Introduction
Prompt referral for mechanical (primary percutaneous coronary intervention [pPCI]) or pharmacological (fibrinolytic) reperfusion represents the gold standard emergency treatment for patients experiencing ST-elevation myocardial infarction (STEMI) [1–3]. However, in a considerable proportion of STEMI patients, successful restoration of infarct-related artery (IRA) patency is not followed by adequate myocardial blood flow at a tissue level. This condition is known as no-reflow and seems to be related to microvascular obstruction (MVO) [4]. No-reflow represents one of the most challenging conditions for interventional cardiologists and has a strong negative impact on in-hospital and long-term clinical outcome of STEMI patients treated with pPCI or fibrinolysis, negating the benefits of prompt and effective reopening of the IRA [5–10]. Interestingly, no-reflow has been observed also in NSTEMI patients and during elective PCI, particularly when performed on saphenous vein grafts [11].
No-reflow can be assessed with both invasive and noninvasive techniques. On the basis of coronary angiography, no-reflow is usually defined as a Thrombolysis in Myocardial Infarction (TIMI) flow grade <3 or 3 in the presence of a myocardial blush grade (MBG) 0 to 1 despite effective mechanical or pharmacological restoration of IRA patency [12,13]. In the setting of STEMI, an ST-segment elevation resolution of less than 70% 60 to 90 minutes after pPCI on the surface electrocardiogram (ECG) is usually considered suggestive of no-reflow [14]. Myocardial contrast echocardiography (MCE) uses ultrasound to visualise contrast microbubbles that freely flow within patent microcirculation; no-reflow is detected as lack of intramyocardial contrast opacification [15]. Cardiac magnetic resonance (CMR), with gadolinium to assess regional cardiac perfusion, diagnoses no-reflow through: (1) lack of gadolinium enhancement during first pass; and (2) lack of gadolinium enhancement within a necrotic region, identified by late gadolinium hyper-enhancement [16]. The physiopathology of no-reflow is complex, multifactorial and still incompletely understood. In humans, no-reflow is likely to be due to a variable combination of four major pathogenetic components: (1) distal atherothrombotic embolisation from both culprit plaque and thrombus; (2) ischaemic injury; (3) reperfusion injury; and (4) individual susceptibility of the coronary microcirculation. Concerning distal embolisation, emboli of different sizes can originate from epicardial coronary culprit plaque and thrombus. Of note, experimental studies showed a significant and irreversible reduction in myocardial blood flow when microspheres obstruct more than 50% of coronary capillaries [17]. Large emboli (with a diameter >200 µm) are the most likely to significantly obstruct pre-arterioles and, therefore, reduce myocardial blood flow at a tissue level [17,18].
In this review we will summarise the most important predictors of no-reflow related to distal embolisation, as well as available evidence concerning the most important nonpharmacological procedural strategies to prevent distal embolisation and, thus, no-reflow during PCI. As a result of the multifactorial physiopathology of no-reflow, multiple pharmacological agents specifically targeting different physiopathological pathways, such as antiplatelet and vasodilator drugs, have also been tested for prevention of no-reflow, especially in the setting of STEMI. However, a systematic and comprehensive review of the use of pharmacological agents to prevent no-reflow is beyond the scope of the present article.
Procedural Predictors of Distal Embolisation-Related No-Reflow
Several parameters have been shown to be able to predict no-reflow occurrence probably owing, at least in part, to their ability to predict distal embolisation during PCI.
