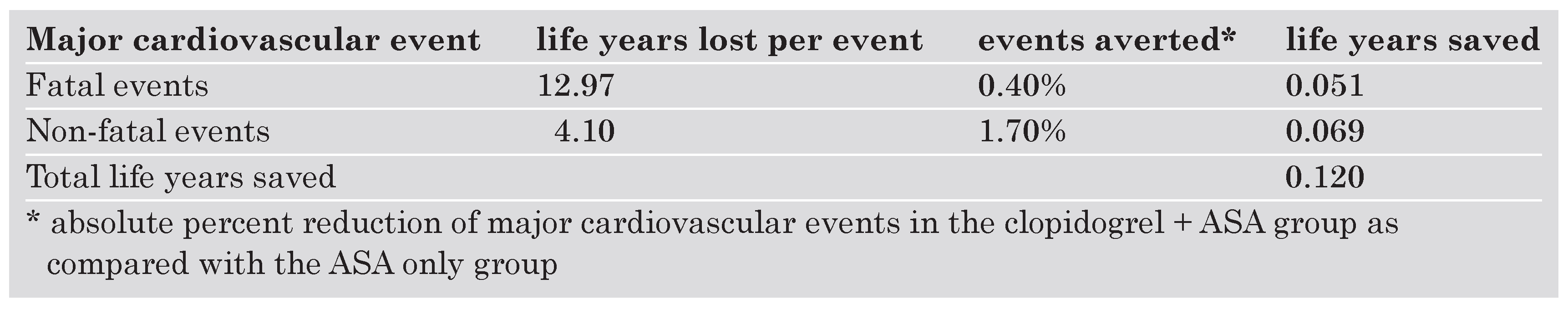Zusammenfassung
Hintergrund und Zielsetzung: Die Ergebnisse der Clopidogrel in Unstable Angina to prevent Recurrent Events (CURE)-Studie haben gezeigt, dass der Einsatz von Clopidogrel zusätzlich zur Standardtherapie mit Acetylsalicylsäure (ASA) zu einem Rückgang der kardiovaskulären Ereignisse Herzinfarkt, Hirnschlag und Todesfall infolge eines kardiovaskulären Ereignisses bei Patienten mit dem akuten Koronarsyndrom führt. Ziel dieser Studie war es, die Kosten-Effektivität dieser Strategie zu ermitteln.
Material und Methoden: Der RessourcenVerbrauch wurde aus den Case Report Forms (CRFs) der CURE-Studienpatienten erhoben. Erfasst wurden initiale Hospitalisationen, Folgehospitalisationen, Studienmedikation und Begleitmedikation. Die initialen und Folgehospitalisationen wurden mittels eines geblindeten, automatisierten Verfahrens in diagnosebezogene Fallgruppen, Diagnosis Related Groups (DRG), eingeordnet. Der Verbrauch an Studienmedikation wurde aufgrund der Behandlungstage erhoben. Zur Schätzung des Verbrauchs an Begleitmedikation wurden empfohlene Dosen und Behandlungsdauern verwendet, da aus den CRFs nur hervorging, ob eine entsprechende Begleitmedikation angeordnet wurde. Zur Bewertung der Hospitalisationen wurden die DRG-spezifischen Kostengewichte mit der Basisrate der Swiss All Patient Diagnosis Related Groups (APDRG) multipliziert. Die Bewertung der Studienund Begleitmedikation erfolgte zu Detailhandelspreisen. Als Mass für die Wirksamkeit wurde die Anzahl gewonnener Lebensjahre mittels der DEALE-Methode geschätzt.
Ergebnisse: Die durchschnittlichen Kosten sind bei Patienten, die mit Clopidogrel + ASA behandelt wurden, mit CHF 14 839 etwas höher als bei Patienten, die nur mit ASA behandelt wurden (CHF 14 380). Dies ist bedingt durch die Kosten der Studienmedikation (CHF 775), welche teilweise kompensiert werden durch Einsparungen während der initialen und der Folgehospitalisationen in der Höhe von CHF 327. Die übrigen Kosten waren in beiden Gruppen gleich. Die Behandlung mit Clopidogrel führte zu 0,12 gewonnenen Lebensjahren. Die inkrementelle Kosteneffektivität – d.h. die inkrementellen Kosten pro gewonnenes Lebensjahr – betrug CHF 3810. Die Clopidogrel+ ASA-Therapie ist also kosteneffektiv und ist vergleichbar mit anderen kosteneffektiven Therapien kardiovaskulärer Krankheiten.
