Outcome After Simultaneous PCI and Left Atrial Appendage Occlusion
Abstract
Background
Methods
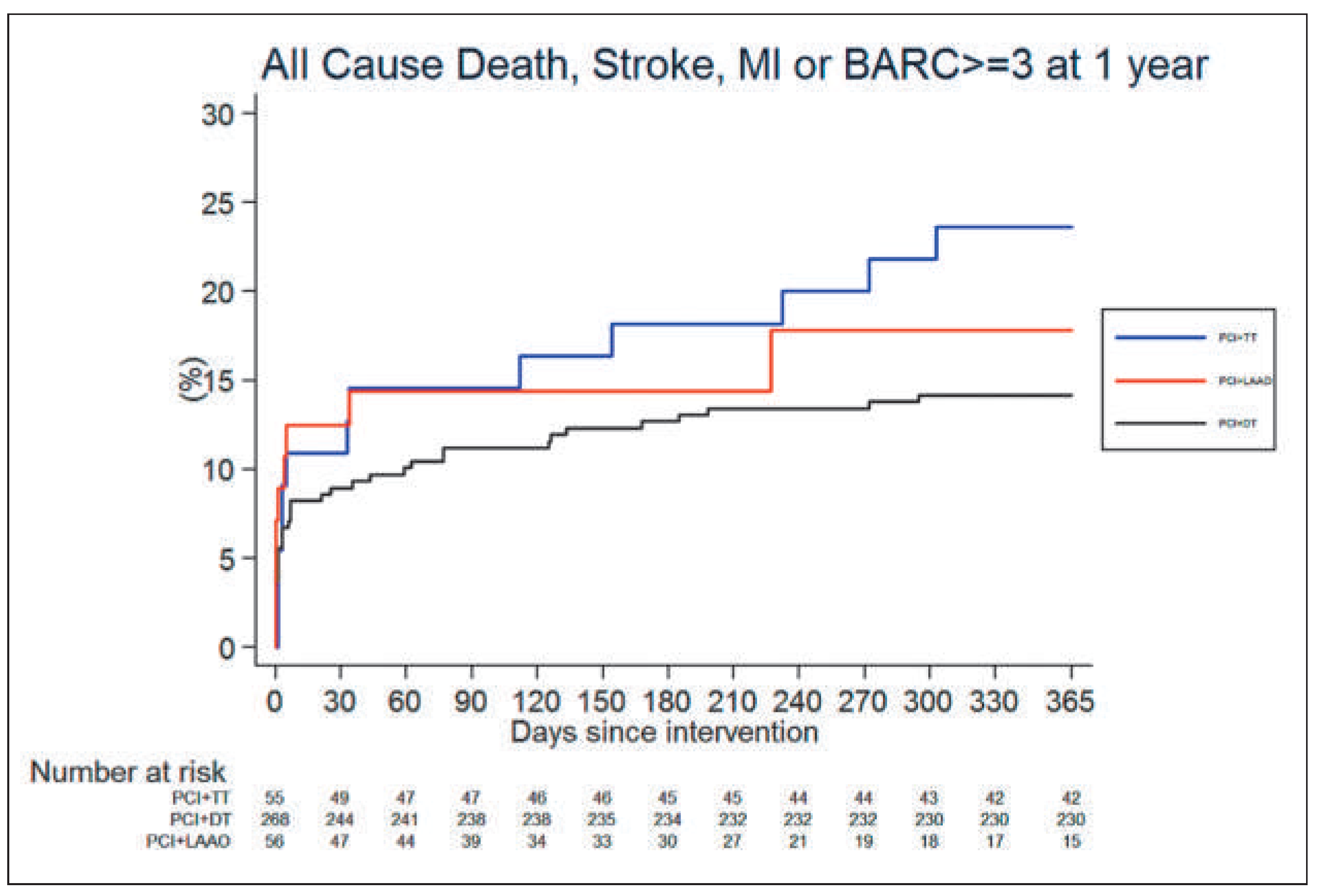
Results
Discussion
Authors’ Contribution
Funding / potential competing interests
References
- Budaj, A.; Flasinska, K.; Gore, J.M.; Anderson FAJr Dabbous, O.H.; Spencer, F.A.; et al. Magnitude of and risk factors for in-hospital and postdischarge stroke in patients with acute coronary syndromes: findings from a Global Registry of Acute Coronary Events. Circulation. 2005, 111, 3242–3247. [Google Scholar] [CrossRef]
- Wyse, D.G.; Waldo, A.L.; DiMarco, J.P.; Domanski, M.J.; Rosenberg, Y.; Schron, E.B.; et al. A comparison of rate control and rhythm control in patients with atrial fibrillation. N Engl J Med. 2002, 347, 1825–1833. [Google Scholar] [PubMed]
- Fox, K.A.A.; Eagle, K.A.; Gore, J.M.; Steg, P.G.; Anderson, F.A; GRACE and GRACE2 Investigators. The Global Registry of Acute Coronary Events 1999 to 2009 –, G.R.A.C.E. Heart. 2010, 96, 1095–1101. [Google Scholar] [CrossRef]
- Pilgrim, T.; Kalesan, B.; Zanchin, T.; Pulver, C.; Jung, S.; Mattle, H.; et al. Impact of atrial fibrillation on clinical outcomes among patients with coronary artery disease undergoing revascularisation with drug-eluting stents. EuroIntervention. 2013, 8, 1061–1071. [Google Scholar] [CrossRef] [PubMed]
- Lamberts, M.; Olesen, J.B.; Ruwald, M.H.; Hansen, C.M.; Karasoy, D.; Kristensen, S.L.; et al. Bleeding after initiation of multiple antithrombotic drugs, including triple therapy, in atrial fibrillation patients following myocardial infarction and coronary intervention: a nationwide cohort study. Circulation. 2012, 126, 1185–1193. [Google Scholar] [CrossRef] [PubMed]
- Lamberts, M.; Gislason, G.H.; Lip, G.Y.; Lassen, J.F.; Bjerring Olesen, J.; Mikkelsen, A.P.; et al. Antiplatelet therapy for stable coronary artery disease in atrial fibrillation patients on oral anticoagulant: A nationwide cohort study. J Am Coll Cardiol. 2012, 59, E512–E512. [Google Scholar] [CrossRef]
- Dewilde, W.J.; Oirbans, T.; Verheugt, F.W.; Kelder, J.C.; De Smet, B.J.; Herrman, J.P.; et al. Use of clopidogrel with or without aspirin in patients taking oral anticoagulant therapy and undergoing percutaneous coronary intervention: an open-label, randomised, controlled trial. Lancet. 2013, 381, 1107–1115. [Google Scholar] [CrossRef]
- Holmes DRJr Schwartz, R.S. Leh atrial appendage occlusion eliminates the need for warfarin. Circulation. 2009, 120, 1919–1926. [Google Scholar] [CrossRef]
