Introduction
Prevention of venous thromboembolism (VTE) is receiving increasing attention as a patient safety issue. Many medical centers are appointing physicians as Patient Safety Officers whose task is to minimise preventable adverse events. In the past, VTE prevention among hospitalised patients was a task relegated to generalists, without much oversight by specialists in cardiovascular medicine, angiology, hypertension, cardiac surgery, vascular surgery, or pediatric cardiology. However, the situation is changing rapidly. Organisations that certify and accredit hospitals will soon mandate enforcement of prophylaxis protocols. In the United States, the National Quality Forum has embarked upon a 3-year-study which will culminate in VTE prophylaxis recommendations that the Joint Commission on Hospital Accreditation will declare as obligatory for hospital licensing and recertification. As specialists, we stand at a fork in the road. We can ignore the changes the surround us, or we can embrace these changes and use our expertise to ensure that prophylaxis protocols against VTE are evidence based and suitable for broad application in our hospitals. We also have a special obligation to lead by example.
Rarely will we be consulted on how to optimise prophylaxis in a hospitalised patient. However, our colleagues in general medicine and general surgery will pay careful attention to the orders we write for our own patients. Do we order thromboprophylaxis when our patients are hospitalised for congestive heart failure or pneumonia? And when we consult on patients with cardiovascular disorders, do we simply address the narrow and specific question that we were asked, or do we provide an additional suggestion for VTE prophylaxis when we discover hospitalised patients who are not being protected? We have a unique opportunity to promote VTE prophylaxis among the individual patients whom we hospitalise, among the broader population of patients on whom we consult, and among patients whom we will never meet but who will receive prevention based upon the guideline committees on which we serve.
