Abstract
Thrombosis formation is based on Virchow’s triad of vascular endothelial damage, blood flow stasis, and blood hypercoagulability. In patients with venous thromboembolism (VTE), prothrombotic states play an important pathophysiological role. They are the consequence of acquired or genetic thrombophilia, or of disorders with activated coagulation or inflammation systems. Elevated levels of tissue factor (TF) carrying cell-derived microvesicles contribute to the prothrombotic state. Patients with activated inflammatory or apoptotic systems have an increased risk for VTE. Most recognised VTE risk factors are associated with a prothrombotic state. Risk factor assessment of hospitalised patients aims at identifying patients at high risk for VTE, because thromboprophylactic interventions effectively and safely prevent VTE. Specific clinical settings are associated with an increased risk for VTE, such as prior thrombosis, long distance travel, oral contraceptives, and hormone replacement therapy.
Zusammenfassung
Die venöse Thrombusformation basiert auf dem Virchow-Trias des vaskulären Endothelschadens, der Blutfluss-Stase und der Hyperkoagulabilität. Bei Patienten mit einer venösen Thromboembolie spielt der prothrombotische Zustand eine herausragende pathophysiologische Rolle. Er ist häufig die Konsequenz einer genetischen oder erworbenen Thrombophilie oder von Erkrankungen mit einer aktivierten Gerinnungsoder Entzündungskaskade. Die erhöhte Präsenz «tissue factor» (gewebethromboplastin)-tragender Mikropartikel im zirkulierenden Blut scheint zu diesem prothrombotischen Zustand beizutragen. So haben Patienten mit einem aktivierten Entzündungssystem ein erhöhtes venöses Thromboembolie-Risiko. Auch sind die meisten nachgewiesenen klinischen Risikofaktoren mit einem prothrombotischen Zustand vergesellschaftet. Die Identifizierung individueller Risikofaktoren bei hospitalisierten Patienten ist unerlässlich, da thromboprophylaktische Massnahmen wirksam sind und sicher die venöse Thromboembolie verhindern können. Zudem sind spezifische klinische Szenarien mit einem erhöhten Thromboembolie-Risiko verbunden, wie etwa lange Flugreisen, die Einnahme oraler Kontrazeptiva oder die Hormon-Substitutionstherapie.
Key words: Prävention; venöse Thromboembolie; Risikofaktor-Identifikation
Introduction