Coronary angiography allows direct visualisation of luminal thrombus on culprit coronary stenosis. Pre-thrombectomy/pre-pPCI features of luminal thrombus as assessed with coronary angiography predict no-reflow occurrence in STEMI patients undergoing pPCI [19]. Interestingly, in STEMI patients undergoing mechanical thrombectomy as adjunct to standard pPCI, a high residual thrombus burden after thrombectomy has also been recently demonstrated to independently predict post-pPCI no-reflow occurrence [20]. Moreover, a reference lumen diameter bigger than 4 mm was an independent predictor of no-reflow in a study by Yip et al. [19]. In a recent large retrospective registry of acute coronary syndrome patients, PCI on bifurcation coronary lesions and PCI on complex coronary lesions as assessed with coronary angiography were both associated with higher no-reflow risk, probably due to a high risk of distal embolisation from coronary culprit plaque. However, both NSTEMI and STEMI patients were included in this registry [21]. Coronary angiography also allows prediction of distal embolisation and no-reflow during elective PCI performed on saphenous vein grafts. In a study by Liu et al., the presence of extensive graft disease, large plaque volume and presence of complicated/ulcerated plaque were all associated with higher risk of distal embolisation and periprocedural myocardial infarction in patients undergoing PCI in saphenous vein grafts [22]. A study by Sdringola et al. confirmed and further expanded these results by showing an increased risk of no-reflow/slow-flow in patients undergoing PCI in saphenous vein grafts in the presence of extensive graft disease and/or complicated/ulcerated plaque [23]. Coronary angiography represents the gold standard technique for the diagnosis of coronary stenosis. However, coronary angiography does not allow direct visualisation of coronary plaque and vessel walls. Intravascular ultrasound (IVUS) and optical coherence tomography (OCT) are catheter-based techniques that provide high-resolution cross-sectional images of the lumen and vessel wall, thus allowing assessment of plaque burden and of plaque morphological features. A recent study by Li et al. using IVUS demonstrated, in a series of 120 STEMI patients, that plaque area, plaque volume, presence of eccentric plaque, presence of soft/lipid-rich plaque and plaque rupture were all predictors of no-reflow [24]. Moreover, presence of uncalcified plaques associated with backward ultrasound attenuation seems to be associated with higher risk of no-reflow in STEMI patients [25,26]. Prediction of no-reflow with OCT is still controversial. In a little series from Tanaka et al. the presence of a thincap fibroatheroma (i.e., a plaque with lipid content in≥2 quadrants and the thinnest part of the fibrous cap measuring <65 µm) was found to be a predictor of no-reflow in NSTEMI patients undergoing urgent PCI [27]. This association was not confirmed in a more recent study by Ikenaga et al. in 39 STEMI patients. In this study the longitudinal length of the lipid pool was found to be the only OCT predictor of no-reflow [28]. Interestingly, IVUS and OCT data seem to be able to predict no-reflow occurence mainly due to an increased risk of distal embolisation from coronary plaque.
In a recent study by Carol et al. on STEMI patients, late clinical presentation (i.e., first medical contact >12 hours after symptom onset) was associated with a higher prevalence of old organised thrombus on pathological analysis after thrombus-aspiration during pPCI [29]. Organised thrombus is known to be an independent predictor of both in-hospital and long-term mortality in STEMI patients undergoing pPCI [30,31]. Moreover, organised thrombus, as compared with fresh thrombus, was associated with a higher risk of macroscopic distal embolisation during angiography and with a lower rate of complete ST segment resolution after pPCI in a recent study by Verouden et al. [32]. This could explain, at least in part, the high risk of no-reflow observed in late-presenting STEMI patients.
Interestingly, increased risk of severe ischaemic- and reperfusion-related injury could also contribute to the high risk of no-reflow observed in this subgroup of STEMI patients. Therefore, prompt referral for reperfusion with reduction of ischaemia time surely represents a key strategy to prevent no-reflow occurrence, reduce infarct size and allow myocardial salvage in STEMI patients.
Management of Distal Embolisation to Prevent No-Reflow
As seen before, distal embolisation from coronary thrombus or plaque has a key role in no-reflow physiopathology. Multiple nonpharmacological procedural strategies have been tested in a clinical setting in an effort to prevent distal embolisation and, thus, no-reflow.
Thrombectomy Devices
The use of manual or mechanical thrombectomy devices to reduce the risk of distal embolisation during pPCI has been investigated in several clinical trials. Manual thrombectomy is usually performed using dedicated catheters compatible with a 6 or 7 French guiding catheter on 0.014’’ guide-wires and allows direct retrieval of intraluminal thrombus (
Figure 1). Mechanical thrombectomy devices like Angiojet® use high-pressure backward saline jets to create a vacuum at the tip of the catheter to break up and remove thrombus.