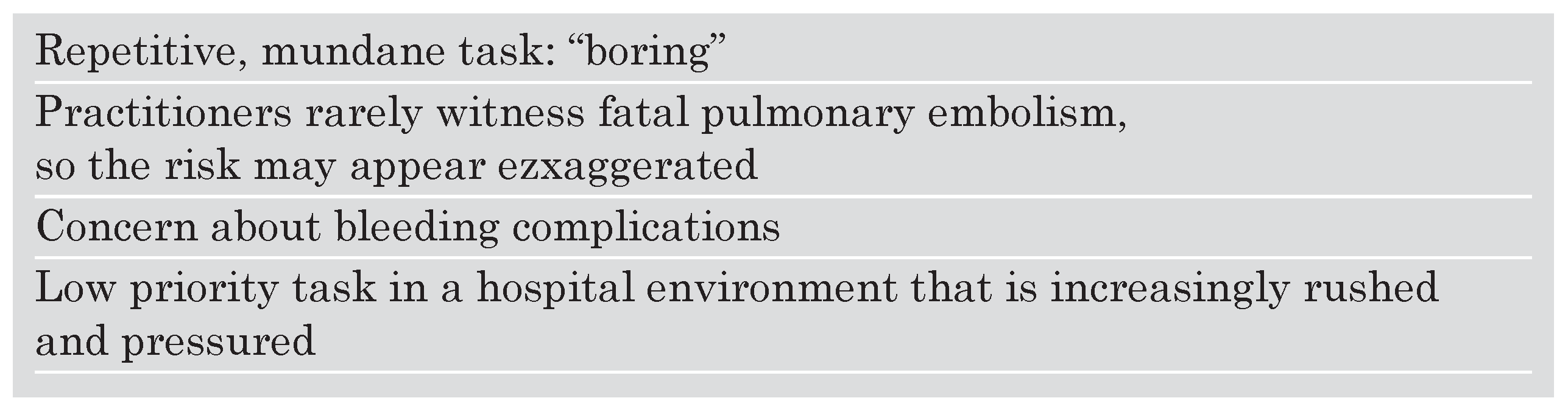
I continue to be amased by the lack of implementation of VTE prophylaxis among some segments of our physician community. Surgeons have endorsed prophylaxis and have successfully woven protocols into everyday routine. Surgeons use peer pressure and train their House Officers to implement VTE prophylaxis. However, internal medicine physicians and medical subspecialists perform poorly when their patient charts are audited to determine whether VTE prophylaxis is routinely ordered. This is especially surprising because internists and subspecialists take pride in staying up to date with evidence based medicine. I have tried to figure out why barriers to prophylaxis exist (
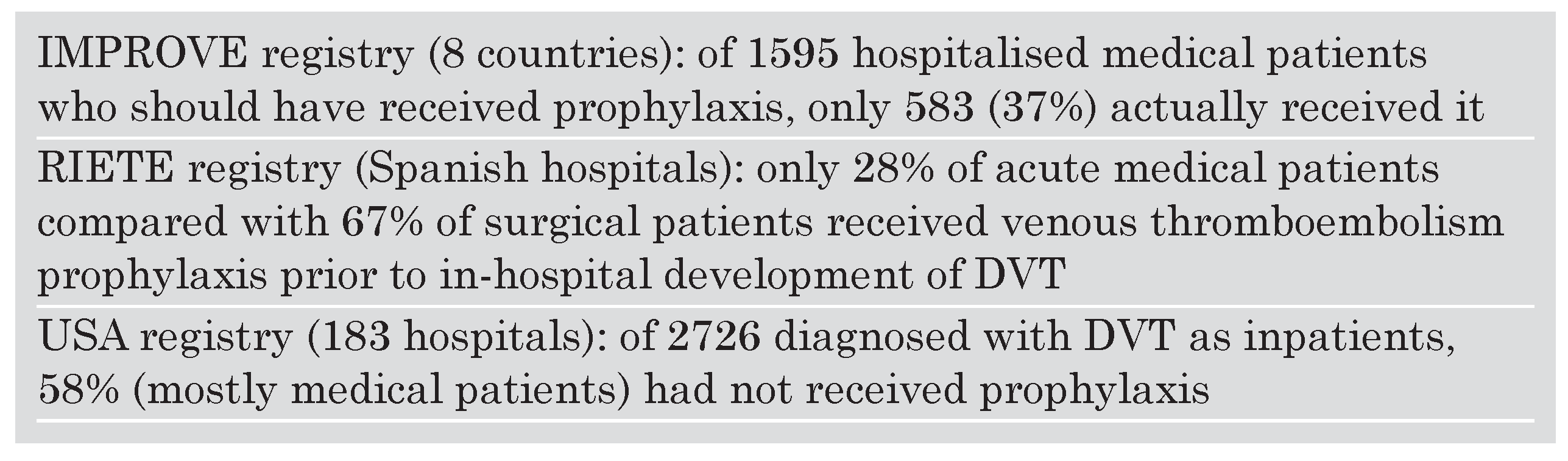
Table 1). While the barriers I have listed constitute my personal speculation and apply to my practice environment and culture, underutilisation of VTE prophylaxis remains an international problem and not an issue confined to the United States (
Table 2).
In a prospective United States registry of 5451 patients at 183 hospitals with deep venous thrombosis (DVT) confirmed by venous ultrasound examination, 71% had not received VTE prophylaxis prior to developing DVT. Of 2726 diagnosed with DVT as inpatients, 58% (mostly medical patients) had not received prophylaxis [
1].
Effective pharmacological and mechanical measures have been proven to decrease asymptomatic DVT rates among hospitalised patients [
2]. Yet some have argued that prior studies in hospitalised medical patients have never shown that prophylaxis results in a significant decrease in total mortality, cardiovascular death, or symptomatic DVT. VTE prophylaxis trials have shared the limited objective of reducing rates of asymptomatic DVT. Until recently, skeptics have questioned whether a decrease in asymptomatic DVT translates into a decrease in mortality.