- Nietlispach, F.; Gloekler, S.; Krause, R.; Shakir, S.; Schmid, M.; Khattab, A.A.; et al. Amplatzer left atrial appendage occlusion: single center 10–year experience. Catheter Cardiovasc Interv. 2013, 82, 283–289. [Google Scholar] [CrossRef] [PubMed]
- Reddy, V.Y.; Sievert, H.; Halperin, J.; Doshi, S.; Buchbinder, M.; et al. Percutaneous left atrial appendage closure vs Warfarin for atrial fibrillation – a randomized clinical trial. JAMA. 2014, 312, 1988–1998. [Google Scholar] [CrossRef] [PubMed]
- Mehran, R.; Rao, S.V.; Bhatt, D.L.; Gibson, C.M.; Caixeta, A.; Eikelboom, J.; et al. Standardized bleeding definitions for cardiovascular clinical trials: a consensus report from the Bleeding Academic Research Consortium. Circulation. 2011, 123, 2736–2747. [Google Scholar] [CrossRef] [PubMed]
| Baseline characteristics | PCI + LAAO | PCI + DT | PCI + TT | p-value |
|---|---|---|---|---|
| N = 379 | N = 56 | N = 268 | N = 55 | |
| Age years (SD) | 76.4 ± 7.2 | 72.0 ± 9.1 | 73.1 ± 8.1 | 0.003 |
| Sex, male n (%) | 14 (25.0%) | 68 (25.4%) | 18 (32.7%) | 0.513 |
| BMI mean kg/m2 (SD) | 27.7 ± 4.6 | 27.0 ± 4.5 | 28.7 ± 5.4 | 0.086 |
| Cardiovascular risk factors | ||||
| Hypertension n (%) | 52 (92.9%) | 171 (63.8%) | 42 (76.4%) | <0.001 |
| Diabetes mellitus n (%) | 18 (32.1%) | 52 (19.4%) | 16 (29.1%) | 0.055 |
| Clinical features | ||||
| Renal failure (creatinine ≥200 μmol/l) n (%) | 7 (12.7%) | 4 (1.7%) | 1 (2.0%) | <0.001 |
| Congestive heart failure | 9 (21.4%) | 110 (41.0%) | 26 (47.3%) | 0.025 |
| Prior stroke or TIA | 13 (31.0%) | 26 (9.7%) | 9 (16.4%) | 0.001 |
| LV ejection fraction mean (SD) | 54.3 ± 11.7 | 50.7 ± 12.6 | 48.4 ± 14.3 | 0.082 |
| CHA2DS2 | 2.9 ± 1.3 | 1.9 ± 1.2 | 2.3 ± 1.2 | <0.001 |
| CHA2DS2-vasc score | 3.5 ± 2.2 | 3.6 ± 1.3 | 4.2 ± 1.3 | 0.030 |
| Clinical Outcome | All patients N = 379 | PCI+LAAO N = 56 | PCI+DT N = 268 | PCI+TT N = 55 |
|---|---|---|---|---|
| 30-day follow-up | ||||
| Death n (%) | 12 (3.2) | 0 (0.0) | 7 (2.6) | 5 (9.1) |
| Cardiac events | ||||
| Cardiac death n (%) | 9 (2.4) | 0 (0.0) | 5 (1.9) | 4 (7.4) |
| Myocardial infarction (MI) n (%) | 10 (2.7) | 0 (0.0) | 10 (3.7) | 0 (0.0) |
| – Q-wave MI n (%) | 5 (1.3) | 0 (0.0) | 5 (1.9) | 0 (0.0) |
| – Non Q-wave MI n (%) | 5 (1.3) | 0 (0.0) | 5 (1.9) | 0 (0.0) |
| Neurologic events | ||||
| Ischaemic stroke | 4 (1.1) | 2 (3.6) | 2 (0.7) | 0 (0.0) |