Introduction
Patients with acute coronary syndrome are conventionally treated with aspirin to prevent new major cardiovascular events (myocardial infarction, stroke, cardiovascular death). Despite this treatment, there still remains a considerable risk of such events in these patients. Given the clinical and socioeconomic impact of major cardiovascular events there is a need to reduce further these risks in a broad spectrum of patients both when they first present with acute coronary syndromes and in the long term. The addition of clopidogrel, a thienopyridine derivative, to the standard treatment offers a new therapeutic approach to these patients. The Clopidogrel in Unstable Angina to Prevent Recurrent Events (CURE) study was a phase III multicenter, randomized, double-blind, placebo-controlled parallel group clinical trial [
1]. In this trial 482 centers in 28 countries were participating. A total of 12 562 patients with acute coronary syndromes (unstable angina or myocardial infarction without ST-segment elevation) who had presented within 24 hours after the onset of symptoms were randomized to receive clopidogrel (300 mg immediately, followed by 75 mg once daily) (6259 patients) or placebo (6303 patients) in addition to acetylsalicyl-acid (ASA) for 3 to 12 months. The mean age of the patients was 64.2 years and 61.7% were males. The mean followup was 9 months. The first primary outcome – a composite of death from cardiovascular causes, nonfatal myocardial infarction, or stroke – occurred in 9.3 percent of the patients in the clopidogrel group and 11.4 percent of the patients in the placebo group thus showing a 20 percent relative reduction of the risks of major cardiovascular events [
1]. Of these events, 5.1% were fatal and 4.2% were nonfatal in the clopidogrel group and 5.5% were fatal and 5.9% were nonfatal in the placebo group.
When considering the introduction of a novel treatment not only the clinical benefit, but also the costs associated with this treatment have to be taken into account. Very often interventions that improve outcomes also increase costs. In such cases it is necessary to show that the treatment provides good value for money. Under the constraints of limited health-care budgets affording scarce personal and financial resources, pharmacoeconomical analyses are used increasingly in healthcare policy decisions and are now required for drug reimbursement by some authorities [
2,
3,
4]. While improving clinical outcomes the addition of clopidogrel to ASA in the treatment of patients with acute coronary syndrome also impacts on the treatment costs of these patients. The aim of this study was to evaluate the cost-effectiveness of this treatment in a Swiss setting.
Materials and Methods
Data available from the CURE trial [
1] were used to estimate resource use and valued with Swiss unit costs. Therefore the time horizon was that of the clinical trial, ie 9 months. The costs included were limited to direct medical costs (hospitalisations and medications only) as no data on direct non medical costs or indirect costs were recorded in the CURE trial. Cost estimates require two types of data to be assessed. First the medical resource items and their quantities were identified and extracted from the CURE trial data. Second, treatment patterns and medical resource items obtained from the CURE data were validated for Switzerland and Swiss cost values were assigned to each resource use item. Effectiveness was estimated as the number of life years saved using the Declining Exponential Approximation of Life Expectancy (DEALE) method [
5]. With this information, costs and incremental cost-effectiveness of clopidogrel on top of standard therapy (including ASA) versus ASA alone was calculated.
Cost assessment
Resource use was obtained from the case report forms (CRFs) of all patients in the CURE trial and grouped into initial hospitalisation, re-hospitalisations, study drug, and concomitant medications. The initial and rehospitalisations were assigned to a diagnosis related group (DRG) in a blinded automated fashion. DRGs are a sort of patient classification system that groups patients with similar characteristics together [
6]. These groups are constructed to be clinically meaningful and homogenous with respect to treatment costs. Hospital inpatients are first grouped into one of 25 Major Diagnostic Categories and then further assigned to one specific sub-group (DRG)[
7]. DRG assignment requires detailed clinical information including diagnoses, procedures, complications, co-morbidity, signs and symptoms, and discharge status. Patients with similar presenting problems, similar treatment patterns, and resource use are grouped in the same DRG. Patients in different DRGs are different with respect to their clinical problems, treatment patterns, and resource use. This concept allows the assessment of hospitalisation costs depending on the patient characteristics and irrespective of the hospital a patient is treated in. Average costs per DRG are comparable in different hospitals. However, the case-mix, ie the distribution of patients among DRGs may vary between hospitals and is an important cause of cost differences between hospitals.
Study drug uptake was assessed based on one loading dose plus the number of days on treatment with normal daily doses and compliance was taken into account. The use of concomitant medication was only recorded as yes / no answers on the CRFs without information on doses or duration. It was therefore assumed that drugs were given continuously between observations and daily defined doses were applied to estimate typical doses of concomitant medication for each therapeutic class. Drugs given inpatient during hospitalisations are covered by the cost of the DRGs.