A post-hoc analysis of patients enrolled in the Prospective Evaluation of Dalteparin Efficacy for Prevention of VTE in Immobilised Patients Trial (PREVENT) examined mortality rates in patients who developed asymptomatic DVT compared with those who did not develop DVT [
3]. Overall, 3706 patients were enrolled at 219 hospitals in 26 countries. These were hospitalised medical patients at risk for DVT and they were randomised to dalteparin 5000 units once daily versus placebo. Of asymptomatic patients alive on day 21, 1738 had undergone technically adequate ultrasound examinations of both proximal and distal leg veins. These 1738 patients constituted the study group for analysis of asymptomatic DVT. In this population, 1540 had no DVT, 118 had calf DVT, and 80 had proximal DVT.
By day 90 of PREVENT, the death rate was 1.9% in the no DVT group, 3.4% in the calf DVT group, and 13.8% in the proximal DVT group. The hazard ratio for death in the asymptomatic proximal DVT group was 7.3 (95% CI = 3.8 to 15.3; p <0.0001) compared with the no DVT group. The association of asymptomatic proximal DVT with increased mortality remained highly significant after adjusting for differences in baseline demographics and clinical variables. The increased mortality rate in asymptomatic calf DVT compared with no DVT trended toward but did not achieve statistical significance. These findings underscore the clinical relevance of asymptomatic DVT, especially proximal DVT, and support the common practice of targeting asymptomatic DVT as an appropriate endpoint in clinical trials of thromboprophylaxis.
There also appears to be a gender bias against women, who do not receive VTE prophylaxis as frequently as men [
4]. This finding is based upon observations from the prospective United States registry of 5451 patients with ultrasound confirmed DVT. Of these patients, 2619 developed DVT while being hospitalised for reasons other than DVT or were diagnosed with acute DVT as outpatients within 30 days of hospital discharge. Men were 21% more likely than women to receive prophylaxis within 30 days prior to acute DVT (61% vs 56%, OR 1.21; p = 0.017). The observed gender difference was present in all age groups. The gender difference was present in both academic and community hospitals and throughout all regions of the United States. The gender difference also persisted after multivariable analysis that adjusted for cancer, surgery, prior DVT, trauma, and age. Men and women did receive mechanical prophylaxis with graduated compression stockings or intermittent pneumatic compression with similar frequency. However, women received pharmacological prophylaxis less often than men. These findings indicate that hospitalised women should be not be overlooked when considering pharmacological VTE prevention.
One barrier to consistent, universal prophylaxis among hospitalised patients may be the needlessly cumbersome and outdated classic approach to risk stratification. This strategy, which is not practical in contemporary clinical practice, categorises patients into 1 of 4 risk groups and then prescribes differing prophylaxis regimens (or no prophylaxis) according to the degree of risk. This approach worked well in a previous era when low risk patients were commonly hospitalised for prolonged lengths of stay. Nowadays, this time-consuming and often ambiguous scheme seems outdated. In modern practice, essentially all patients hospitalised for more than one night are at high risk of developing VTE [
5].
As specialists in vascular medicine and surgery, we should advocate streamlining and simplifying our approach to VTE prophylaxis. Virtually every patient with an anticipated hospital stay of 48 hours or more warrants prophylaxis against VTE. Patients should be immunised against VTE, with the same philosophy used to promote vaccination, hand washing, and disposing of sharp needles. The subtleties of distinguishing low risk from medium risk from high risk are distracting and impede focusing on the main task: effective and safe prophylaxis for all hospitalised patients with protocols that are routine, rapidly implemented, and practical [
5].
Pharmacological prophylaxis should form the foundation for any prophylaxis program among hospitalised patients. For those with bleeding problems or whose risks of bleeding make this approach risky, mechanical prophylaxis should be utilised with graduated compression stockings, intermittent pneumatic compression devices, or both. For patients at very high risk of VTE, combined pharmacological and mechanical prophylaxis should be ordered.
