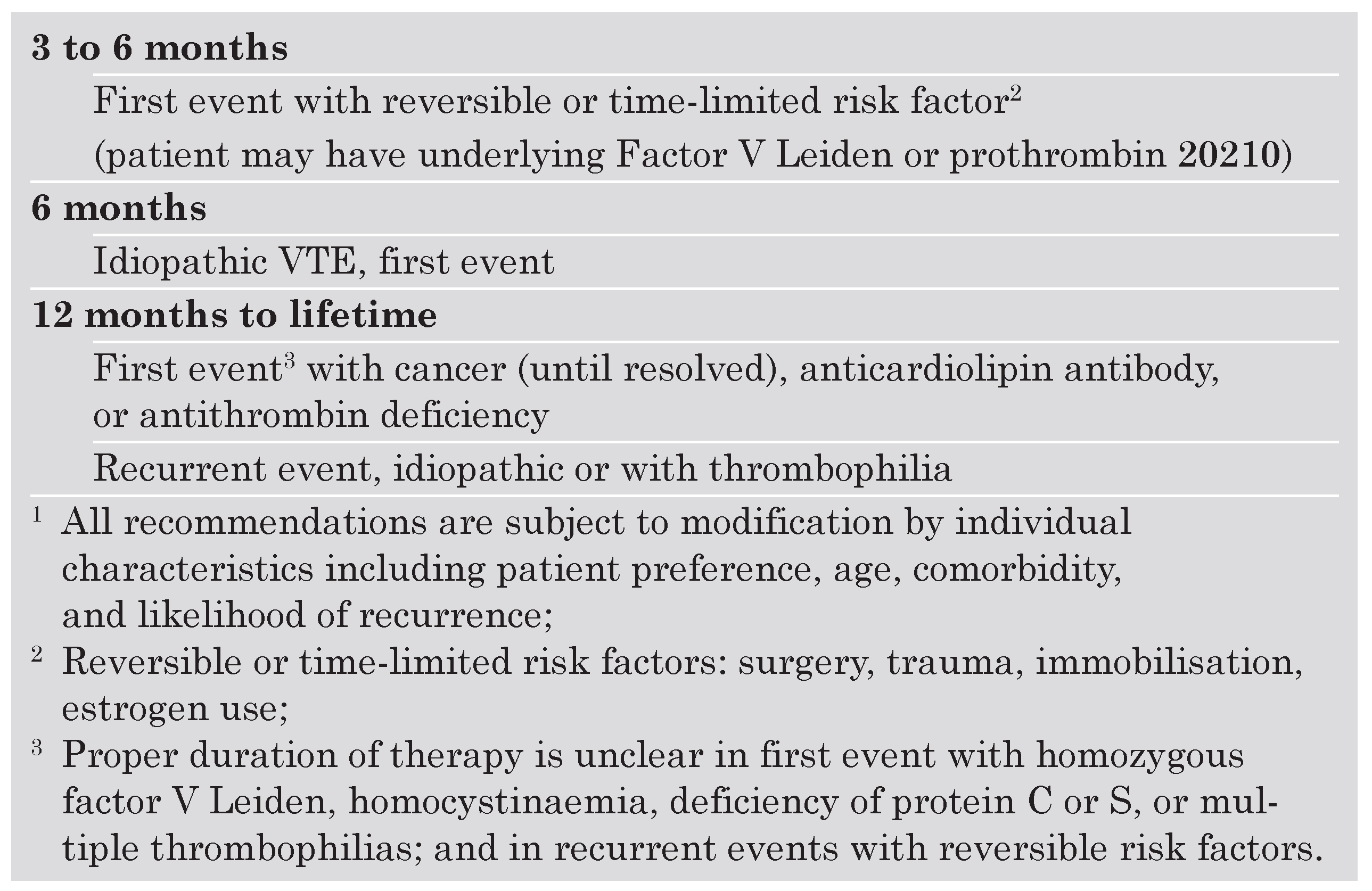
Duration of oral anticoagulation following DVT
- -
- according to a meta-analysis of 25 studies [23], recurrent DVT or PE is rather rare during anticoagulant treatment (8.8%; 95% confidence interval [CI]: 5.0–14.1%) with a fatality rate of only 0.4% (95% CI: 0.2–0.6%);
- -
- recurrences occur preferentially during the initial three weeks after the start of treatment, and concern mainly patients with cancer (odds ratio [OR] 2.7), chronic cardiovascular disease (OR 2.3), chronic respiratory disease (OR 1.9) and other clinically significant medical disease (OR 1.8) [24];
- -
- -
- risk of recurrent VTE is 40% lower in patients treated for 12–24 weeks compared with those treated for 3–6 weeks without significant difference in major bleeding risk [27];
- -
- -
- after a follow-up of two years, the recurrence rate was 18.1% in patients treated for 6 weeks compared to 9.5% in those treated for 6 months [29];
- -
- in a small group of selected high-risk patients with idiopathic DVT [30], the annual recurrence rate was 27.4% in patients given oral anticoagulants for three months compared with 1.3% in patients treated for two years; in the latter group, major haemorrhage occurred in 3.8% compared with 0 in the shorter treatment duration group;
- -
- after two years of follow-up, DVT patients who were treated for one year had a recurrence rate of 15.7% compared to 15.8% in patients treated for three months [31], suggesting that the clinical benefit associated with extending the duration of anticoagulant therapy to one year is not maintained after the therapy is discontinued;
- -
- after a second episode of VTE, one study showed that the cumulative recurrence rate was 20.7% after 4 years in patients treated for 6 months compared with 2.6% in those treated indefinitely; the corresponding rates of major bleeding were 2.7% and 8.6% [32].
Conclusions and perspectives
References
- Brandjes, D.P.; Heijboer, H.; Buller, H.R.; de Rijk, M.; Jagt, H.; ten Cate, J.W. Acenocoumarol and heparin compared with acenocoumarol alone in the initial treatment of proximal-vein thrombosis. N Engl J Med 1992, 327, 1485–1489. [Google Scholar] [CrossRef] [PubMed]
- Barritt, D.W.; Jordan, S.C. Anticoagulant drugs in the treatment of pulmonary embolism. A controlled trial. Lancet 1960, 1, 1309–1312. [Google Scholar] [CrossRef]
- Hommes, D.W.; Bura, A.; Mazzolai, L.; Buller, H.R.; ten Cate, J.W. Subcutaneous heparin compared with continuous intravenous heparin administration in the initial treatment of deep vein thrombosis. A meta-analysis. Ann Intern Med 1992, 116, 279–284. [Google Scholar] [CrossRef] [PubMed]
- Bounameaux, H.; de Moerloose, P. Is laboratory monitoring of low-molecular-weight heparin therapy necessary? No. J Thromb Haemost 2004, 2, 551–554. [Google Scholar] [CrossRef]