In the REMEDIA trial thrombectomy with a simple a manual aspiration catheter in a pPCI setting was associated with reduced risk of both angiographic and ECG no-reflow and better post-pPCI myocardial perfusion as assessed with MCE [33,34]. A larger clinical trial, the TAPAS trial, randomly assigned 1071 STEMI patients to either standard PCI or PCI with manual thrombectomy. The TAPAS trial confirmed data from the REMEDIA trial by showing a reduction in angiographic no-reflow occurrence in patients undergoing manual thrombectomy during pPCI. This trial further expanded REMEDIA trial results by showing a significant reduction in 1-year cardiovascular mortality in patients treated by use of manual thrombus aspiration [35]. However, in a more recent clinical trial, by Lagerqvist et al., that randomised 7244 STEMI patients to manual thrombectomy followed by pPCI or pPCI alone, thrombus aspiration was not associated with a significant reduction in overall 1-year mortality. Furthermore, thrombus aspiration did not significantly reduce the 1-year rate of a composite of death from any cause, rehospitalisation for myocardial infarction and stent thrombosis [36]. Moreover, 5-year follow-up data from a real-world, large-scale clinical registry, the KREDO-Kyoto AMI registry, were recently published and showed that thrombus aspiration was not able to significantly reduce 5-year mortality in STEMI patients undergoing pPCI [37]. The INFUSE-AMI trial is a recent 2×2 factorial design trial including 452 STEMI patients referred for pPCI who were randomly assigned to either intracoronary abciximab or no abciximab and to either manual aspiration thrombectomy as adjunct to standard pPCI or standard pPCI alone. Thrombus aspiration was not associated with a significant reduction in 30-day infarct size as assessed with CMR [38]. Of note, in more recent studies, the widespread use of bivalirudin and glycoprotein IIb/IIIa inhibitors could have diluted the beneficial effect of manual thrombectomy on long-term clinical outcome. At present, clinical evidence seems to support the use of manual thrombectomy to prevent no-reflow occurrence even if benefit on mortality/long-term clinical outcome remains unclear. Therefore, manual thrombectomy is still a class IIa recommendation in 2012 European Society of Cardiology (ESC) guidelines in the setting of pPCI and may be proposed to STEMI patients with a high angiographic thrombus burden [39].
Data on the use of mechanical thrombectomy devices as adjunct to standard PCI are still conflicting. In a study by Ali et al., 480 STEMI patients were randomly assigned to mechanical thrombectomy as adjunct to PCI or standard PCI alone. Despite effective thrombus removal, mechanical thrombectomy was not associated with improved ECG reperfusion outcomes. Moreover, no benefits on 30-day clinical outcome were observed in patients undergoing mechanical thrombectomy [40]. However, in the JETSTENT trial by Migliorini et al., rheolytic thrombectomy was associated with an increased rate of complete resolution of ST-segment elevation and better 6-month and 1-year clinical outcomes [41]. Even results of studies directly comparing manual and mechanical thrombectomy are not univocal. In a study by Parodi et al., 80 STEMI patients were randomly assigned to either manual or mechanical thrombectomy. Mechanical thrombectomy was associated with significantly better post-pPCI reperfusion [42]. However, in the recent COCOTH study by Giglioli et al., randomising 185 STEMI patients to either manual or mechanical thrombectomy as adjunct to standard PCI, no differences in both ECG and angiographic reperfusion outcomes were observed between the two study groups [43]. In summary, the inconclusive results of these studies do not support routine use of the more expensive mechanical thrombectomy devices. However, mechanical thrombectomy devices could represent a valid therapeutic option in selected cases, such as manual thrombectomy device failure or massive intracoronary thrombosis.