To value initial and subsequent hospitalisations each DRG was assigned a specific cost value. For this, the Swiss All Patient Diagnosis Related Groups (APDRG) cost weights were applied. DRG costing was elaborated by APDRG Switzerland, a working group that was established in 1998 with the aim to promote the use of DRGs in Switzerland. Each APDRG is assigned a number of points – the cost weights. These weights are then multiplied with the amount of one point, currently CHF 6049 [8, 9], to calculate the hospital costs per DRG. The costs and cost weights were assessed based on the detailed records of 8 hospitals and key indicators of further 18 hospitals. They include full operating hospital costs (without investments) per APDRG. These are not the charges paid by the health insurers but serve as reference costs for the negotiation of charges, as in Switzerland the hospitals are refunded only about half their operating costs by compulsory health insurance. The APDRGs are an updated and refined version of the original DRGs that have been developed at Yale University, first introduced in New Jersey and 1983 adapted by Medicare. The APDRGs were chosen mainly because of the positive experiences in France, Belgium, and Australia. For the time being APDRGs are used in the hospitals of the Canton of Vaud and in some hospitals of the Canton of Zurich [
7].
The study drug and concomitant medications during the follow-up were valued according to the official drug list (Spezialitätenliste) or at retail prices. Comedications prescribed during hospitalisation are covered by the hospitalisation costs as assessed above.
Effectiveness
The number of life years saved per fatal and nonfatal event avoided was assessed and multiplied with the absolute percent reductions of fatal and nonfatal events respectively. The DEALE approximation was used to estimate the number of life years saved in avoided fatal events. The DEALE approach is based on the assumption that survival follows a simple declining exponential function with a constant annual mortality rate. In such a circumstance, life expectancy is equal to the reciprocal of the annual mortality rate. Furthermore, if several risk factors are operative, then overall mortality can be computed simply as the sum of the individual rates [
5]. Life years saved per avoided fatal event were calculated by adding the excess mortality rate specific to the acute coronary syndrome and the population mortality rate of a Swiss population with similar age and gender distribution and taking the reciprocal of this sum. Excess mortality was obtained using the annualised death rate from all causes of the patients in the placebo arm of the CURE study from which the population mortality of an international population with similar country, age, and gender distribution as in the CURE study was subtracted. Country specific mortality rates were calculated based on the WHO life tables for 191 countries [
10]. The number of life years lost per nonfatal major cardiovascular event was estimated at 4.1 years based on the literature [11, 12]. Haldemann et al. [
11] report a reduction of life expectancy of 4.1 years in patients with nonfatal myocardial infarction or stroke based on analyses of data from the CAPRIE trial [
13]. Peters et al. show data on life expectancy in patients with myocardial infarction and stroke in 60 and 70 years old males and females [
12]. Based on these data and taking a weighted average with the similar proportions for gender and events as in the CURE study, the average life expectancy in patients with a history of a major cardiovascular event can be roughly estimated at 8.9 years, which is 4.1 years less than the average life expectancy of a patient at inclusion into the CURE study.
Sensitivity analysis
One-way sensitivity analysis was performed on incremental cost-effectiveness to test the robustness of the results and to identify the parameters with the most important impact on the results. For this, the relative risk of major cardiovascular events with clopidogrel as compared with placebo, 0.80, was varied within the 95% percent confidence interval from 0.72 to 0.90. In addition, hospitalisation costs, study medication costs and comedication costs were varied ± 10% one at a time while all other parameters were held constant. The cost of bleedings is normally included in the DRGs. Treatment with clopidogrel leads to a somewhat higher risk of major bleeding episodes (3.7% of patients with major bleeding episodes in the clopidogrel group as compared to 2.7% in the placebo group) [
1]. In order to account for possible extra costs of bleeding a sensitivity analysis was performed. For this, each major bleeding episode was assigned an extra day of hospitalisation at a daily cost of CHF 1100.