Assessment of individual risk factors (RF) for venous thromboembolism (VTE) is not only important for diagnosing VTE [1] but it helps selecting patients who benefit from VTE prophylaxis. For hospitalised patients undergoing orthopedic and general surgery, RF assessment and thromboprophylaxis have been defined and recommended on an evidencebased ground [2]. There is less evidence for acutely ill medical patients, a more heterogeneous target group, and different approaches to VTE prophylaxis have been proposed. In this chapter, the contribution of various risk factors to the process of thrombosis formation are reviewed, and widely adopted RF assessment strategies for surgical and medical patients are summarised. The clinical relevance of congenital and acquired thrombophilia, as well as their screening indications are discussed. Recurrent VTE, prolonged air travel, oral contraceptives, and hormone replacement therapy will also be summarised.
Pathophysiological influence of risk factors on the occurrence of VTE
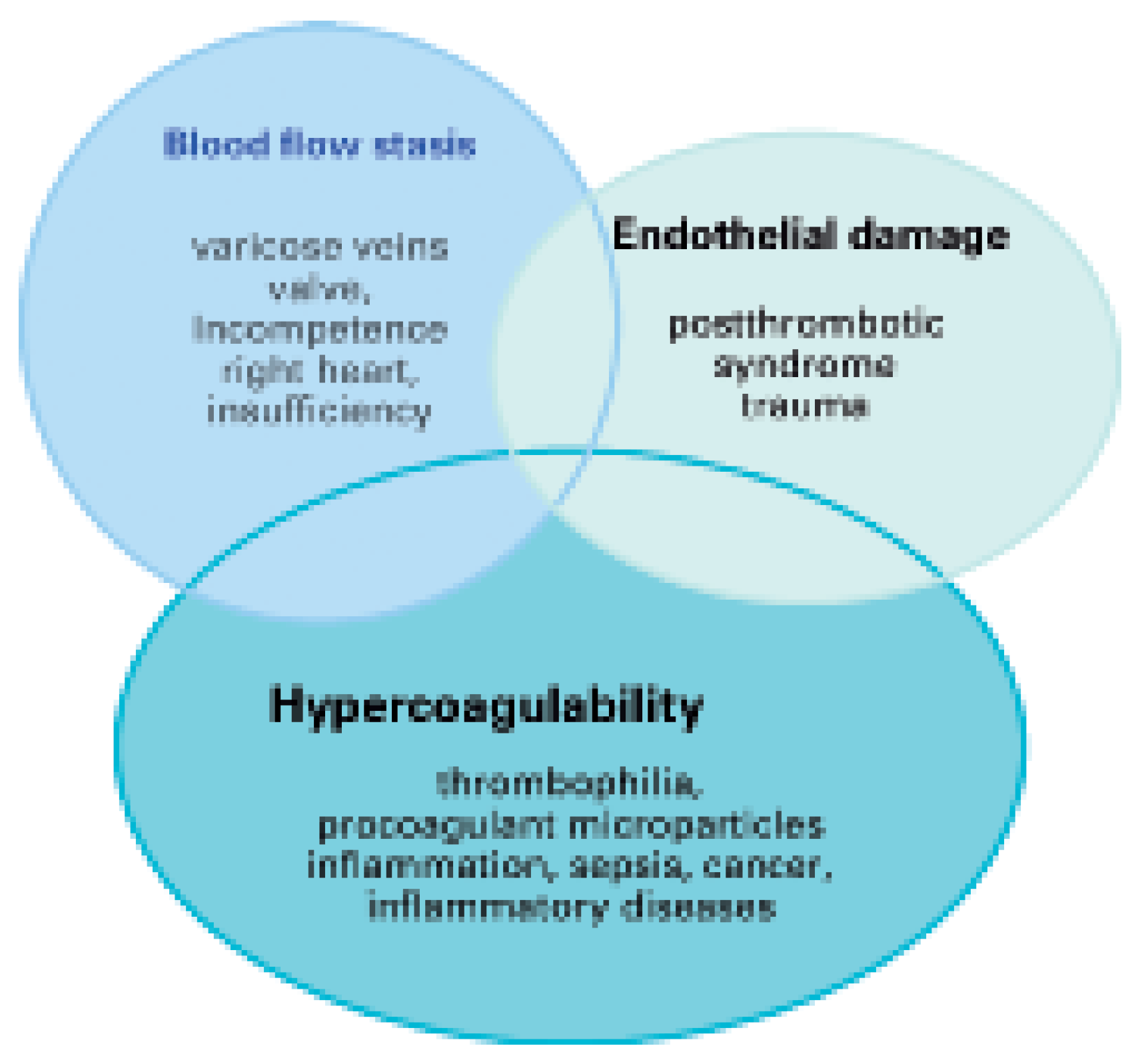
Traditionally, the occurrence of VTE is explained by Virchow’s triad which includes three conditions for the formation of thrombosis (Figure 1) [3]. Blood flow stasis is caused by ectatic venous diameters with valve incompetence as in chronic venous insufficiency or due to systemic right heart insufficiency. Vascular endothelial damage occurs after a previous thrombosis or thrombophlebitis. Blood hypercoagulability is caused by genetic or acquired thrombophilia, chronically activated states of coagulation, inflammation or infection, cell apoptosis, and cancerinduced tissue factor release [4]. In contrast to arterial thrombosis, venous thrombosis rarely occurs at sites of vascular endothelial injury; more frequently, it is triggered by activation of the coagulation system with subsequent fibrin deposition or platelet activation. Venous thrombi mostly consist of fibrin with entrapped erythrocytes and only few thrombocytes [5]. This might be the reason, why platelet inhibitors are less effective in preventing venous thrombosis than anticoagulant drugs.
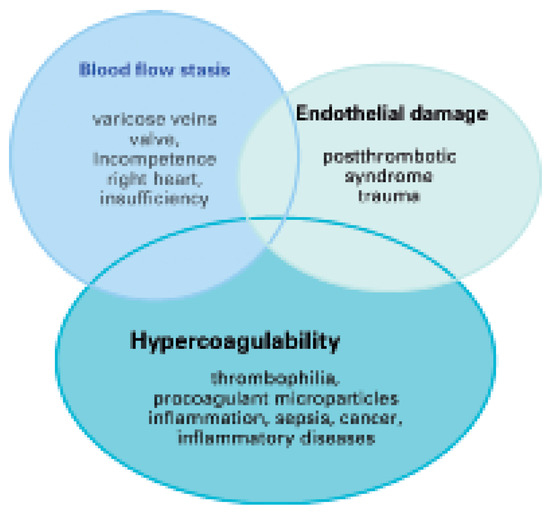
Figure 1.
Virchow’s triad of pathophysiological mechanisms as occuring in specific diseases emphasising hypercoagulability as a frequently involved state.