Distal Protection Devices
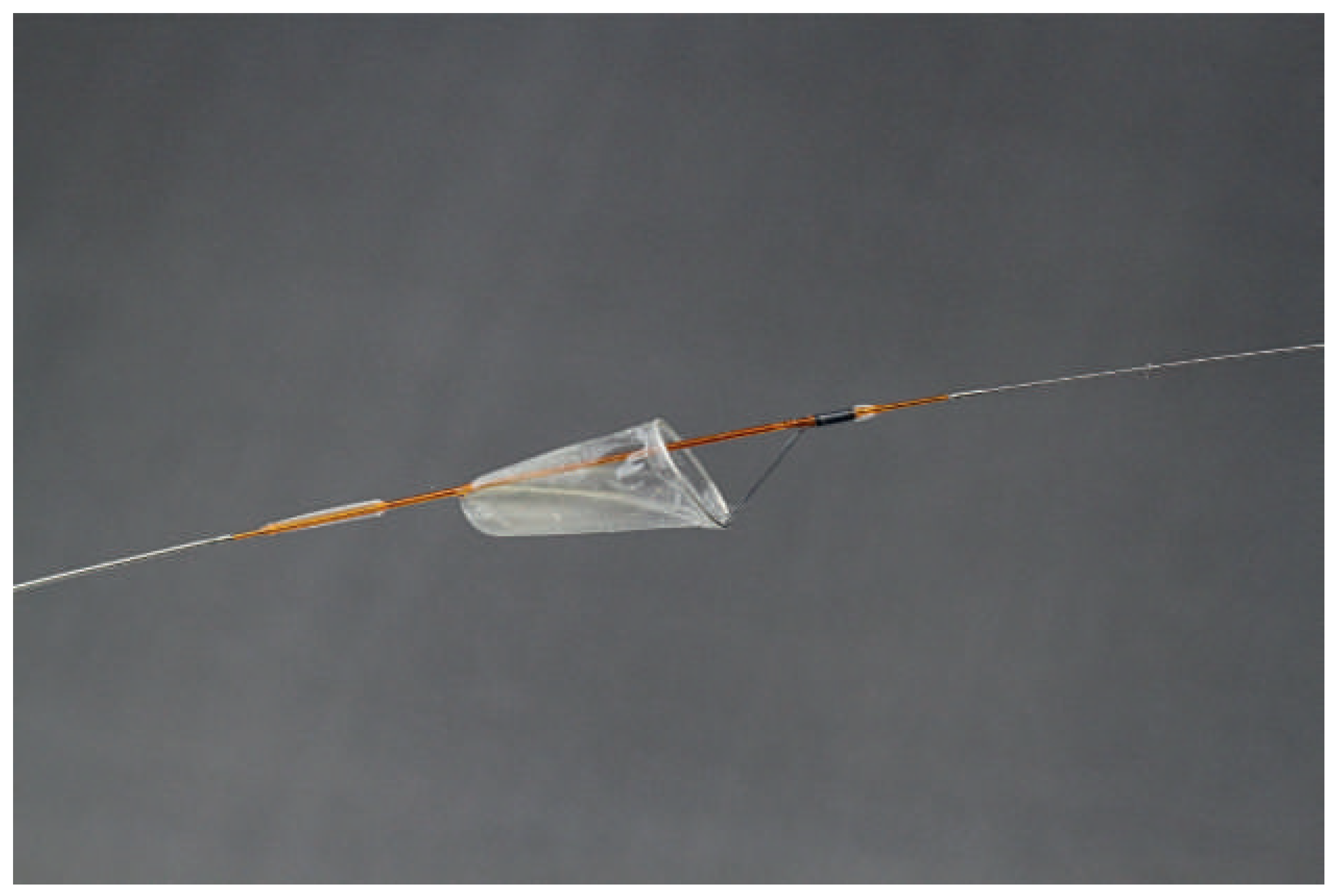
Distal protection devices used in the setting of PCI essentially consist of a filter device placed between the target lesion and the distal vasculature (
Figure 2). Filter-based distal protection devices allow blood flow during PCI and prevent distal migration of microparticles whose diameter is greater than pore size (usually 100–150 μm). Distal protection devices have not been shown to reduce no-reflow occurrence and improve prognosis in STEMI patients treated with pPCI in two large scale clinical trials, the EMERALD trial and the DEDICATION trial [44,45]. Therefore they have a class III recommendation in 2012 ESC guidelines in this setting [39].