Results
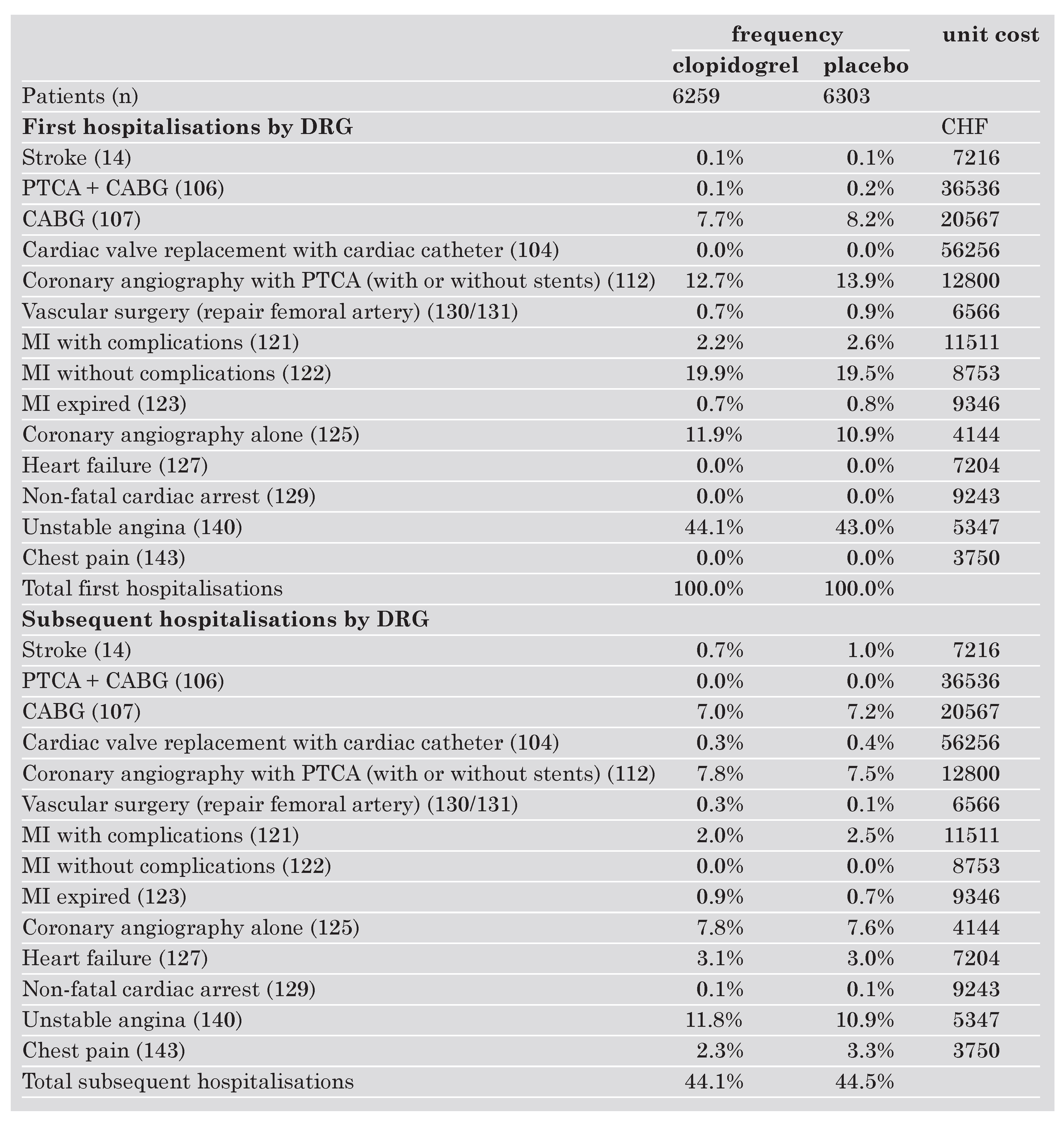
The resources used by the patients in the clopidogrel group and in the placebo group, respectively, as well as the unit costs are shown in the Table 1. According to the protocol all patients were initially hospitalised. Most hospitalisations were attributed to unstable angina (DRG 140) (44% in the clopidogrel group, 43% in the placebo group) and myocardial infarction without complications (DRG 122) (19% in each group). There were slightly more hospitalisations in the placebo group for the coronary angiography with PTCA (DRG 112) (13.9% vs 12.7%), the CABG (DRG 107) (8.2% vs 7.7%), the MI with complications (DRG 121) (2.6% vs 2.2%) than in the group clopidogrel on top of standard therapy. For hospitalisations in coronary angiography alone (DRG 125), the inverse was true (11.9% in the clopidogrel on top of standard therapy group and 10.9% in the placebo group). Subsequent hospitalisations occurred in 44% of all patients (2761 subsequent hospitalisations in the clopidogrel group and 2802 in the placebo group). There were considerably more subsequent hospitalisations for stroke (DRG 14), myocardial infarction with complications (DRG 121), and chest pain (DRG 143) in the placebo group whereas the rate of hospitalisations for unstable angina (DRG 140) was markedly higher in the clopidogrel group.
