Abstract
Imaging of myocardial perfusion with SPECT, SPECT/CT and PET/CT is widely used for the detection of flow-limiting epicardial lesions and risk stratification of patients with suspected or known CAD. While regional reductions in radiotracer uptake during stress as compared to rest identify flow-limiting effects of the most advanced focal epicardial lesions, the haemodynamic significance of less severe obstructive CAD lesions in multivessel disease or the presence of subclinical and nonobstructive CAD may be missed. The concurrent ability of PET/CT to determine regional myocardial blood flow (MBF) in ml/g/min at rest and during pharmacologically induced hyperaemic flows allows the calculation of the myocardial flow reserve (MFR). Adding the hyperaemic MBF and MFR to the conventional visual analysis of myocardial perfusion (1.) signifies reductions in coronary vasodilator capacity, as functional precursor of the CAD process, and determines its response to preventive medical intervention, (2.) provides important prognostic information in subclinical – and clinically manifest CAD, as well as in cardiomyopathy, (3.) improves the identification and characterisation of the extent and severity of CAD burden; and (4.) contributes to denote the flow-limiting effect of single lesions in multivessel CAD. The diagnostic scope of PET/CT, however, extends beyond myocardial flow to the identification of hibernating stunning myocardium in ischaemic cardiomyopathy, cardiac sarcoid involvement, and inflammatory coronary plaque burden. It is anticipated that with the advent of PET/MRI (magnetic resonance imaging) further advances and refinement in the comprehensive assessment of cardiovascular pathology will ensue.
Introduction
An article form the series “Atherosclerosis and inflammation”
Myocardial perfusion scintigraphic imaging (MPS) with SPECT, SPECT/CT and PET/CT is the mainstay for the detection of flow-limiting epicardial lesions and risk stratification of patients with suspected or known CAD in clinical practice [1–3]. Regional reductions in radiotracer uptake during stress as compared to rest commonly signify flow-limiting effects of the most advanced focal epicardial lesions, while the haemodynamic significance of less severe obstructive CAD lesions in multivessel disease and/or the presence of subclinical and non-obstructive CAD may not be detected. The concurrent ability of PET/ CT to determine regional myocardial blood flow (MBF) in ml/g/min during different forms of vasomotor stress and at rest enables the calculation of the myocardial flow reserve (MFR). Adding the hyperaemic MBF and MFR to the conventional visual analysis of myocardial perfusion (1.) signifies reductions in coronary vasodilator capacity, as functional precursor of the CAD process, and determines its response to preventive medical intervention, (2.) provides important prognostic information in subclinical and clinically manifest CAD as well as in cardiomyopathy, (3.) improves the identification and characterisation of the extent and severity of CAD burden, and (4.) contributes to denote the flow-limiting effect of single lesions in multivessel CAD [1, 4, 5]. The diagnostic scope of PET/CT, however, expands beyond myocardial flow to the identification of hibernating stunning myocardium in ischaemic cardiomyopathy as described in depth more recently [6], cardiac sarcoid involvement, and inflammatory coronary plaque burden. This review aims to summarise the contributions of cardiac PET and PET/CT in the identification and characterisation of subclinical or clinically manifest CAD with its diagnostic and prognostic implications, its potential influence on the clinical decision-making process in CAD patients, evolving role in the detection of cardiac sarcoid involvement as well as “vulnerable” coronary plaque burden.
Perfusion and flow in CAD detection
Although contrast CT coronary angiography with 128 slice or dual source CT is commonly used for the non-invasive detection of CAD [7], invasive coronary angiography with quantitative evaluation of luminal narrowing is still regarded as the “gold standard” for the evaluation of the severity of obstructive luminal narrowing [8]. Seminal investigations by Gould et al. [9–11] have demonstrated that coronary flow at rest remains normal and is related to cardiac workload [12] unless the epicardial luminal diameter is reduced by 90%. A compensatory vasodilation of the coronary microcirculation, aiming to compensate the increase in vascular resistance at the site of an advanced epicardial stenosis, provides an explanation for this. Conversely, there is an inverse relationship between increasing severity of epicardial lesions with ≥50% luminal obstruction and decreases of hyperaemic myocardial blood flow (MBF) during pharmacologic vasodilation, myocardial flow reserve (MFR), and stress-induced regional myocardial perfusion defects [13–16]. This inverse relationship between epicardial stenosis severity and hyperaemic flows, however, may be confounded by collateral flow supply, relatively preserved MFR owing to physical exercise and/or beneficial effects of preventive medical care with statins and/or ACE inhibitors on vasodilator effects of coronary arteriolar vessels, resulting in a marked variability in hyperaemic flow increases [1, 13, 15, 17]. In clinical practice, therefore, no simple association between the severity of obstructive CAD lesions and hyperaemic MBFs exists [13–15, 18–21]. The clinical decision-making process regarding interventional or surgical procedures to restore myocardial perfusion of blood flow is generally based on the identification of stress induced myocardial ischaemia, or invasively measured fractional myocardial flow reserve [1, 22].
The ability of PET/CT in concert with tracer kinetic modelling to concurrently assess regional myocardial blood flow (MBF) in ml/g/min at rest and during pharmacologically stimulated hyperaemia enables the quantification of the myocardial flow reserve (MFR) that expands the scope of conventional myocardial perfusion imaging from the detection of advanced, obstructive epicardial CAD to the identification and description of CAD burden in multivessel disease as well as to early functional stages of the atherosclerotic process or microvascular dysfunction [1, 3, 4] (Table 1).

