Severe Pulmonary Embolism: Surgical Aspects
Abstract
Introduction
Classification of Pulmonary Embolism
Surgical Technique
Surgical Technique for Acute Pulmonary Embolism
Surgical Technique for Chronic Pulmonary Embolism
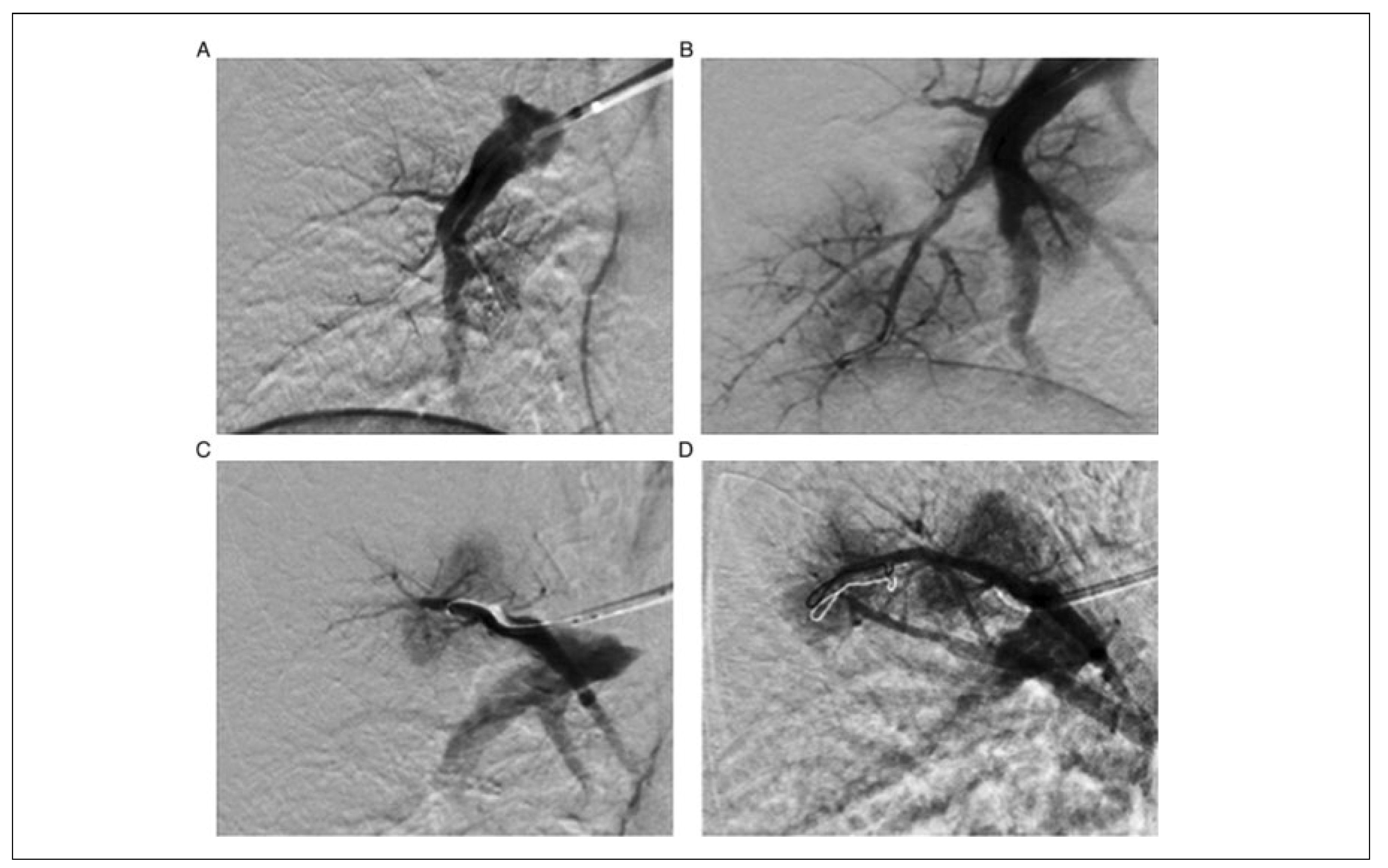
Percutaneous Pulmonary Angioplasty
Surgery in Acute Pulmonary Embolism: Indications and Outcome
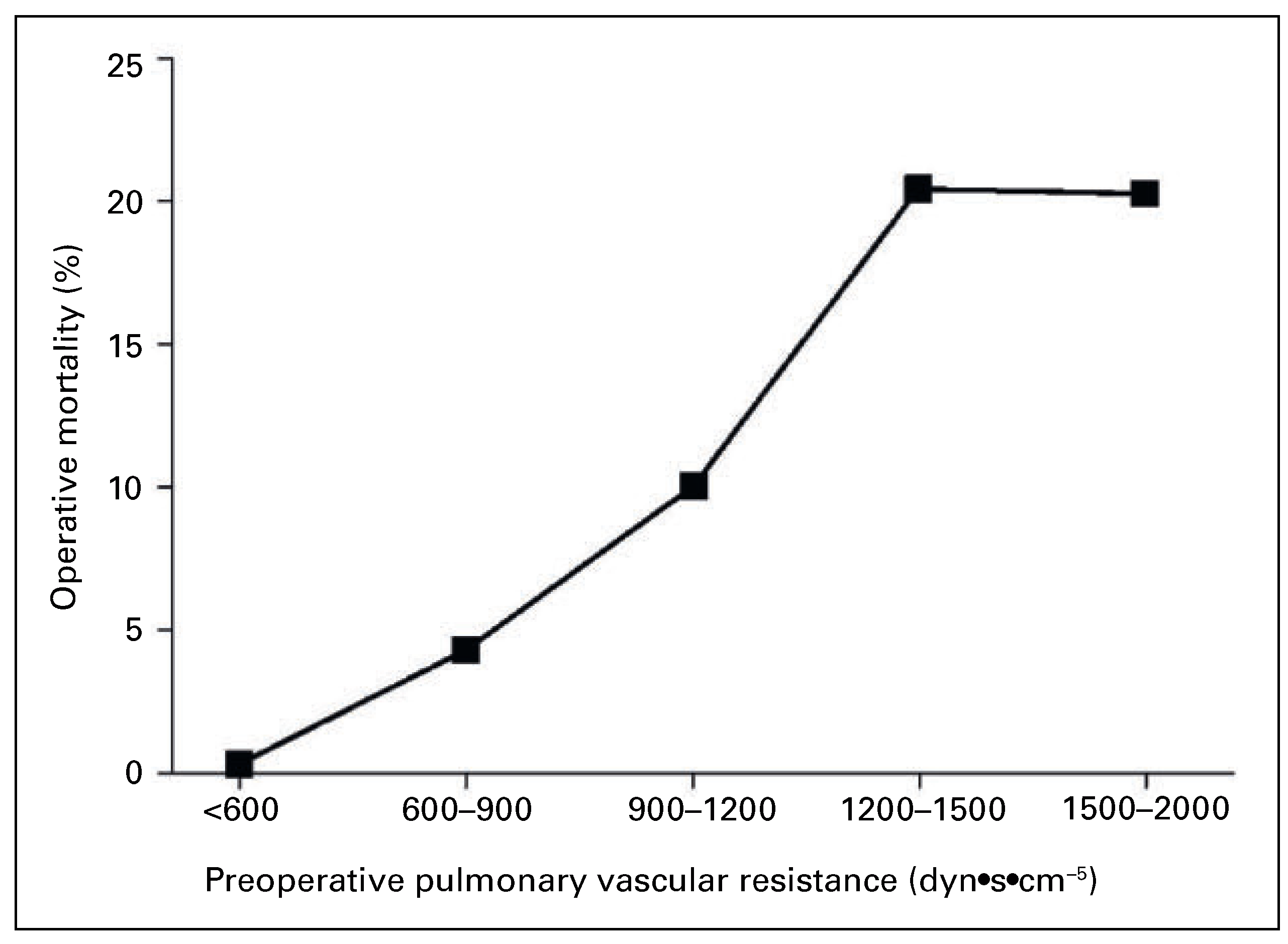
Surgery in Chronic Thromboembolic Hypertension: Indications and Outcome
Percutaneous Pulmonary Angioplasty in Chronic Thromboembolic Hypertension: Indications and Outcome
Conclusion
Disclosure Statement
References
- Trendelenburg, F. Über die operative Behandlung der Embolie der Lungenarterie. Archiv für Klinische Chirurgie 1924, 133, 312–359. [Google Scholar]
- Kirschner, M. Ein durch die Trendelenburgsche Operation geheilter Fall von Embolie der Art. Pulmonalis. Archiv für klinische Chirurgie. 1924, 133, 312–359. [Google Scholar]
- Gibbon, J. The Development of the Heart-Lung Apparatus. Am. J. Surg. 1978, 135, 608–619. [Google Scholar] [CrossRef] [PubMed]
- Cooley, D.A.; Beall, A.C. Surgical treatment of acute massive pulmonary embolism using temporary cardiopulmonary bypass. Dis. Chest. 1962, 41, 102–104. [Google Scholar] [CrossRef] [PubMed]
- Taylor Thompson, B. Overview of acute pulmonary embolism in adults. In: UpToDate: Waltham, MA. (Accessed on May 27, 2016).
- Fedullo, P.F. Overview of the treatment of chronic thromboembolic pulmonary hypertension. In: UpToDate, Waltham, MA. (Accessed on May 27, 2016).