| TIA | 1 (0.3) | 0 (0.0) | 1 (0.4) | 0 (0.0) |
| Unclear neurologic event | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Bleeding Events | ||||
| BARC 3a | 12 (3.2) | 4 (7.1) | 6 (2.2) | 2 (3.6) |
| BARC 3b | 4 (1.1) | 1 (1.8) | 3 (1.1) | 0 (0.0) |
| BARC 3c | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| BARC 4 | 1 (0.3) | 0 (0.0) | 1 (0.4) | 0 (0.0) |
| BARC 5a | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| BARC 5b | 2 (0.5) | 0 (0.0) | 1 (0.4) | 1 (1.9) |
| Composite Outcomes | ||||
| Death / MI / ischaemic stroke | 24 (6.3) | 2 (3.6) | 17 (6.3) | 5 (9.1) |
| Cardiac death / MI / ischaemic stroke | 21 (5.6) | 2 (3.6) | 15 (5.6) | 4 (7.4) |
| Death / MI / ischaemic stroke / BARC bleeding 3–5 | 37 (9.8) | 7 (12.5) | 24 (9.0) | 6 (10.9) |
| Cardiac death / MI/ ischaemic stroke/ BARC bleeding 3–5 | 34 (9.0) | 7 (12.5) | 22 (8.2) | 5 (9.2) |
| 1-year follow-up | ||||
| Death n (%) | 30(8.2) | 2 (6.3) | 18 (6.7) | 10 (18.2) |
| Cardiac events | ||||
| Cardiac death n (%) | 17 (4.7) | 1 (1.9) | 9 (3.4) | 7 (13.0) |
| Myocardial infarction (MI) n (%) | 13 (3.5) | 1 (3.4) | 11 (4.1) | 1 (2.0) |
| – Q-wave MI n (%) | 5 (1.3) | 0 (0.0) | 5 (1.9) | 0 (0.0) |
| – Non Q-wave MI n (%) | 8 (2.2) | 1 (3.4) | 6 (2.3) | 1 (2.0) |
| Neurologic events | ||||
| Ischaemic stroke | 7 (1.9) | 2 (3.6) | 5 (1.9) | 0 (0.0) |
| TIA | 1 (0.3) | 0 (0.0) | 1 (0.4) | 0 (0.0) |
| Unclear neurologic event | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Bleeding Events | ||||
| BARC 3a | 13 (3.5) | 4 (7.1) | 7 (2.6) | 2 (3.6) |
| BARC 3b | 6 (1.6) | 1 (1.8) | 4 (1.5) | 1 (2.0) |
| BARC 3c | 1 (0.3) | 0 (0.0) | 1 (0.4) | 0 (0.0) |
| BARC 4 | 1 (0.3) | 0 (0.0) | 1 (0.4) | 0 (0.0) |
| BARC 5a | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| BARC 5b | 2 (0.5) | 0 (0.0) | 1 (0.4) | 1 (1.9) |
| Composite outcomes | ||||
| Death / MI / ischaemic stroke | 46 (12.5) | 5 (8.9) | 30 (11.2) | 11 (20.0) |
| Cardiac death / MI / ischaemic stroke | 35(9.5) | 4 (7.1) | 23 (8.6) | 8 (14.9) |
| Death / MI / Ischaemic stroke / BARC bleeding 3–5 | 60 (16.1) | 9 (16.1) | 38 (14.2) | 13 (23.6) |
| Cardiac death / MI / ischaemic stroke / BARC bleeding 3–5 | 50 (13.5) | 9 (16.1) | 31 (11.6) | 10 (18.5) |
| Cox regression with reference group as PCI + DT | Age-adjusted analysis | ||
|---|---|---|---|
| PCI + LAAO | PCI + TT | ||
| HR (95%CI) p-value | HR (95%CI) | p-value | |
| 30-day follow-up | |||