- Leizorovicz, A.; Simonneau, G.; Decousus, H.; Boissel, J.P. Comparison of efficacy and safety of low molecular weight heparins and unfractionated heparin in initial treatment of deep venous thrombosis: A meta-analysis. BMJ 1994, 309, 299–304. [Google Scholar] [CrossRef][Green Version]
- Simonneau, G.; Sors, H.; Charbonnier, B.; et al. A comparison of low-molecular-weight heparin with unfractionated heparin for acute pulmonary embolism. The THESEE Study Group. N Engl J Med 1997, 337, 663–669. [Google Scholar] [CrossRef]
- Koopman, M.M.; Prandoni, P.; Piovella, F.; et al. Treatment of venous thrombosis with intravenous unfractionated heparin administered in the hospital as compared with subcutaneous low-molecular-weight heparin administered at home. The Tasman Study Group. N Engl J Med 1996, 334, 682–687, Erratum in: N Engl J Med 1997, 337, 1251. [Google Scholar] [CrossRef]
- Beer, J.H.; Burger, M.; Gretener, S.; Bernard-Bagattini, S.; Bounameaux, H. Outpatient treatment of pulmonary embolism is feasible and safe in a substantial proportion of patients. J Thromb Haemost 2003, 1, 186–187. [Google Scholar] [CrossRef]
- Halkin, H.; Lubetsky, A. Warfarin dose requirement and CYP2C9 polymorphisms. Lancet 1999, 353, 1972–1973. [Google Scholar] [CrossRef] [PubMed]
- Bounameaux, H.; Perneger, T. Fondaparinux: A new synthetic pentasaccharide for thrombosis prevention. Lancet 2002, 359, 1701–1710. [Google Scholar] [CrossRef]
- Büller, H.R.; Davidson, B.L.; Decousus, H.; et al. Fondaparinux or enoxaparin for the initial treatment of symptomatic deep venous thrombosis: A randomized trial. Ann Intern Med 2004, 140, 867–873. [Google Scholar] [CrossRef]
- Büller, H.R.; Davidson, B.L.; Decousus, H.; et al. Subcutaneous fondaparinux versus intravenous unfractionated heparin in the initial treatment of pulmonary embolism. N Engl J Med 2003, 349, 1695–1702, Erratum in: N Engl J Med 2004, 350, 423. [Google Scholar]
- Fiessinger, J.N.; Huisman, M.; Davidson, B.L. Ximelagatran vs low-molecular-weight heparin and warfarin for the treatment of deep vein thrombosis: A randomized trial. JAMA 2005, 293, 681–689. [Google Scholar] [CrossRef] [PubMed]
- Schulman, S.; Wahlander, K.; Lundstrom, T.; et al. Secondary prevention of venous thromboembolism with the oral direct thrombin inhibitor ximelagatran. N Engl J Med 2003, 349, 1713–1721. [Google Scholar] [CrossRef] [PubMed]
- Landefeld, C.S.; Beyth, R.J. Anticoagulant-related bleeding: Clinical epidemiology, prediction, and prevention. Am J Med 1993, 95, 315–328. [Google Scholar] [CrossRef]
- Torn, M.; Bollen, W.L.; van der Meer, F.J.; va der Wall, E.E.; Rosendaal, F.R. Risks of oral anticoagulant therapy with increasing age. Arch Intern Med 2005, 165, 1527–1532. [Google Scholar] [CrossRef]