In recent years, the concept of hypercoagulability has been supported by the identification of two clinically relevant factors: (1.) acquired and genetic deficiencies of various coagulation and lysis glycoproteins [6,7,8,9,10,11], and (2.) by the detection of procoagulant circulating cell-derived microparticles (MP) carrying tissue factor (TF) and other coagulation proteins [12,13,14]. TF as a transmembrane molecule forms a complex with coagulation factor VIIa and activates the extrinsic coagulation system. It is expressed in adventitial cells and inhibits bleeding from the damaged endothelium [15]. It is usually not released into the circulation, but it is expressed by activated endothelial cells following denudation of the vascular endothelial layer [16]. When TF is present in circulating blood, it occurs in two forms: (1.) a soluble shed part [17], and (2.) bound to the surface of MPs derived from thrombocytes, monocytes, macrophages, leukocytes, and endothelial cells. Elevated levels of soluble TF and TF-carrying MPs have been assessed in patients with sepsis, cancer and diseases with an activated inflammatory status [18,19,20,21].
Risk factor assessment for thromboprophylactic treatment of hospitalised patients
According to the Seventh American College of Chest Physician (ACCP) Conference on Antithrombotic and Thrombolytic Therapy [2], the prevalence of VTE among hospitalised patients is high and it is difficult to predict which patient will develop symptomatic thromboembolic complications. Screening of all patients at risk by non-invasive tests is ineffective and expensive. Unprevented VTE, on the other side, may lead to fatal PE and symptomatic DVT with considerable costs and increased risk for recurrent VTE. Thromboprophylactic treatment, effectively and safely reduces the incidence of in-hospital acquired VTE [2].
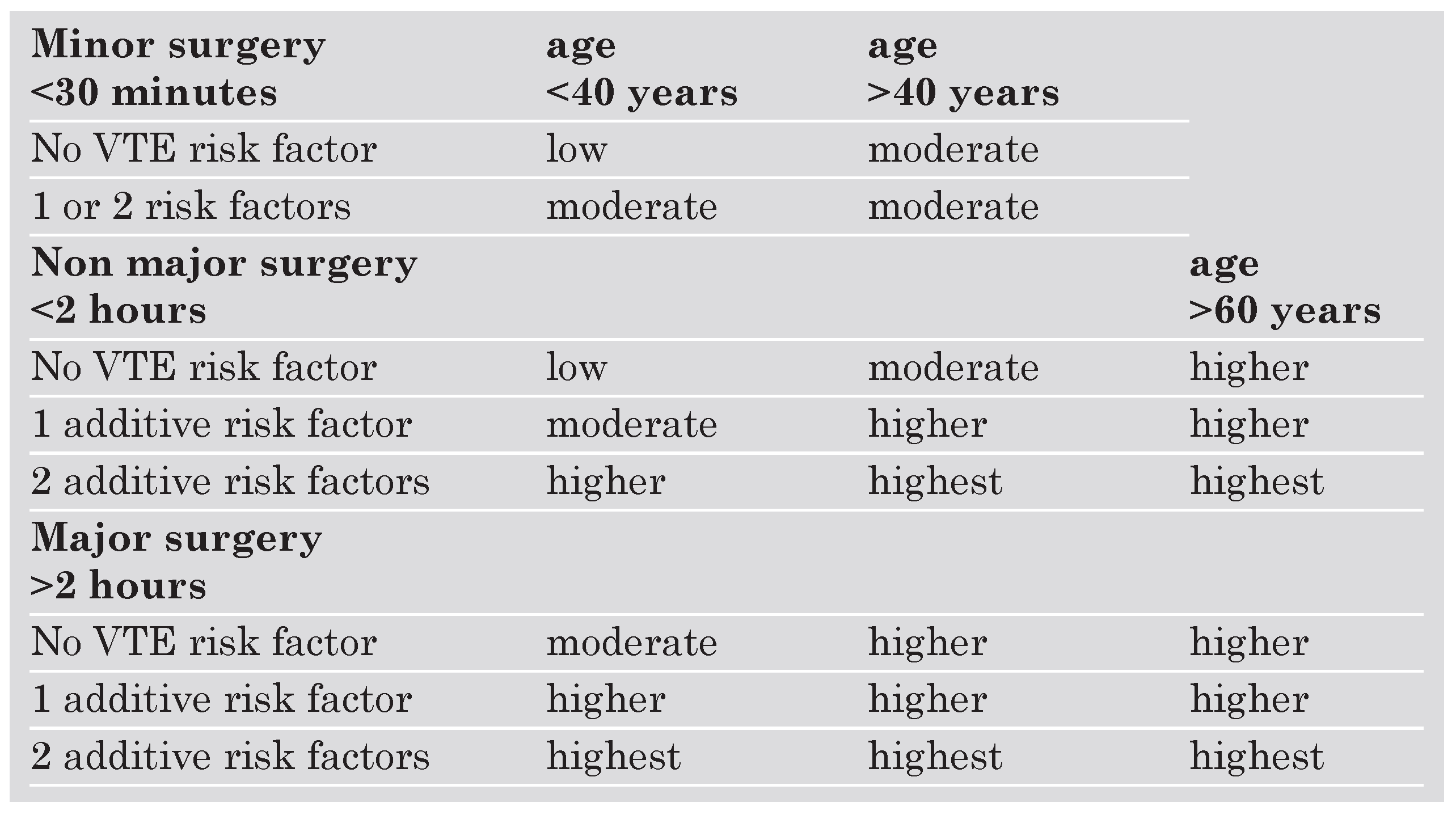
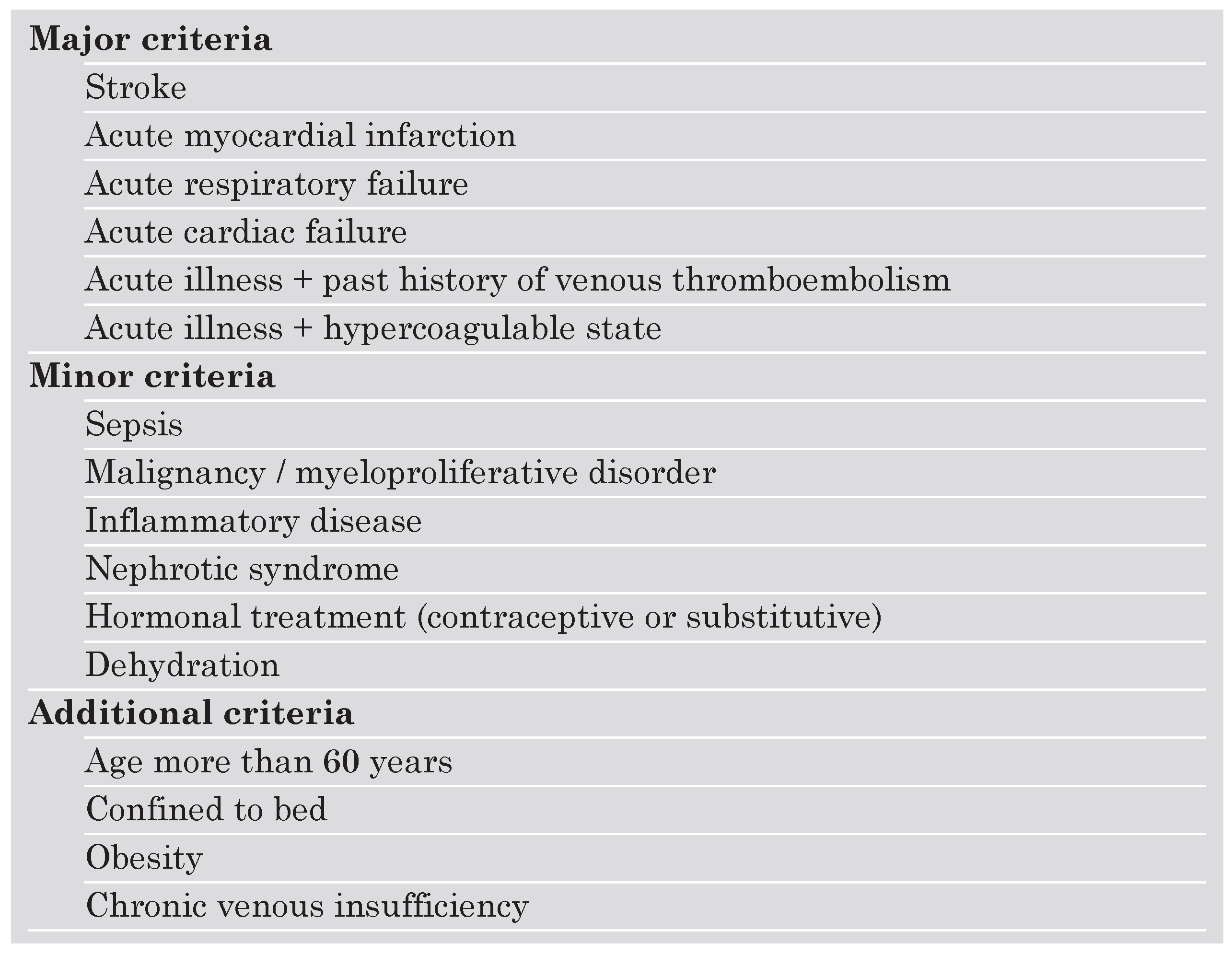