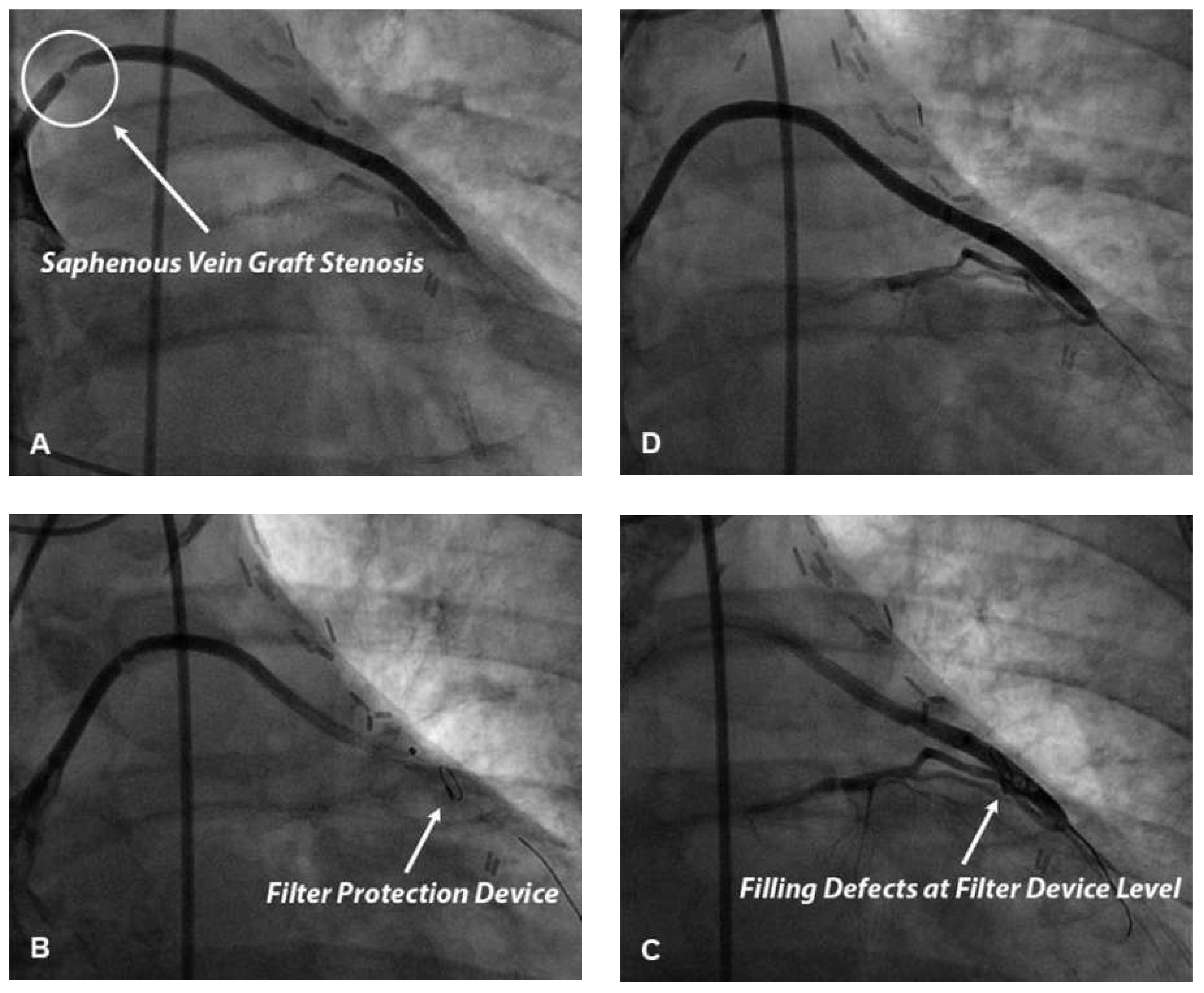
The use of filter distal protection devices has been demonstrated to reduce the occurrence of both no-reflow and periprocedural myocardial infarction in elective PCI performed in saphenous vein grafts (
Figure 3) [46]. Distal embolisation during elective PCI in saphenous vein grafts, as opposed to native coronary arteries, is more likely to be due to large necrotic and/or lipid emboli essentially originating from vessel wall plaque. Differences in amount and/or composition of embolised material could explain, at least in part, differences in results of clinical trials investigating the use of distal protection devices in pPCI and elective saphenous vein graft PCI. In this setting distal balloon occlusion devices and proximal occlusion devices seem to be as effective as filter devices in preventing both no-reflow and periprocedural myocardial infarction and, therefore, represent a valid alternative to a filter device [47,48].