Low dose unfractionated heparin, administered in a fixed dosing regimen of 5000 units injected subcutaneously every 8 hours, can halve VTE rates. For surgical patients, the first dose is administered 2 hours before the skin incision. Prophylaxis should be continued for at least a week because the peak incidence of postoperative VTE is 5–10 days following surgery. In medical patients, heparin 5000 units 3 times daily appears equivalent in efficacy and safety to low molecular weight heparin administered once daily.
Low molecular weight heparins and fondaparinux, an anti-Xa agent, provide the convenience of once daily dosing. They are administered as fixed low doses. These drugs, in contrast to unfractionated heparin, also reduce the possible catastrophic side effect of heparin induced thrombocytopenia, which is rare with low molecular weight heparin and which has never been confirmed with fondaparinux. Because of rigorously conducted clinical trials, these agents are being used for VTE prevention in both surgical [
6] and medical [
7] patients.
The PREVENT Trial is the largest VTE prophylaxis study ever undertaken among hospitalised patients with medical illnesses [
8]. Patients were randomised to receive either subcutaneous dalteparin 5000 units daily or placebo for 14 days. They underwent venous ultrasonography at day 21. The incidence of VTE was reduced from 5.0% in the placebo group to 2.8% in the dalteparin group, a relative risk reduction of 45% (p = 0.0015). The observed benefit was maintained at 90 days. The major bleeding rate was 0.5% in the dalteparin group compared with 0.2% in the placebo group. Thus, dalteparin 5000 units once daily halved the VTE rate with a low risk of bleeding.
There has been some hesitation to order fixed dosing of low molecular weight heparin among obese or elderly patients. Therefore, Kucher and colleagues undertook a subgroup analysis of the PREVENT Trial [
9]. The objective was to determine whether a fixed rather than weight-based dosing might cause decreased efficacy in obese patients and decreased safety in elderly patients. The standard definition for obesity was used: a Body Mass Index of 30 or greater for men and 28.6 or greater for women (calculated as weight in kilograms divided by the square of height in meters). The primary endpoint was a composite of symptomatic VTE, fatal pulmonary embolism, sudden death, or asymptomatic proximal DVT by day 21.
Dalteparin reduced the primary endpoint for all subgroups of Body Mass Index except for a Body Mass Index of 40 or greater. Dalteparin was also effective in all age subgroups, including patients older than 80 years. With respect to safety, dalteparin was not associated with an increase in major haemorrhage in either obese or elderly patients. Thus, this study indicates that prophylaxis with fixed low dose dalteparin is appropriate for many hospitalised medical patients and does not require dose adjustment for a wide range of ages and Body Mass Index.
With adherence to prophylaxis protocols, prevention of VTE can usually be achieved in the hospital setting. Primary prevention of VTE will be cost effective by avoiding the expenses associated with diagnosis and treatment of acute DVT and pulmonary embolism [
10].
Mechanical prophylaxis with graduated compression stockings or intermittent pneumatic compression devices [
15] has not been studied as extensively or rigorously as pharmacological prophylaxis. There is a rapid increase in use of the ultimate form of mechanical prevention, inferior vena caval filters. It is difficult to sort out how often filters are used for primary prevention of VTE compared with their use for secondary prevention after an acute DVT or pulmonary embolism.
Until recently, inferior vena caval filters were permanently implanted, without an option for removal. Concern has always centered on whether permanently implanted filters would lead to creation of large venous collaterals that could enable DVT to embolise to the lungs. There has also been a worry that filters can serve as a nidus for DVT on the filter itself and promote venous insufficiency and postphlebitic syndrome. Though more clinical trials are needed, it is likely that some of these potential problems with permanent filters may diminish with retrievable inferior vena caval filters.
Retrievable filters are being used more frequently for primary VTE prevention, especially in patients at high risk of VTE who are scheduled for operations that carry a high risk of postoperative DVT or pulmonary embolism. In my outpatient office practice, I often recommend placement of a retrievable filter for currently anticoagulated patients who have suffered VTE and who will undergo gastric bypass surgery or extensive lung cancer or pelvic cancer surgery.