The ACCP 2004 recommendations for thromboprophylaxis of hospitalised patients are implemented on a group-specific routine approach rather than on individual risk assessment. This strategy allows the treating physician to find the recommended thromboprophylactic interventions for a specific target patient group. For surgical patients, one widely accepted way to stratify risk is the classification based on a simplified system that includes four categories of risk according to age (<40 y, >40 y, <60 y, >60 y), duration of the operation (<30 min, <2 h, >2 h), and presence of additional risk factors (Table 1). For hospitalised acutely ill medical patients, it might be more useful to assess an individually tailored RF profile that includes both predisposing and exposing factors. Predisposing factors (PF) are persistent patient characteristics such as age, history of previous VTE, varicose veins, or thrombophilia, whereas exposing factors (EF) are transient, such as trauma, chemotherapy, acute heart failure, or acute respiratory failure. Among patients presenting with VTE, 50% have had at least one predisposing RF. The incidence of inhospital acquired VTE in acutely medical ill patients is 10 to 15% [22]. For medical patients, a number of RF scores have been used as decision making tools to consider prophlaxis [23,24,25]. Problematic is that none of these scores have been validated and that some of the included factors are not clearly defined. Moreover, factor interaction and combination of various factors are unknown regarding their impact on the effective risk of VTE. Another risk assessment score has been used in a recent Swiss survey evaluating the routine use of thromboprophylaxis in acutely ill medical patients of eight different hospital centers in Switzerland [26]. The score used a classification of major, minor and additive risk factors (Table 2). Surprisingly, prophylaxis has been underand overused in about 45% of hospitalised medical high risk patients from Swiss academic and non-academic hospitals. Therefore, there is an urgent need to implement VTE prophylaxis among hospitalised medical patients in Switzerland.