| Death | n.e | 3.81 (1.20–12.12) | 0.023 |
| Cardiac death | n.e | 4.05 (1.08–15.14) | 0.037 |
| Myocardial infarction | n.e | n.e. | |
| Ischaemic stroke or TIA | 2.86 (0.46–17.94) 0.262 | n.e. | |
| Ischaemic stroke or MI | 089 (0.19–4.11) 0.878 | n.e. | |
| BARC bleeding ≥3 | 1.92 (0.66–5.61) 0.234 | 1.31 (0.37–4.70) | 0.678 |
| Death / MI / ischaemic stroke | 0.48 (0.11–2.12) 0.336 | 1.40 (0.52–3.80 | 0.507 |
| Cardiac death / MI / ischaemic stroke | 0.59 (0.13–2.63) 0.493 | 1.28 (0.42–3.85) | 0.664 |
| Death / MI / ischaemic stroke / BARC bleeding 3–5 | 1.27 (0.54–2.99) 0.578 | 1.19 (0.49–2.92) | 0.697 |
| Cardiac death / MI / ischaemic stroke / BARC bleeding 3–5 | 1.45 (0.61–3.45) 0.397 | 1.16 (0.44–3.07) | 0.761 |
| 1-year follow-up | |||
| Death n (%) | 0.51 80.12–2.209 0.363 | 2.89 (1.33–6.26) | 0.007 |
| Cardiac death n (%) | 0.49 (0.06–3.91) 0.499 | 3.98 (1.48–10.70) | 0.006 |
| Myocardial infarction n (%) | 0.50 (0.06–4.00) 0.518 | 0.46 (0.06–3.55) | 0.454 |
| Ischaemic stroke or TIA | 1.58 (0.31–8.10) 0.584 | n.e. | |
| Ischaemic stroke or MI | 1.09 (0.31–3.86) 0.894 | 0.33 (0.04–2.50) | 0.283 |
| BARC bleeding ≥3 | 1.48 (0.53–4.16) 0.455 | 1.39 (0.46–4.23) | 0.559 |
| Death / MI / ischaemic stroke | 0.84 (0.32–2.18) 0.714 | 1.80 (0.90–3.60) | 0.095 |
| Cardiac death / MI / ischaemic stroke | 0.88 (0.30–2.60) 0.824 | 1.70 (0.76–3.81) | 0.194 |
| Death / MI / ischaemic stroke / BARC bleeding 3–5 | 1.17 (0.56–2.45) 0.671 | 1.68 (0.89–3.15) | 0.107 |
| Cardiac death / MI / ischaemic stroke / BARC bleeding 3–5 | 1.43 (0.67–3.05) 0.349 | 1.72 (0.84–3.50) | 0.138 |
| Cox regression with reference group as PCI + DT | Baseline characteristics-adjusted analysis* | |||
| PCI + LAAO | PCI + TT | |||
| HR (95%CI) | p-value | HR (95%CI) | p-value | |
| 30-day follow-up | ||||
| Death | n.e | 4.47 (1.31–15.26) | 0.017 | |
| Cardiac death | n.e | 4.93 (1.24–19.69) | 0.024 | |
| Myocardial infarction | n.e | n.e. | ||
| Ischaemic stroke or TIA | 1.71 (0.21–14.11) | 0.621 | n.e. | |
| Ischaemic stroke or MI | 0.89 (0.18–4.27) | 0.883 | n.e. | |
| BARC bleeding ≥3 | 2.12 (0.71–6.30) | 0.178 | 1.21 (0.33–4.37) | 0.772 |
| Death / MI / ischaemic stroke | 0.55 (0.12–2.42) | 0.427 | 1.30 (0.47–3.54) | 0.614 |
| Cardiac death / MI / ischaemic stroke | 0.65 (0.14–2.90) | 0.571 | 1.18 (0.39–3.59) | 0.770 |
| Death / MI / ischaemic stroke / BARC bleeding 3–5 | 1.44 (0.61–3.42) | 0.404 | 1.10 (0.45–2.70) | 0.840 |