- Bounameaux, H.; de Moerloose, P.; Sarasin, F.P. Optimal duration of oral anticoagulant therapy following deep vein thrombosis of lower limbs. Blood Coagul Fibrinol 1996, 7, 507–514. [Google Scholar] [CrossRef]
- Warkentin, T.E.; Greinacher, A. Heparin-induced thrombocytopenia: Recognition, treatment, and prevention: The Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy. Chest 2004, 126, 311–337S, Erratum in: Chest 2005, 127, 416. [Google Scholar] [CrossRef]
- Lee, W.M.; Larrey, D.; Olsson, R.; et al. Hepatic findings in long term clinical trials of ximelagatran. Drug Saf 2005, 28, 351–370. [Google Scholar] [CrossRef] [PubMed]
- Ridker, P.M.; Goldhaber, S.Z.; Danielson, E.; et al. Long-term, low-intensity warfarin for the prevention of recurrent venous thromboembolism: A randomized. Double-blind, placebo-controlled trial. N Engl J Med 2003, 348, 1425–1434. [Google Scholar] [CrossRef]
- Ridker, P.M. Long-term low-dose warfarin use is effective in the prevention of recurrent venous thromboembolism: Yes. J Thromb Haemost 2004, 2, 1034–17. [Google Scholar] [CrossRef] [PubMed]
- Kearon, C.; Ginsberg, J.S.; Kovacs, M.J.; et al. Extended Low Intensity Anticoagulation for Thrombo-Embolism Investigators. Comparison of low-intensity warfarin therapy with conventional-intensity warfarin therapy for long-term prevention of recurrent venous thromboembolism. N Engl J Med 2003, 349, 631–639. [Google Scholar] [CrossRef]
- Douketis, J.D.; Kearon, C.; Bates, S.; Duku, E.K.; Ginsberg, J.S. Risk of fatal pulmonary embolism in patients with treated venous thromboembolism. JAMA 1998, 279, 458–462. [Google Scholar] [CrossRef]
- Douketis, J.D.; Foster, G.A.; Crowther, M.A.; Prins, M.H.; Ginsberg, J.S. Clinical risk factors and timing of recurrent venous thromboembolism during the initial 3 months of anticoagulant therapy. Arch Intern Med 2000, 160, 3431–3436. [Google Scholar] [CrossRef]
- Sarasin, F.P.; Bounameaux, H. Duration of oral anticoagulant therapy after proximal deep vein thrombosis: A decision analysis. Thromb Haemost 1994, 71, 286–291. [Google Scholar] [CrossRef]
- Sarasin, F.P.; Bounameaux, H. Prolonged oral anticoagulant therapy in Factor V Leiden carriers with a first episode of deep vein thrombosis? BMJ 1998, 316, 95–99. [Google Scholar] [CrossRef]
- Pinède, L.; Cucherat, M.; Duhaut, P.; Ninet, J.; Pasquier, J.; Boissel, J.P. Comparison of long versus short duration of oral anticoagulant therapy after a first episode of venous thromboembolism: A meta-analysis of randomized controlled trials. J Intern Med 2000, 247, 553–562. [Google Scholar] [CrossRef]
- Research Committee of the British Thoracic Society. Optimum duration of anticoagulation for deep-vein thrombosis and pulmonary embolism. Lancet 1992, 340, 873–876. [Google Scholar] [CrossRef]