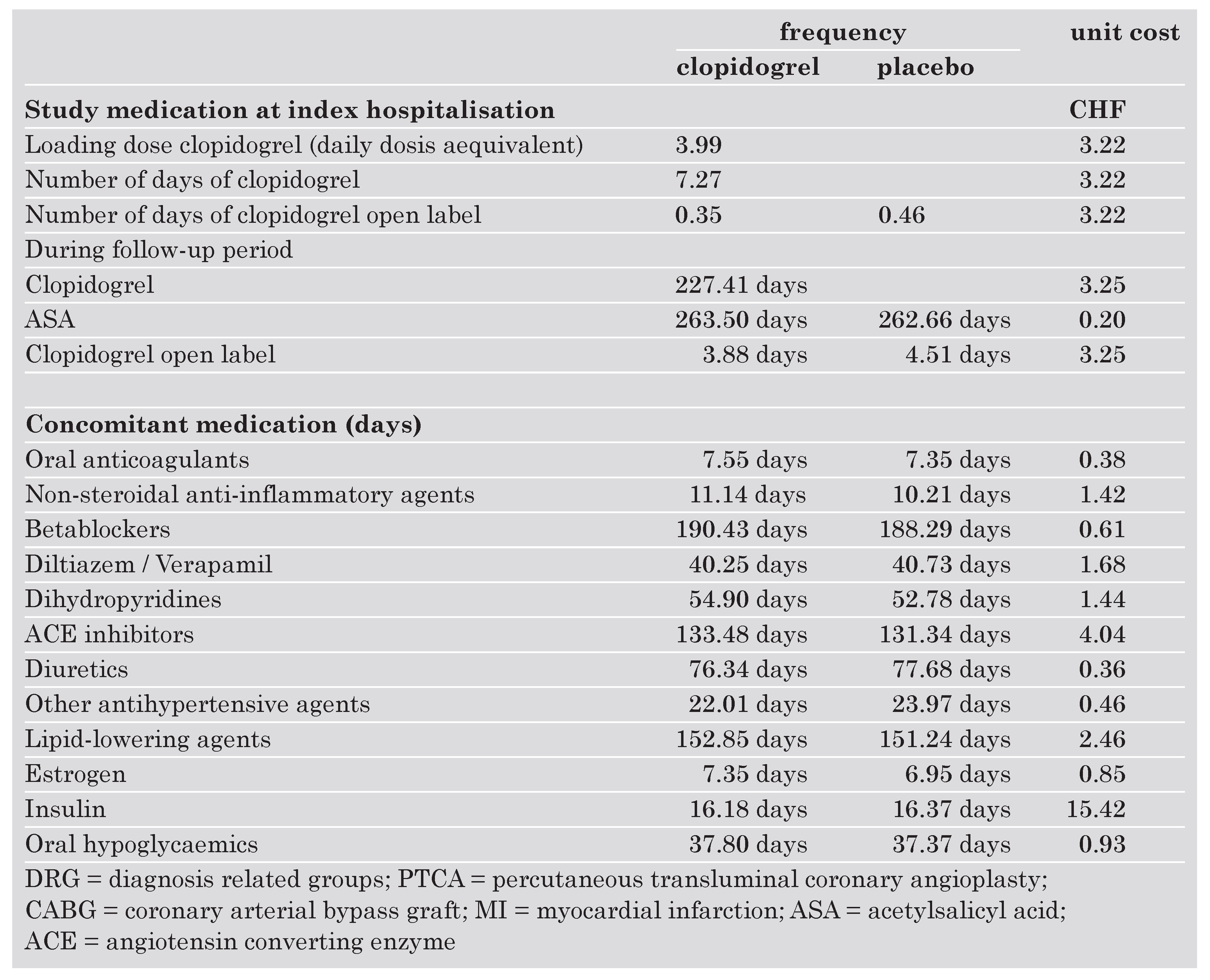
At index hospitalisation after a loading dose, clopidogrel was administered on 7.3 days and 0.4 days open label in the study group. In the placebo group there was an open label use of clopidogrel of 0.5 days per patient. Followup was very similar in both groups as can be seen from the number of days of ASA given in each group.
Table 1. Resource use and unit costs.