| Cardiac death / MI / ischaemic stroke / BARC bleeding 3–5 | 1.61 (0.67–3.86) | 0.3285 | 1.00 (0.38–2.66) | 0.995 |
| 1-year follow-up | ||||
| Death n (%) | 0.52 (0.11–2.37) | 0.399 | 2.93 (1.33–6.45) | 0.008 |
| Cardiac death n (%) | 0.40 (0.05–3.53) | 0.409 | 4.33 (1.57–11.95) | 0.005 |
| Myocardial infarction n (%) | 0.54 (0.07–4.28) | 0.556 | 0.43 (0.05–3.34) | 0.417 |
| Ischaemic stroke or TIA | 1.33 (0.22–7.98) | 0.754 | n.e. | |
| Ischaemic stroke or MI | 1.17 (0.33–4.22) | 0.806 | 0.29 (0.04–2.19) | 0.228 |
| BARC bleeding ≥3 | 1.52 (0.53–4.39) | 0.435 | 1.35 (0.44–4.14) | 0.600 |
| Death / MI / ischaemic stroke | 0.96 (0.37–2.54) | 0.941 | 1.68 (0.83–3.37) | 0.147 |
| Cardiac death / MI / ischaemic stroke | 0.99 (0.33–2.92) | 0.979 | 1.60 (0.71–3.60) | 0.258 |
| Death / MI / ischaemic stroke / BARC bleeding 3–5 | 1.32 (0.63–2.79) | 0.460 | 1.57 (0.83–2.97) | 0.164 |
| Cardiac death / MI / ischaemic stroke / BARC bleeding 3–5 | 1.57 (0.73–3.37) | 0.250 | 1.49 (0.73–3.06) | 0.274 |
© 2015 by the author. Attribution - Non-Commercial - NoDerivatives 4.0.
Share and Cite
Körmendy, D.; Pilgrim, T.; Pulver, C.; Shakir, S.; Zanchin, T.; Gloekler, S.; Nietlispach, F.; Rat-Wirtzler, J.; Moschovitis, A.; Khattab, A.A.; et al. Outcome After Simultaneous PCI and Left Atrial Appendage Occlusion. Cardiovasc. Med. 2015, 18, 96. https://doi.org/10.4414/cvm.2015.00300
Körmendy D, Pilgrim T, Pulver C, Shakir S, Zanchin T, Gloekler S, Nietlispach F, Rat-Wirtzler J, Moschovitis A, Khattab AA, et al. Outcome After Simultaneous PCI and Left Atrial Appendage Occlusion. Cardiovascular Medicine. 2015; 18(3):96. https://doi.org/10.4414/cvm.2015.00300
Chicago/Turabian StyleKörmendy, Dezsö, Thomas Pilgrim, Cédric Pulver, Samera Shakir, Thomas Zanchin, Steffen Gloekler, Fabian Nietlispach, Julie Rat-Wirtzler, Aris Moschovitis, Ahmed A. Khattab, and et al. 2015. "Outcome After Simultaneous PCI and Left Atrial Appendage Occlusion" Cardiovascular Medicine 18, no. 3: 96. https://doi.org/10.4414/cvm.2015.00300
APA StyleKörmendy, D., Pilgrim, T., Pulver, C., Shakir, S., Zanchin, T., Gloekler, S., Nietlispach, F., Rat-Wirtzler, J., Moschovitis, A., Khattab, A. A., Stortecky, S., Büllesfeld, L., Räber, L., Wenaweser, P., Windecker, S., & Meier, B. (2015). Outcome After Simultaneous PCI and Left Atrial Appendage Occlusion. Cardiovascular Medicine, 18(3), 96. https://doi.org/10.4414/cvm.2015.00300