Excimer Coronary Laser
The excimer coronary laser involves a laser beam in the field of ultraviolet (308 nm). The absorption depth is of 0.05 mm, thus allowing a very short space of action. Current laser catheters may be concentric or eccentric according to the laser beam orientation, and may vary in size: concentric catheters have a diameter of 0.9, 1.4, 1.7 or 2 mm whereas eccentric catheters have a diameter of 1.7 or 2 mm. Finally, energy production may vary as the smaller laser catheter is also the most powerful, producing a beam of 80 mJ/mm2/80 hertz. The use of excimer coronary lasers in STEMI patients undergoing pPCI could be of interest for many reasons: rapid removal of thrombus with vaporisation of procoagulant reactants, reduction of distal embolisation risk and debulking of underlying plaque. In the CARMEL registry 151 high-risk STEMI patients were enrolled, including patients with cardiogenic shock, rescue PCI patients and patients presenting with degenerated saphenous vein grafts and/or complex lesion morphology. The use of an excimer coronary laser was associated with high procedural and device success rates, a low complication rate and a significant increase in TIMI flow grade. Maximal laser gain was achieved in lesions with a high thrombus burden [49]. A registry by Dave et al. confirmed these positive results by showing a significant increase in MBG, and high angiographic and ECG reperfusion rates in STEMI patients treated with and excimer coronary laser [50]. Despite these encouraging results, data from large clinical trials supporting the use of excimer coronary lasers to prevent no-reflow occurrence during pPCI are still lacking. One small randomised study by Dorr et al., in a population of 27 STEMI patients, showed a shorter TIMI frame count in patients treated with an excimer coronary laser as compared with a conventional treatment group, treated with balloon angioplasty and stent implantation alone [51]. The LASER-AMI trial, which is now on-going, compares use of the excimer coronary laser with manual thrombus aspiration in STEMI patients undergoing pPCI to prevent ECG no-reflow occurrence. Results from this trial should better clarify the role of the excimer coronary laser in this clinical setting [52].
STENTYS® Self-Apposing Stent
The STENTYS® Self-Apposing stent is a self-expanding nitinol stent. STENTYS® is compatible with 6 French guiding catheters and is delivered using a rapid-exchange delivery system over a conventional 0.014” guide-wire. STENTYS® is available in a bare metal version and in a drug-eluting version eluting paclitaxel, and gradually conforms to vessel wall shape. The use of a self-expanding stent in the setting of pPCI could be of interest for many reasons. First of all, in STEMI patients, thrombus dissolution behind the stent struts and progressive vessel wall relaxation after vasoconstriction which characterise the acute phase of STEMI could lead to stent undersizing and, therefore, to incomplete stent apposition. Previous studies demonstrated that acute and late incomplete stent apposition play a key role in the pathogenesis of acute and late / very late stent thrombosis [53–55]. STENTYS® is characterised by a progressive increase in stent diameter, if unconstrained. The use of STENTYS® in the pPCI setting could, therefore, reduce the risk of both incomplete stent apposition and stent thrombosis. Concerning no-reflow occurrence, aggressive stent deployment could lead to plaque disruption and distal embolisation from culprit coronary plaques. The ability of STENTYS® to grow in volume within the first hours to days after pPCI allows more gentle stent deployment (with lower stent balloon inflation pressures), which could lead to a reduced risk of plaque disruption or thrombus dislodgement and, therefore, of distal embolisation related no-reflow. At present, studies specifically focusing on no-reflow occurrence following STENTYS® implantation in pPCI setting are missing. Thus, future studies comparing risk of distal embolisation and no-reflow in STEMI patients undergoing self-expanding stent implantation or conventional balloon-expandable stent implantation during pPCI are needed. The APPOSITION V trial is now on-going and will be the first randomised trial powered on clinical endpoints and directly comparing bare metal STENTYS® with a conventional balloon-expandable stent (MULTI-LINK®) in patients presenting with STEMI undergoing pPCI [56]. At present, STENTYS® could represent a valid therapeutic option for STEMI patients undergoing pPCI, especially in the presence of specific anatomical subsets in which good stent apposition is unlikely to be achieved with conventional balloon-expandable stents.