A review of the United States National Hospital Discharge Survey showed a 20-fold increase in inferior vena caval filter placement over the past two decades [
11]. Almost half the filters were placed in patients who had established DVT without pulmonary embolism.
In the United States acute DVT registry of 5451 patients, 14% of all patients underwent filter placement [
12]. These filters were placed as permanent devices, before retrievable filters became available. This high rate of filter placement suggests that in actual clinical practice, filters are inserted for indications that are broader than the two classic indications: (1.) contraindications to anticoagulation, and (2.) failure of anticoagulation. The largest trial of permanent vena caval filter placement followed patients for 8 years and found that filters reduced the risk of pulmonary embolism but increased the risk of DVT. Filters had no effect on survival [
13].
As specialists with an interest and stake in optimal VTE prevention, we must determine how to change physician behavior (
Table 3) to achieve a true consensus on VTE prophylaxis. We can use protocols and consensus statements as a starting point, but the most challenging aspect remains devising strategies that translate evidence-based medicine into actual implementation in daily clinical practice. In the real world, the physician’s orders on an individual patient are the real measure of whether the lessons about VTE prevention have been communicated effectively.
At my hospital, Brigham and Women’s Hospital, we have an excellent educational network, and VTE management is a major clinical interest for many physicians. For the past decade, our computer program has been programmed to suggest VTE prophylaxis if a computer order is entered for “bed rest”. Nevertheless, audits of our own clinical practice reveal that many high risk patients for VTE have not received prophylaxis orders. Therefore, we undertook a randomised clinical trial of high risk patients who were not receiving VTE prophylaxis [
14].