![Cardiovascmed 07 00174 i001 Cardiovascmed 07 00174 i001]()
![Cardiovascmed 07 00174 i002 Cardiovascmed 07 00174 i002]()
The concomitant medications included medicines of 12 therapeutic classes and were very similar in both groups. Most important were betablockers, lipid lowering agents and angiotensin converting enzyme inhibitors.
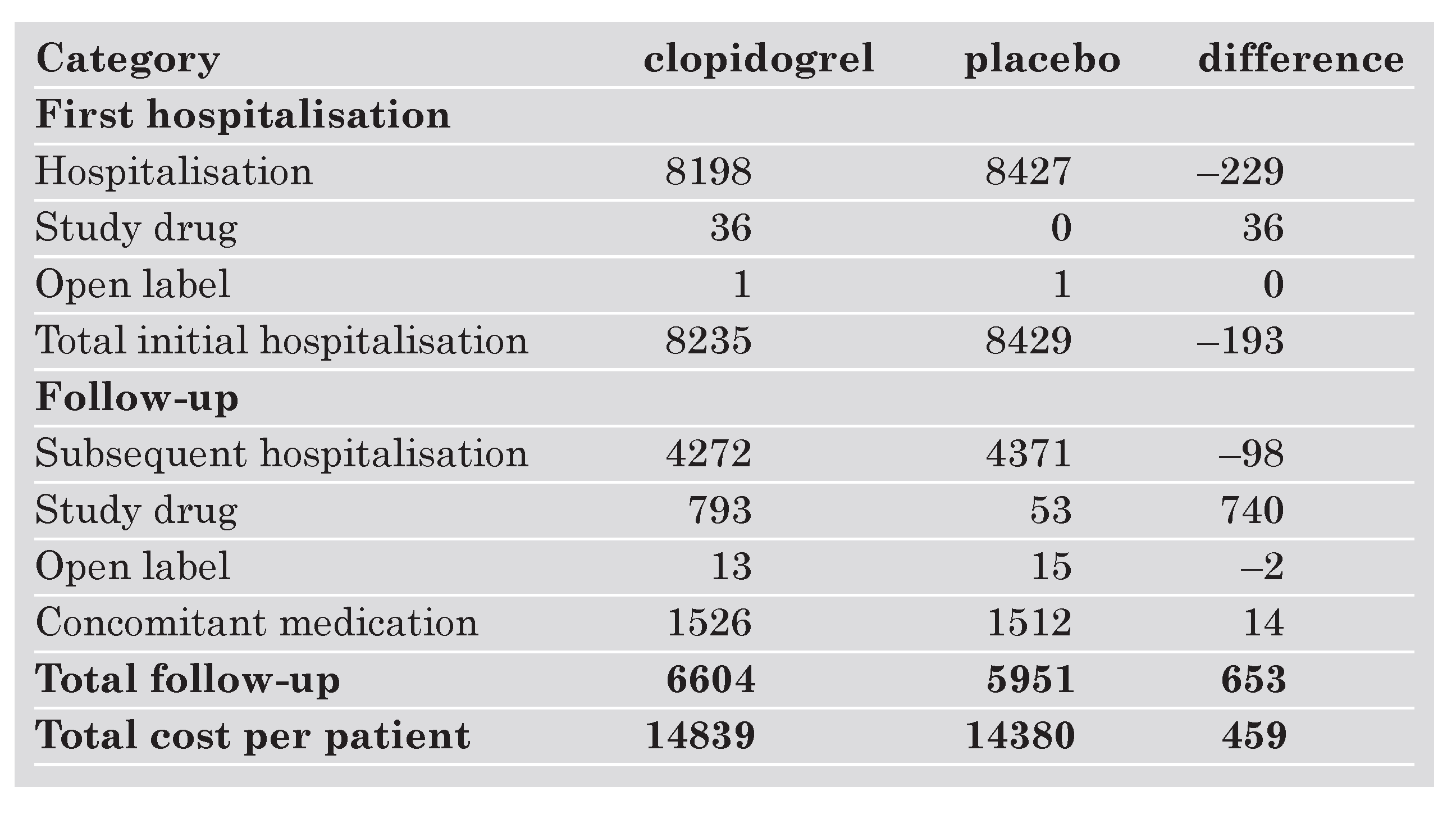
Table 2 shows the costs per patient by component of clopidogrel on top of standard therapy compared to ASA alone. Patients in the clopidogrel arm have on average slightly higher costs than patients treated with placebo, CHF 14 839 as compared with CHF 14 380. This is due to the acquisition costs of clopidogrel (CHF 775, not shown in the Table 2 but included in the costs of study drug together with ASA). Nevertheless these acquisition costs are partly offset by savings during the initial and subsequent hospitalisations, which amounted to CHF 327. The costs of concomitant medication and open label drug use were quite similar in both groups.