Table 1.
Risk factor classification for surgical patients based on a system that includes four categories of risk (low, moderate, high, highest) according to age, duration of the operation, and presence of additional risk factors. VTE = venous thromboembolism.

Table 2.
Risk factor classification for acutely ill medical patients as applied in a Swiss survey [26]. Indication for thromboprophylaxis was given in the presence of at least one major, or two minor, or one minor plus one additional criterion.
Hospitals with adequate information system resources may apply an electronic alert system, enabling the recognition of high risk patients without prophylaxis orders. Kucher et al. demonstrated that the application of a software program, that is linked to the patient database and identifies RF of consecutive hospitalised patients by alerting the responsible physician, results in a 40% reduction of in-hospital acquired VTE [27]. Disadvantages of individual RF assessment scoring such as logistic complexity and suboptimal compliance are eliminated by the implementation of an electronic alert system and may provide increased safety in identifying individual patients who do require thromboprophylaxis.
Thrombophilia and concomitant risk factors
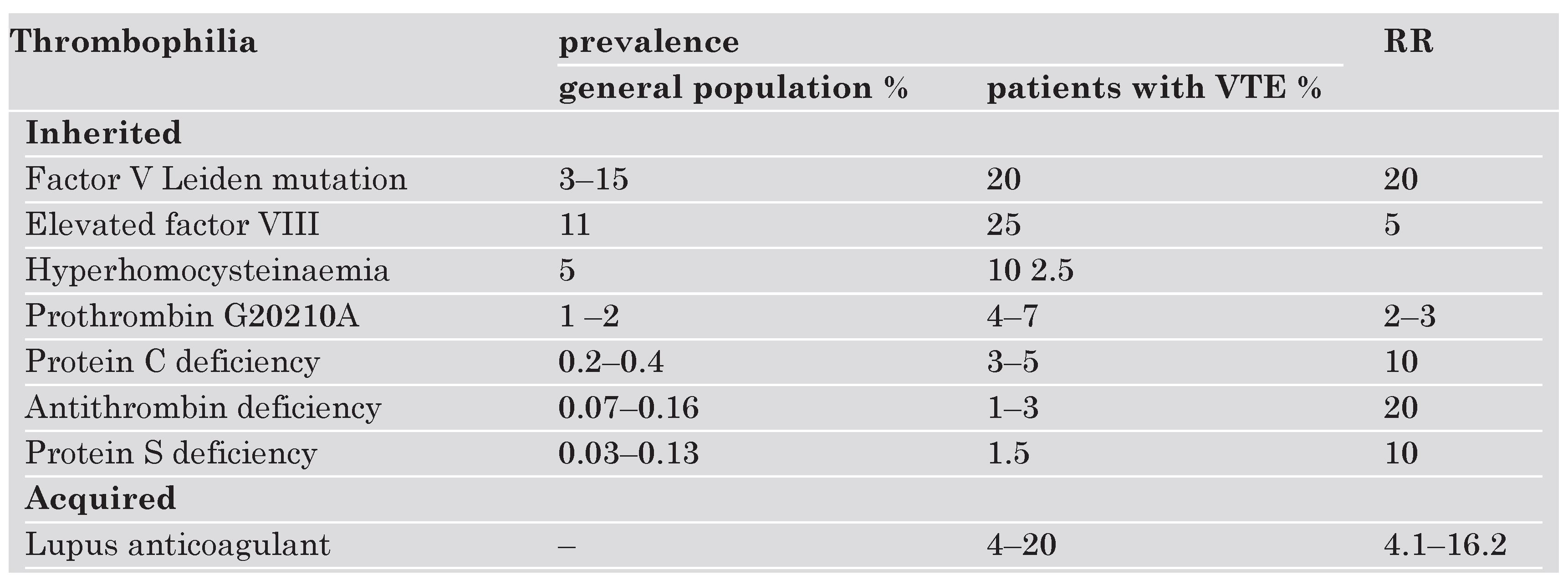
Thrombophilia is one major risk factor causing hypercoagulable states. Thrombophilic factors are either genetic or acquired. The most frequent hereditary defects are factor VIII elevation, activated protein C (APC) resistance due to the factor V Leiden mutation, hyperhomocysteinaemia, and prothrombin G 20210A mutation. Prevalence among the normal population and in patients with VTE and relative risks are presented in Table 3 [28]. A number of other genetic factors have been associated with an increased risk for VTE or with recurrence of VTE such as low levels of tissue factor pathway inhibitor (TFPI) [29], a polymorphism of the promotor of plasminogen activator inhibitor-1 gene (PAI-1 4G/5G) [30], high levels of thrombin-activatable fibrinolysis inhibitor (TAFI) [31], factor VII-activating protease (FSAP) variant Marburg [32] and dysfibrinogenaemia. These factors, however, are of lesser clinical relevance due to either their low prevalence within the population or to weak evidence. Hyperhomocysteinaemia and elevated levels of factors I, VIII, XI, and APC-resistance may be hereditary or acquired. Anti-phospholipid antibodies are acquired and often occur with autoimmune disease, for example lupus erythematosus [33]. The risk of VTE with underlying thrombophilia is substantially higher when additional risk such as cancer, chemotherapy, surgery, prior VTE are present [34].

Table 3.
Prevalences of thrombophilia among the normal population as well as in patients with VTE and relative risks (RR): Factor V Leiden mutation is both frequent and associated with high relative risk (28).
In recent years, many thrombophilic factors have been identified as independent risk factors for VTE, calling for a consensus on who should be screened. Recommendations for prophylactic treatment of patients at risk issued by an International Faculty of thrombosis-related specialists [28] include all first idiopathic episodes of VTE, patients with a first episode of VTE aged below 50 years, patients with recurrent VTE, patients with VTE at unusual sites (upper extremity, mesenteric, hepatic veins) and a number of additional clinical settings as shown in Table 4.