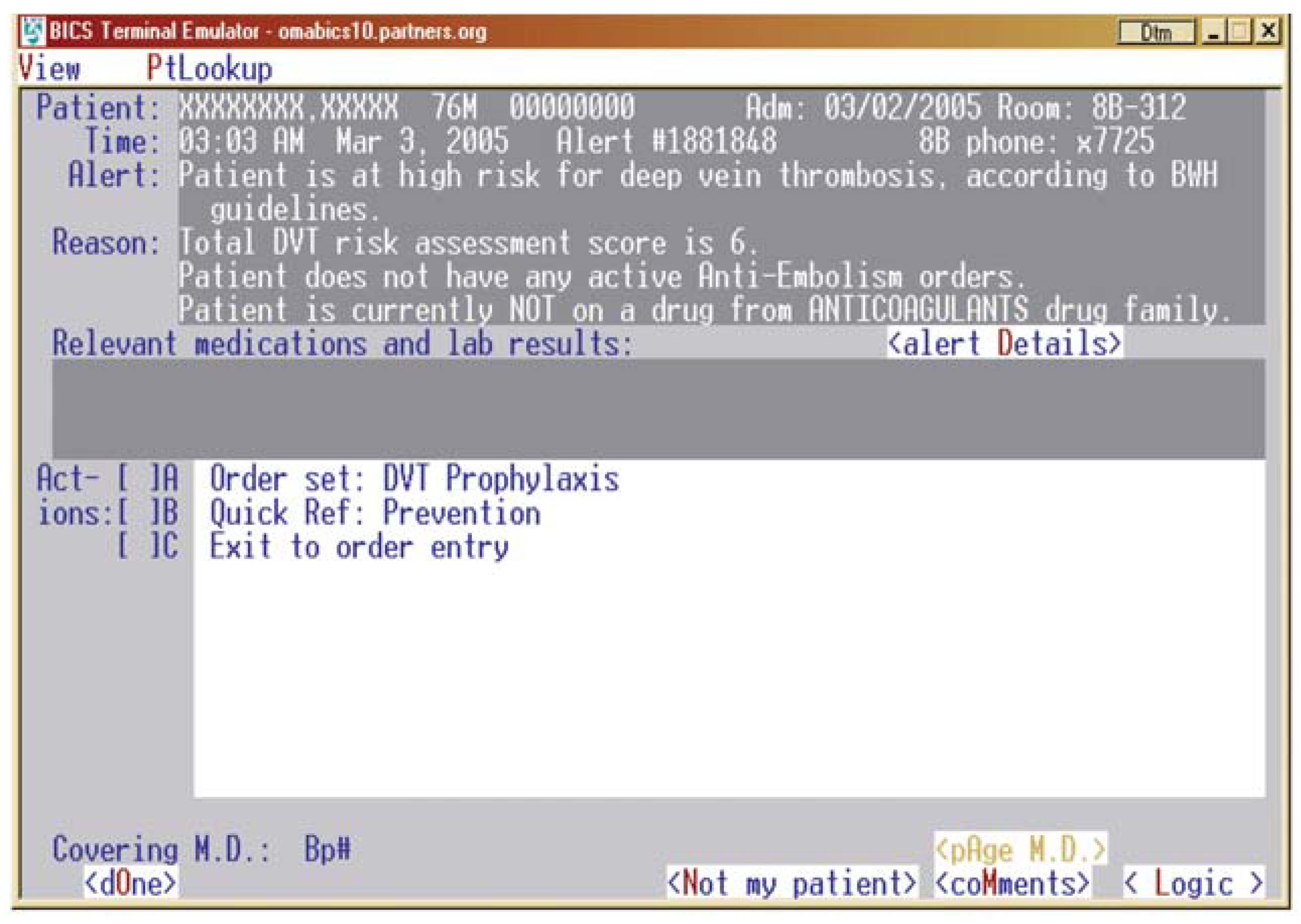
The primary end point of the trial was to reduce clinically diagnosed and objectively confirmed VTE. We worked closely with our Informations Systems Department so that our hospital computers could identify high risk patients and determine whether prophylaxis orders had been written. For those high risk patients without prophylaxis orders, the computer randomised patients to an intervention group or to a control group. In the intervention group, the physician responsible for care of a high risk patient not receiving prophylaxis received an electronic alert suggesting prophylaxis (
Figure 1), whereas the physicians of patients in the control group received no electronic alert. We devised a point score system to identify patients at high risk for VTE. We defined “high risk” as patients with 4 or more score points, and we assigned points as follows: prior VTE (3), cancer (3), hypercoagulability (3), major
surgery (2), advanced age (1), obesity (1), bed rest (1), hormone replacement therapy or oral contraceptives (1).
During our trial, 2506 patients at high risk for VTE and without prophylaxis orders were identified and enrolled: 1255 to the intervention group and 1251 to the control group. Of note, among these 2506 high risk patients not receiving prophylaxis, 83% were medical, 13% were surgical, and 4% were trauma. This emphasises that failure to implement prophylaxis is predominantly a problem on general medical and subspecialty medical services.
In the intervention group, the responsible physician was required to acknowledge the alert and could then order or withhold prophylaxis. Despite the alert, two-thirds of alerted physicians did not initiate VTE prophylaxis. While some of these high risk patients may have had contraindications to pharmacologic prophylaxis, we have no obvious explanation for failure to order mechanical prophylaxis with graduated compression stockings or intermittent pneumatic compression devices. The physicians responsible for control group patients did not receive an alert.