- Schulman, S.; Rhedin, A.S.; Lindmarker, P.; et al. A comparison of six weeks with six months of oral anticoagulant therapy after a first episode of venous thromboembolism. N Engl J Med 1995, 332, 661–665. [Google Scholar] [CrossRef] [PubMed]
- Kearon, C.; Gent, M.; Hirsh, J.; et al. Extended anticoagulation prevented recurrence after a first episode of idiopathic venous thromboembolism. N Engl J Med 1999, 340, 901–907. [Google Scholar] [CrossRef]
- Agnelli, G.; Prandoni, P.; Santamaria, M.G.; et al. Three months versus one year of oral anticoagulant therapy for idiopathic deep venous thrombosis. Warfarin Optimal Duration Italian Trial Investigators. N Engl J Med 2001, 345, 165–169. [Google Scholar] [CrossRef] [PubMed]
- Schulman, S.; Granqvist, S.; Holmstrom, M.; et al. The duration of oral anticoagulant therapy after a second episode of venous thromboembolism. N Engl J Med 1997, 336, 393–398. [Google Scholar] [CrossRef]
- Büller, H.R.; Agnelli, G.; Hull, R.D.; Hyers, T.M.; Prins, M.H.; Raskob, G.E. Antithrombotic therapy for venous thromboembolic disease. Chest 2004, 126, 401–428S. [Google Scholar] [CrossRef] [PubMed]
- Palareti, G.; Legnani, C.; Cosmi, B.; Coccheri, S. Predictive Value of D-dimer test for recurrent venous thromboembolism after anticoagulation withdrawal in subjects with a previous idiopathic event and in carriers of congenital thrombophilia. Circulation 2003, 108, 313–318. [Google Scholar] [CrossRef]
- Prandoni, P.; Lensing, W.A.; Prins, M.H.; et al. Residual thrombosis as a predictive factor of recurrent venous thromboembolism. Ann Intern Med 2002, 137, 955–960. [Google Scholar] [CrossRef]
- Beyth, R.J.; Quinn, L.M.; Landefeld, C.S. Prospective evaluation of an index for predicting the risk of major bleeding in outpatients treated with warfarin. Am J Med 1998, 105, 91–99. [Google Scholar] [CrossRef] [PubMed]
 |
 |
 |
 |
© 2006 by the author. Attribution - Non-Commercial - NoDerivatives 4.0.
Share and Cite
Bounameaux, H.; Righini, M. Therapy of Venous Thromboembolism: Anticoagulant Treatment. Cardiovasc. Med. 2006, 9, 117. https://doi.org/10.4414/cvm.2006.01158
Bounameaux H, Righini M. Therapy of Venous Thromboembolism: Anticoagulant Treatment. Cardiovascular Medicine. 2006; 9(3):117. https://doi.org/10.4414/cvm.2006.01158
Chicago/Turabian StyleBounameaux, Henri, and Marc Righini. 2006. "Therapy of Venous Thromboembolism: Anticoagulant Treatment" Cardiovascular Medicine 9, no. 3: 117. https://doi.org/10.4414/cvm.2006.01158
APA StyleBounameaux, H., & Righini, M. (2006). Therapy of Venous Thromboembolism: Anticoagulant Treatment. Cardiovascular Medicine, 9(3), 117. https://doi.org/10.4414/cvm.2006.01158