- Eini, Z.M.; Houri, Z.; Cohen, I.; Sion, R.; Tamir, A.; Sasson, L.; Mandelberg, A. Massive Pulmonary Emboli in Children. Does Fiber-optic-Guided Embolectomy have a role? Review of the Literature and Report of Two Cases. Chest 2013, 143, 544–549. [Google Scholar] [CrossRef] [PubMed]
- Dartevelle, P.; Fadel, E.; Mussot, S.; Chapelier, A.; Hervé, P.; de Perrot, M.; et al. Chronic thromboembolic pulmonary hypertension. Eur. Respir. J. 2004, 23, 637–648. [Google Scholar] [CrossRef] [PubMed]
- Mayer, E.; Klepetko, W. Techniques and Outcome of Pulmonary Endarterectomy for Chronic Thromboembolic Pulmonary Hypertension. Proc. Am. Thorac. Soc. 2006, 3, 589–593. [Google Scholar] [CrossRef] [PubMed]
- Lang, I.M.; Madani, M. Update on Chronic Thromboembolic Pulmonary Hypertension. Circulation 2014, 130, 508–518. [Google Scholar] [CrossRef] [PubMed]
- Kearon, C.; Akl, E.A.; Comerota, A.J.; Prandoni, P.; Bounameaux, H.; Goldhaber, S.Z.; et al. Antithrombotic therapy for VTE disease. Antithrombotic Therpay and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012, 141 (2 Suppl), e419S–e496S. [Google Scholar] [CrossRef] [PubMed]
- Stein, P.D.; Alnas, M.; Beemath, A.; Patel, N. Outcome of Pulmonary Embolectomy. Am. J. Cardiol. 2007, 99, 421–423. [Google Scholar] [CrossRef] [PubMed]
- Meneveau, N.; Séronde, M.F.; Blonde, M.C.; Legalery, P.; et al. Management of Unsuccessful Thrombolysis in Acute Massive Pulmonary Embolism. CHEST 2006, 129, 1043–1050. [Google Scholar] [CrossRef] [PubMed]
- Torbicki, A. Pulmonary Thromboembolic Disease. Clinical Management of Acute and Chronic Disease. Rev. Esp. Cardiol. 2010, 63, 832–849. [Google Scholar] [CrossRef] [PubMed]
- Kim, N.H.; Delcroix, M.; Jenkins, D.P.; Channick, R.; Dartevelle, P.; Jansa, P.; et al. Chronic Thromboembolic Pulmonary Hypertension. JACC 2013, 62, D92–9. [Google Scholar] [CrossRef] [PubMed]
- Cannon, J.E.; Su, L.; Kiely, D.G.; Page, K.; Toshner, M.; Swietlik, E.; et al. Dynamic Risk Stratification of Patient Long-Term Outcome Aher Pulmonary Endarterectomy: Results From the United Kingdom National Cohort. Circulation 2016, 133, 1761–1771. [Google Scholar] [CrossRef]
- Freed, D.H.; Thomson, B.M.; Berman, M.; Tsui, S.S.L.; Dunning, J.; Sheares, K.K.; et al. Survival aher pulmonary thrombendarterectomy: Effect of residual pulmonary hypertension. JTCVS 2011, 141, 383–387. [Google Scholar]
- Mizoguchi, H.; Ogawa, A.; Munemasa, M.; Mikouchi, H.; Ito, H.; Matsubara, H. Refined Balloon Pulmonary Angioplasty for Inoperable Patients with Chronic Thromboembolic Pulmonary Hypertension. Circ. Cardiovasc. Interv. 2012, 5, 748–755. [Google Scholar] [CrossRef]
- Andreassen, A.K.; Ragnarsson, A.; Gude, E.; Geiran, O.; Andersen, R. Balloon pulmonary angioplasty in patients with inoperable chronic thromboembolic pulmonary hypertension. Heart 2013, 99, 1415–1420. [Google Scholar] [CrossRef] [PubMed]

© 2017 by the author. Attribution-Non-Commercial-NoDerivatives 4.0.
Share and Cite
Reuthebuch, O. Severe Pulmonary Embolism: Surgical Aspects. Cardiovasc. Med. 2017, 20, 154. https://doi.org/10.4414/cvm.2017.00486
Reuthebuch O. Severe Pulmonary Embolism: Surgical Aspects. Cardiovascular Medicine. 2017; 20(6):154. https://doi.org/10.4414/cvm.2017.00486
Chicago/Turabian StyleReuthebuch, Oliver. 2017. "Severe Pulmonary Embolism: Surgical Aspects" Cardiovascular Medicine 20, no. 6: 154. https://doi.org/10.4414/cvm.2017.00486
APA StyleReuthebuch, O. (2017). Severe Pulmonary Embolism: Surgical Aspects. Cardiovascular Medicine, 20(6), 154. https://doi.org/10.4414/cvm.2017.00486