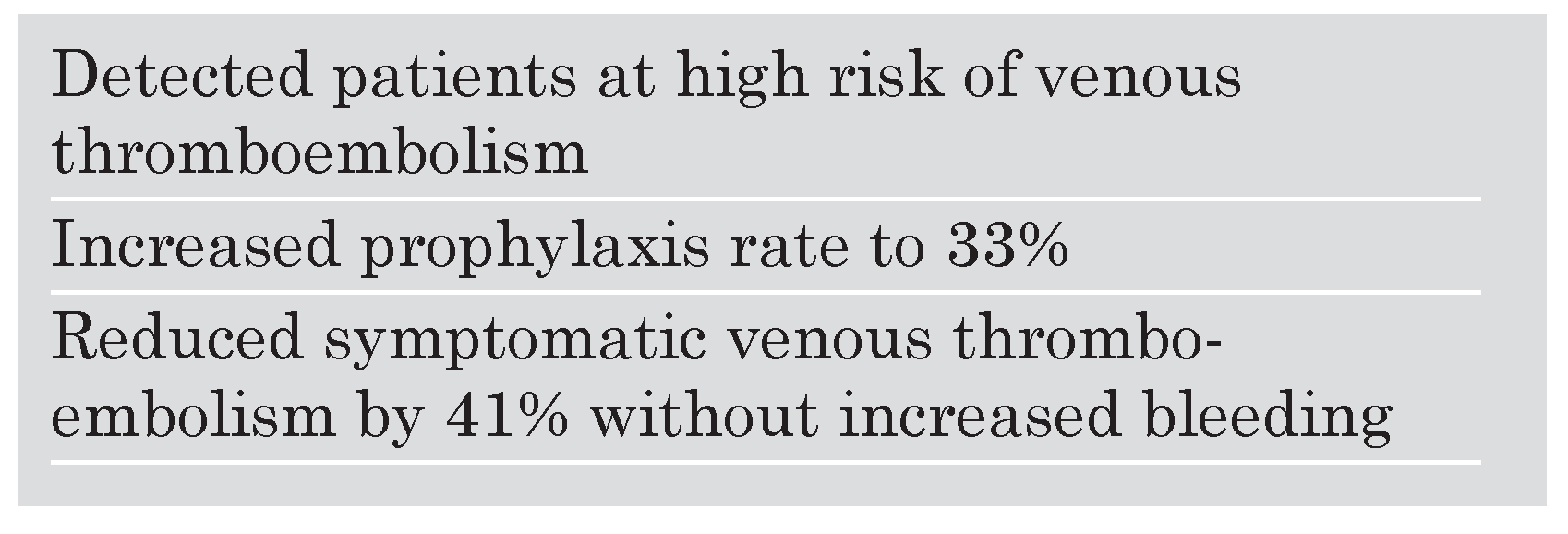
The primary end point of symptomatic and imaging-confirmed VTE occurred in 61 intervention-group and 103 control-group patients. At 90 days, the intervention strategy reduced the risk of VTE by 41%. There was no increased major or minor bleeding in the intervention group. Thus, institution of an electronic alert system increased the use of VTE prophylaxis and markedly reduced the risk of symptomatic VTE (
Table 4).
The beneficial effect of the electronic alert system can be replicated without computer support. To achieve the same objective of lowering the VTE rate, a nurse or physician could round on all overnight admissions, review their charts, and determine whether specific patients are at high risk for developing VTE during hospitalisation. They could then review the written physician orders to determine whether prophylaxis had been instituted. For high risk patients not receiving prophylaxis, the reviewer could then page the responsible physician, point out the patient’s high risk, note the absence of VTE prophylaxis orders, and suggest implementation of preventive measures.
Our electronic alert trial was a test of changing physician behaviour to improve implementation of prophylaxis. It was not a trial to test specific types of prophylaxis.
In summary, failure to utilise VTE prophylaxis remains a problem in high risk general medical and subspecialty medical patients. As part of a multifaceted approach, hospitals with adequate information systems should consider implementation of electronic alerts to increase DVT awareness, increase use of prophylaxis, and decrease rates of DVT and pulmonary embolism. The same strategy can be instituted without any specialised computer systems, as long as a willing physician or nurse reviewer can be recruited. Within a few years, the voluntary aspects of ordering VTE prophylaxis will disappear, as regulatory authorities and insurers demand that VTE prevention becomes obligatory.