Table 4.
Conditions requiring thrombophilia screening for the risk of VTE as recommended in international guidelines [28].
Recurrent VTE is considered as a powerful risk factor, and more than 25% of the patients presenting with acute VTE have had a previous event [4]. The risk of recurrent VTE depends on whether the first episode was provoked by a major reversible RF, such as surgery, metastatic cancer, and chemotherapy or if it was an idiopathic VTE [34].
Oral contraceptives (OC) and hormone replacement therapy (HRT) are important risk factors for VTE. The combination of OC or HRT with thrombophilia is associated with a higher risk for VTE [35]. Transdermal HRT seems to have less effect on coagulation than oral administration, because oral preparations undergo fist-pass hepatic metabolism and have a greater effect on factors produced by the liver than transdermal preparations. In comparison to menstruating women, the incidence of VTE in postmenopausal women is doubled, and the risk further increases with age [35].
Among patients presenting with an acute episode of VTE after a long distance travel, 72% have thrombophilia. Dehydration, immobility by sitting in small spaced seats, and hypobaric hypoxia combined with specific individual RF (obesity, thrombophilia, age, varicose veins, previous episode of VTE), might favour thrombosis formation. Travelling duration of more than 8 hours has been reported to be associated with asymptomatic calf vein thrombosis but only a small risk for the development of symptomatic DVT or fatal PE [36]. According to the ACCP 2004 recommendations, passengers on a flight of >6 hours duration with one predisposing RF are advised to avoid dehydration and constrictive clothing around the lower extremities or the waist, and to practise calf muscle stretching, but routine use of stockings or antithrombotic medication is not advised. For patients with several predisposing factors, stockings with 15–30 mm Hg ankle pressure or prophylactic LMWH injected prior to departure are recommended.
Future prevention efforts must focus on (1.) continuing medical education, (2.) implementation of VTE prevention guidelines into clinical practice, (3.) increase of VTE awareness among health care providers, (4.) increase in the rate of adaquate mechanical and pharmacological prophylaxis.
References
- Wells, P.S.; Hirsh, J.; Anderson, D.R.; Lensing, A.W.; Foster, G.; Kearon, C.; et al. Accuracy of clinical assessment of deep-vein thrombosis. Lancet 1995, 345, 1326–1330. [Google Scholar] [CrossRef]
- Geerts, W.H.; Pineo, G.F.; Heit, J.A.; Bergqvist, D.; Lassen, M.R.; Colwell, C.W.; et al. Prevention of venous thromboembolism: The Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy. Chest 2004, 126, 338S–400S. [Google Scholar] [CrossRef]
- Virchow, R.L.K. (translated by: Matzdorff, et al). Thrombose und Embolie. Science History Publications: Canton, MA, 1998.