MGuard® and MGuard Prime® Stents
MGuard® (stainless steel) and MGuard Prime® (cobalt chromium) stents are bare metal stents equipped with a bio-stable mesh woven from a single strand of 20 µm of polyethylene terephthalate, called MicroNet®, with pore size between 150 and 180 µm. MicroNet® has been developed to trap and seal thrombus and plaque against the vessel wall, thus potentially preventing distal embolisation and no-reflow. In the MASTER trial, 433 STEMI patients presenting within 12 hours from symptom onset and referred for pPCI were randomly assigned to receive a MGuard® stent or a conventional bare metal / drug-eluting stent. Patients treated with MGuard® stent experienced a higher rate of complete ST-segment elevation resolution and a lower rate of angiographic no-reflow. However, 30-day clinical outcome did not differ between the study groups [57]. Future studies are needed to better clarify the role of MicroNet® technology in the setting of pPCI and its eventual benefits on long-term clinical outcome. Development of drug-eluting versions of these stents is on-going.
Direct Stenting
Specific stent deployment techniques could also allow reduction of no-reflow occurrence in STEMI patients throughout a reduction of distal embolisation occurrence. Direct stenting represents the deployment of an intracoronary stent without balloon predilatation. A trial by Loubeyre et al. compared direct stenting with balloon predilatation in 206 STEMI patients treated with pPCI. Direct stenting was associated with a decreased rate of angiographic slow-flow/no-reflow and an increased rate of complete ST-segment elevation resolution as compared with predilatation with an angioplasty balloon [58]. Two recent meta-analyses confirmed the benefits of direct stenting during pPCI on post-reperfusion myocardial microvascular blood flow. In these meta-analyses direct stenting was also associated with a significant reduction in short-term and 1-year mortality, although these data were mostly derived retrospectively from small clinical registries rather than randomised controlled trials [59,60].
In conclusion, available evidence supports direct stenting to prevent no-reflow in STEMI patients. However, only a specific subset of patients (those with optimal distal visualisation of the IRA after guidewire passage) is suitable for this technique in order to avoid stent undersizing.
Deferred Stenting
The DEFER-STEMI trial recently compared deferred stenting (intention-to-stent 4 to 6 hours after balloon angioplasty) with immediate stenting for no-reflow prevention in pPCI. A total of 101 STEMI patients were enrolled. Deferred stenting was significantly associated with lower no-reflow/slow-reflow rates and an increased myocardial salvage index at 6 months, but also with a potentially increased risk of recurrent STEMI [61]. Results from the DEFER-STEMI trial are provocative, but clinical benefits on both no-reflow occurrence and prognosis in STEMI patients as well as the safety of deferred stenting (risk of bail-out stenting) should be confirmed in trials on larger populations.
Conclusions
Distal atherothrombotic coronary embolisation plays a key role in no-reflow physiopathology and multiple nonpharmacological procedural strategies have been tested in clinical practice in order to prevent distal embolisation and, thus, reduce no-reflow occurrence. In the setting of pPCI direct stenting should be preferred when feasible and when there is confidence about the real vessel size. Manual thrombectomy represents a valid therapeutic option in STEMI patients presenting with a high angiographic thrombus burden, even if benefit on mortality/long-term clinical outcome remains unclear. Distal filter protection devices have been demonstrated to reduce both no-reflow and periprocedural myocardial infarction occurrence in elective PCI performed in saphenous vein grafts, and their use should be encouraged in this setting. Future studies are needed to better clarify the role of excimer coronary lasers in pPCI. STENTYS® and MGuard®/McGuard Prime® stents could provide protection against distal embolisation and no-reflow owing to their peculiar mechanical properties. However, future studies are needed in order to better evaluate potential benefits on long-term clinical outcome and, therefore, clarify their role in the setting of STEMI. Finally, concerning deferred stenting, results of the DEFER-STEMI trial are provocative, but need to be confirmed in larger clinical trials specifically addressing safety issues.
Disclosures Statement
No financial support and no other potential conflict of interest relevant to this article was reported.
Abbreviations and Acronyms
| CMR | cardiac magnetic resonance |
| ECG | electrocardiogram |
| IRA | infarct-related artery |
| IVUS | intravascular ultrasound |
| MBG | myocardial blush grade |
| MCE | myocardial contrast echocardiography |
| MVO | microvascular obstruction |
| OCT | optical coherence tomography |
| PCI | percutaneous coronary intervention |
| STEMI | ST-segment elevation myocardial infarction |
| TIMI | Thrombolysis in Myocardial Infarction |