Table 2. Costs by components in CHF.
![Cardiovascmed 07 00174 i003 Cardiovascmed 07 00174 i003]()
The calculation of life years saved is summarised in the Table 3. The annualised compound mortality rate of the patients in the placebo arm of the CURE study (standard therapy) was estimated at 8.25%, the population mortality of an international population with similar country, age and gender distribution was 5.45%. Thus the excessive mortality for acute coronary syndrome was found to be 2.80%. The population mortality of a Swiss population with similar age and gender distribution as in the CURE study was 4.91%. With excess mortality added, the compound mortality for the same population was 7.71%. The corresponding life expectancy is 12.97 years. Treatment with clopidogrel leeds to an absolute reduction of 0.4% of fatal and 1.7% of non fatal cardiovascular events, respectively. Multiplied with 12.97 and 4.1 years of potential life lost, the number of life years saved was estimated at 0.12.
The incremental costs were CHF 459 and combined with the incremental survival of 0.12 years, the incremental cost-effectiveness was CHF 3810 per life year saved.
Table 3. Ursachen kardialer Verkalkungen.
![Cardiovascmed 07 00174 i004 Cardiovascmed 07 00174 i004]()
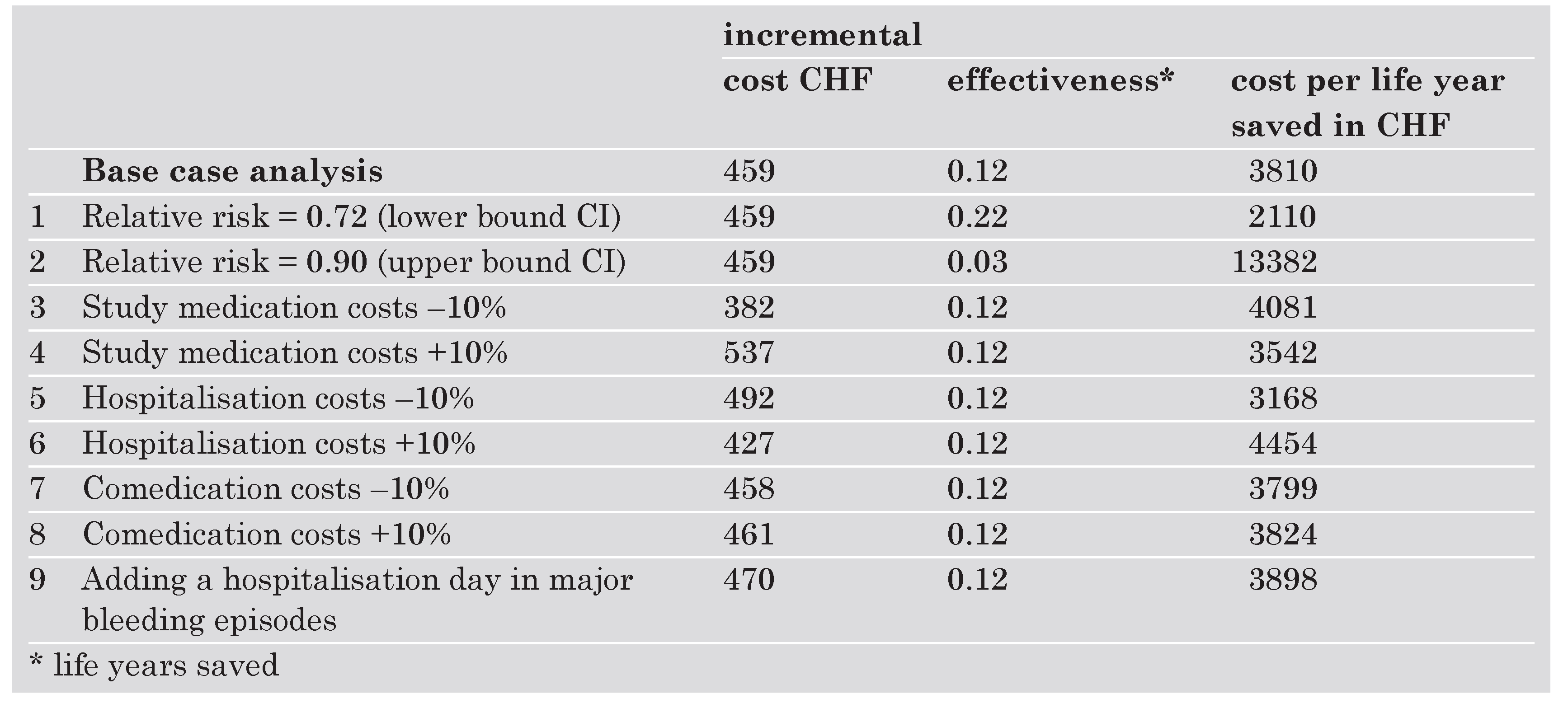
Sensitivity analysis
The results of the sensitivity analyses are shown in the Table 4. The parameter with the greatest impact on cost-effectiveness is the relative risk of major cardiovascular events with clopidogrel. Among the cost parameters the price of study medication had the greatest impact, the impact of hospitalisation costs was small and varying comedication costs had almost no impact on the result at all. Adding an extra hospitalisation day for each major bleeding episode has a small impact on the results although this is probably a overestimate of bleeding costs.