- Kearon, C. Epidemiology of venous thromboembolism. Semin Vasc Med 2001, 1, 7–25. [Google Scholar] [CrossRef] [PubMed]
- Sevitt, S. The structure and growth of valve-pocket thrombi in femoral veins. J Clin Pathol 1974, 27, 517–528. [Google Scholar] [CrossRef]
- Baglin, T.; Luddington, R.; Brown, K.; Baglin, C. Incidence of recurrent venous thromboembolism in relation to clinical and thrombophilic risk factors: Prospective cohort study. Lancet 2003, 362, 523–526. [Google Scholar] [CrossRef] [PubMed]
- Caprini, J.A.; Goldshteyn, S.; Glase, C.J.; Hathaway, K. Thrombophilia testing in patients with venous thrombosis. Eur J Vasc Endovasc Surg 2005, 30, 550–555. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mansilha, A.; Araujo, F.; Severo, M.; Sampaio, S.M.; Toledo, T.; Albuquerque, R. Genetic polymorphisms and risk of recurrent deep venous thrombosis in young people: Prospective cohort study. Eur J Vasc Endovasc Surg 2005, 30, 545–549. [Google Scholar] [CrossRef]
- Emmerich, J.; Vossen, C.Y.; Callas, P.W.; Demers, C.; Naud, S.; Long, G.L.; et al. Chronic venous abnormalities in symptomatic and asymptomatic protein C deficiency. J Thromb Haemost. 2005, 3, 1428–1431. [Google Scholar] [CrossRef][Green Version]
- Dahm, A.; Van Hylckama Vlieg, A.; Bendz, B.; Rosendaal, F.; Bertina, R.M.; Sandset, P.M. Low levels of tissue factor pathway inhibitor (TFPI) increase the risk of venous thrombosis. Blood 2003, 101, 4387–4392. [Google Scholar] [CrossRef]
- Lisman, T.; de Groot, P.G.; Meijers, J.C.; Rosendaal, F.R. Reduced plasma fibrinolytic potential is a risk factor for venous thrombosis. Blood 2005, 105, 1102–1105. [Google Scholar] [CrossRef]
- Chirinos, J.A.; Heresi, G.A.; Velasquez, H.; Jy, W.; Jimenez, J.J.; Ahn, E.; et al. Elevation of endothelial microparticles, platelets, and leukocyte activation in patients with venous thromboembolism. J Am Coll Cardiol. 2005, 45, 1467–1471. [Google Scholar] [CrossRef] [PubMed]
- Myers, D.D.; Hawley, A.E.; Farris, D.M.; Wrobleski, S.K.; Thanaporn, P.; Schaub, R.G.; et al. P-selectin and leukocyte microparticles are associated with venous thrombogenesis. J Vasc Surg 2003, 38, 1075–1089. [Google Scholar] [CrossRef]
- Giesen, P.L.; Rauch, U.; Bohrmann, B.; et al. Blood-borne tissue factor: Another view of thrombosis. Proc. Natl. Acad. Sci. USA 1999, 96, 2311–2315. [Google Scholar] [CrossRef] [PubMed]
- Mackman, N. Arterioscler Thromb Vasc Biol 2004, 24, 1015–1022. [CrossRef]
- Combes, V.; Simon, A.C.; Grau, G.E.; Arnoux, D.; Camoin, L.; Sabatier, F.; et al. In vitro generation of endothelial microparticles and possible prothrombotic activity in patients with lupus anticoagulant. J Clin Invest 1999, 104, 93–102. [Google Scholar] [CrossRef] [PubMed]
- Bogdanov, V.Y.; Balasubramanian, V.; Hathcock, J.; et al. Alternatively spliced human tissue factor: A circulating, soluble, thrombogenic protein. Nat Med 2003, 9, 458–462. [Google Scholar] [CrossRef]
- Nieuwland, R.; Berckmans, R.J.; McGregor, S.; Boing, A.N.; Romijn, F.P.; Westendorp, R.G.; et al. Cellular origin and procoagulant properties of microparticles in meningococcal sepsis. Blood 2000, 95, 930–935. [Google Scholar] [CrossRef]
- Rao, L.V.M. Tissue factor as a tumor procoagulant. Cancer Metastasis Rev 1992, 11, 249–266. [Google Scholar]
- Diamant, M.; Nieuwland, R.; Pablo, R.F.; Sturk, A.; Smit, J.W.; Radder, J.K. Elevated numbers of tissue-factor exposing microparticles correlate with components of the metabolic syndrome in uncomplicated type 2 diabetes mellitus. Circulation 2002, 106, 2442–2447. [Google Scholar] [CrossRef]
- Mallat, Z.; Benamer, H.; Hugel, B.; Benessiano, J.; Steg, P.G.; Freyssinet, J.M. , et al A. Elevated levels of shed membrane microparticles with procoagulant potential in the peripheral circulating blood of patients with acute coronary syndromes. Circulation 2000, 101, 841–843. [Google Scholar] [CrossRef]
- Rosendaal, F.R. Risk factors for venous thrombosis: Prevalence, risk, and interaction. Semin Hematol 1997, 34, 171–187. [Google Scholar] [CrossRef]
- Samama, M.M.; Cohen, A.T.; Darmon, J.Y.; Desjardins, L.; Eldor, A.; Janbon, C.; et al. A comparison of enoxaparin with placebo for the prevention of venous thromboembolism in acutely ill medical patients. Prophylaxis in Medical Patients with Enoxaparin Study Group. N Engl J Med 1999, 341, 793–800. [Google Scholar] [CrossRef]
- Cohen, A.T.; Gallus, A.S.; Lassen, M.R.; et al. Fondaparinux vs placebo for the prevention of venous thromboembolism in acutely ill medical patients (ARTEMIS). J Thromb Haemost 2003. (suppl 1):abstract 2046. [Google Scholar]
- Leizorovicz, A.; Cohen, A.T.; Turpie, A.G.; Olsson, C.G.; Vaitkus, P.T.; Goldhaber, S.Z. PREVENT Medical Thromboprophylaxis Study Group. Randomized, placebo-controlled trial of dalteparin for the prevention of venous thromboembolism in acutely ill medical patients. Circulation 2004, 110, 874–879. [Google Scholar] [CrossRef]
- Chopard, P.; Doerffler-Melly, J.; Hess, U.; Wuillemin, W.A.; Hayoz, D.; Gallino, A.; et al. Venous thromboembolism prophylaxis in acutely ill medical patients: Definite need for improvement. J Intern Med 2005, 257, 352–357. [Google Scholar] [CrossRef] [PubMed]
- Kucher, N.; Koo, S.; Quiroz, R.; Cooper, J.M.; Paterno, M.D.; Soukonnikov, B.; et al. Electronic alerts to prevent venous thromboembolism among hospitalized patients. N Engl J Med 2005, 352, 969–977. [Google Scholar] [CrossRef] [PubMed]
- Nicolaides, A.N. Thrombophilia and venous thromboembolism. International Consensus Statement. Guidelines According to Scientific Evidence. Int Angiol. 2005, 24, 1–26. [Google Scholar] [CrossRef] [PubMed]
- Dahm, A.; van Hylckama Vlieg, A.; Bendz, B.; Rosendaal, F.; Bertina, R.M.; Sandset, P.M. Low levels of tissue factor pathway inhibitor (TFPI) increase the risk of venous thrombosis. Blood 2003, 101, 4387–4392. [Google Scholar] [CrossRef]
- Mansilha, A.; Arau’jo, F.; Severo, M.; Sampaio, S.; Toledo, T.; Henriques, I.; et al. The association between the 4G/5G polymorphism in the promoter of the plasminogen activator inhibitor-1 gene and deep venous thrombosis in young people. Phlebology 2005, 20, 48–52. [Google Scholar] [CrossRef]
- Eichinger, S.; Schonauer, V.; Weltermann, A.; Minar, E.; Bialonczyk, C.; Hirschl, M.; et al. Thrombin-activatable fibrinolysis inhibitor and the risk for recurrent venous thromboembolism. Blood. 2004, 103, 3773–3776. [Google Scholar] [CrossRef]
- Hoppe, B.; Tolou, F.; Radtke, H.; Kiesewetter, H.; Dorner, T.; Salama, A. Marburg I polymorphism of factor VII-activating protease is associated with idiopathic venous thromboembolism. Blood 2005, 105, 1549–1551. [Google Scholar] [CrossRef]
- De Groot, P.G.; Lutters, B.; Derksen, R.H.; Lisman, T.; Meijers, J.C.; Rosendaal, F.R. Lupus anticoagulants and the risk of a first episode of deep venous thrombosis. J Thromb Haemost 2005, 3, 1993–1997. [Google Scholar] [CrossRef] [PubMed]
- Christiansen, S.C.; Cannegieter, S.C.; Koster, T.; Vandenbroucke, J.P.; Rosendaal, F.R. Thrombophilia, clinical factors and recurrent venous thrombotic events. JAMA 2005, 293, 2352–2361. [Google Scholar] [CrossRef] [PubMed]
- Wu, O.; Robertson, L.; Langhorne, P.; Twaddle, S.; Lowe, G.D.; Clark, P.; et al. Oral contraceptives, hormone replacement therapy, thrombophilia and risk of venous thromboembolism: TREATS Study. Thromb Haemost 2005, 94, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Chee, Y.L.; Watson, H.G. Air travel and thrombosis. Br J Haematol 2005, 130, 671–680. [Google Scholar] [CrossRef]
© 2006 by the author. Attribution - Non-Commercial - NoDerivatives 4.0.