Table 4. Ursachen kardialer Verkalkungen
![Cardiovascmed 07 00174 i005 Cardiovascmed 07 00174 i005]()
Discussion
For this cost-effectiveness analysis data collected within the CURE trial were used to estimate resource use. This was combined with Swiss values to assess the costs. This approach has the advantage that bottom-up information from the same source of data can be utilized. The costs of the intervention as well as of medical events associated or avoided with the intervention can be separated and consistent data are obtained. The drawback of this approach is that the data collected in the clinical trial may be incomplete. In this analysis potentially important cost components such as the costs of doctor visits in the follow-up period and nonmedical direct costs were not included since these data were not recorded. Similarly, the costs of major cardiovascular events that occurred beyond the time horizon of the CURE trial were not included either. Therefore potential savings due to avoided events are underestimated. This analysis thus provides a conservative estimation of the cost-effectiveness of treatment with clopidogrel on top of standard therapy.
The CURE study was a international multi-center trial. Resource use for all countries in the study has been pooled in the analysis since the trial was not powered to detect differences between countries. Treatment patterns, however, may vary from country to country. A sensitivity analysis has been conducted where resource utilisation for countries with low intervention rates were excluded. This sensitivity analysis showed that excluding countries with less aggressive treatment strategies does not affect results greatly.
The primary endpoint of the CURE trial was a composite outcome of death from cardiovascular cause or nonfatal myocardial infarction or nonfatal stroke. Each of these fatal and nonfatal major cardiovascular events is associated with a loss of life years. Avoiding such events therefore transforms into saving life years. The number of life years saved was estimated using the DEALE-approach. This approach makes it possible to translate data from various literature sources into a single, unified mortality scale and provides a useful validated and convenient clinical approximation of life years saved.
The results of this analysis show that with an incremental cost of CHF 3810 per life year saved, clopidogrel in combination with ASA is cost effective compared with ASA alone. Using the Coronary Heart Disease Policy Model, a computer simulation of the U.S. population, the incremental cost effectiveness of four strategies in patients over 35 years of age with coronary disease was analysed in the longterm (25 years): ASA for all eligible patients, ASA for all eligible patients plus clopidogrel for patients who were ineligible for ASA, clopidogrel for all patients and the combination of ASA for all eligible patients plus clopidogrel for all patients [
14]. Clopidogrel in combination with ASA was found to have a incremental cost of
$ 61 000 per QALY [
15]. However, these estimates are flawed by extrapolations of shortterm data to the long-term [
16] assuming that patients were given up to 25 years of treatment with clopidogrel. Considering present treatment regimens these estimates are irrelevant. Using a Markov-Model and epidemiological data a Swedish study extrapolated the results of the CURE-trial to estimate the number of life years gained of a one-year treatment period with clopidogrel on top of standard therapy versus standard therapy alone This model predicts an incremental survival of 0.12 years and incremental direct costs of 149 US
$ per patient. The incremental cost-effectiveness ratio is thus US
$ 1290 per life year gained [
17]. On the basis of the CAPRIE trial [
13] a Swiss cost-effectiveness analysis of clopidogrel in the secondary cardiovascular prevention was undertaken. This study covered a time span of two years and found an incremental cost effectiveness of CHF 22 800 per life year gained [
11]. These results support the findings of the present study that the cost-effectiveness of clopidogrel in secondary cardiovascular prevention lies well within the range of other preventive cardiovascular interventions.
This study was supported by Sanofi-Synthélabo (Suisse) SA, Geneva, Switzerland.