Table 1.
Clinical use of PET-determined MFR.
Positron emitting myocardial flow tracers
In clinical practice, PET/CT quantification of regional MBF in ml/g/min is commonly performed with the positron emitting perfusion tracers, such as 13N-ammonia, 82rubidium, or 15O-water [1, 3]. 13N-ammonia myocardial perfusion images afford high contrast resolution that relates to its high first pass myocardial extraction fraction (≈80%) and its relatively long physical half-life (9.8 minutes). These properties of 13N-ammonia lead to a statistically high count images of the myocardium with PET/CT, which permits the evaluation of high quality myocardial perfusion images and accurate MBF quantification [1, 3]. In centres with a relatively high patient volume and without access to a cyclotron, 82rubidium is commonly the preferred perfusion radiotracer owing to its ultrashort 75-second physical half-life and its independency of an onsite cyclotron through the availability of a strontium-82 / rubidium-82 generator system with a 4- to 5-week shelf life. As compared to 13N-ammonia, the lower first-pass extraction (≈60%) of 82rubidium and the more prominent nonlinear myocardial uptake with increasing blood flow, termed “roll-off phenomenon,” manifest in a relatively lower myocardial contrast resolution on PET perfusion images. In clinical practice, however, this does not appear to affect the diagnostic accuracy of 82rubidium for the identification of CAD as it was reported comparable to the one of 13N-ammonia [1, 23]. As regards the flow tracer 15O-labelled water, the static images of the myocardium commonly demonstrate low count density due to its short physical and biological half-life (≈2.4 min) in the myocardium, which hampers its clinical use. Conversely, the high first pass myocardial extraction fraction of 15O-labelled water (≈95%) widely avoids the “roll-off phenomenon” at higher hyperaemic flows and therefore yields a high accuracy in MBF quantification, which renders it as radiotracer of choice for flow research investigations [24, 25]. More recently, flurpiridaz a 18F-labelled positron emitting myocardial flow tracer has been developed for clinical application [26, 27]. The radiotracer has a first pass myocardial extraction fraction higher than 90%, which is also maintained at very high flow rates. In addition, the uptake ratios of the heart and lungs, liver, or blood appear to be more than three times higher than those of 13N-ammonia [28]. These favourable physical and physiological features of flurpiridaz yield high diagnostic a quality of PET/CT myocardial perfusion images [27]. In addition, the long half-life of flurpiridaz ≈110 minutes in conjunction with good image quality, stable kinetics, high extraction over a wide flow range and potential use in centres without a cyclotron may trigger its clinical application, given that flurpiridaz performs well in phase 3 clinical investigations [3, 26].
Enhanced identification of CAD burden
The visual or semi-quantitative evaluation of regional radiotracer uptake of the left ventricle of rest and stress myocardial perfusion scintigraphic images on SPECT, SPECT/CT or PET/CT is in “relative” terms. A reduction in regional radiotracer uptake during stress as compared to rest commonly signifies flow-limiting effects of advanced or severe CAD lesions. Conversely, the myocardial region with the with the highest radiotracer uptake is deemed as the “normal” reference region as compared with stress induced regional perfusion defect. In patients with multivessel disease, however, the defined normal reference region on conventional scintigraphic images may be abnormally perfused as well. In comparison to the other myocardial territories, it may be the region with the least impaired perfusion or hyperaemic flow increases [17]. Combining conventional analysis of scintigraphic myocardial perfusion images with the PET determined hyperaemic MBF and MFR may unravel not only the most advanced or “culprit” lesion but also the flow-limiting effects of the other CAD stenoses of less severity in regions without stress induced perfusion defects on visual perfusion analysis (Figure 1). The concurrent assessment of “relative” perfusion and “absolute” MBF, therefore, may afford not only the detection of the culprit CAD lesion but also the flow-limiting downstream effects of less severe, intermediate or sequential CAD lesions or both in the remaining myocardial regions (Figure 2) [1, 4]. It is important to keep in mind, however, that reductions in hyperaemic MBF and MFR, respectively, are nonspecific as they may not only reflect downstream effects of epicardial lesions but also, at least in part, cardiovascular risk factor related microvascular dysfunction [3, 5, 29]. Furthermore, it remains uncertain whether flow limiting effects of a stenosis contributes mildly, moderately, or severely to a regional reduction in hyperaemic MBF. Due to differences in methodology and radiotracers applied, the optimal cut-off values of PET-determined MFR is still a matter of ongoing debate [1, 5], while for the radiotracer 13N-ammonia or H215O the thresholds for the detection of CAD lesions are commonly reported of 2.0 and 2.5, respectively [30, 31]. Due to the non-specificity of reductions in regional MFRs [5, 17], the flow values need to be interpreted in the clinical context with coronary anatomy and/or microvascular dysfunction in cardiovascular risk and/or CAD individuals [1]. For the time being, it has been suggested that for an epicardial stenosis ≥70%, reductions in MFR <1.7 can be assumed to account, at least in part, to increases in focal epicardial resistance using PET with 13N-ammonia or 82rubidium as myocardial flow tracer [32]. Additional help may also come from the evolving concept to measure a longitudinal decrease in hyperaemic MBFs from the base-to-the apex of the heart [33]. This novel diagnostic approach appears to provide more specific information on epicardial resistance than the conventional MFR [5, 34–37]. Such a diagnostic approach may emerge non-invasive fractional flow reserve, which needs further validation before potential clinical use.
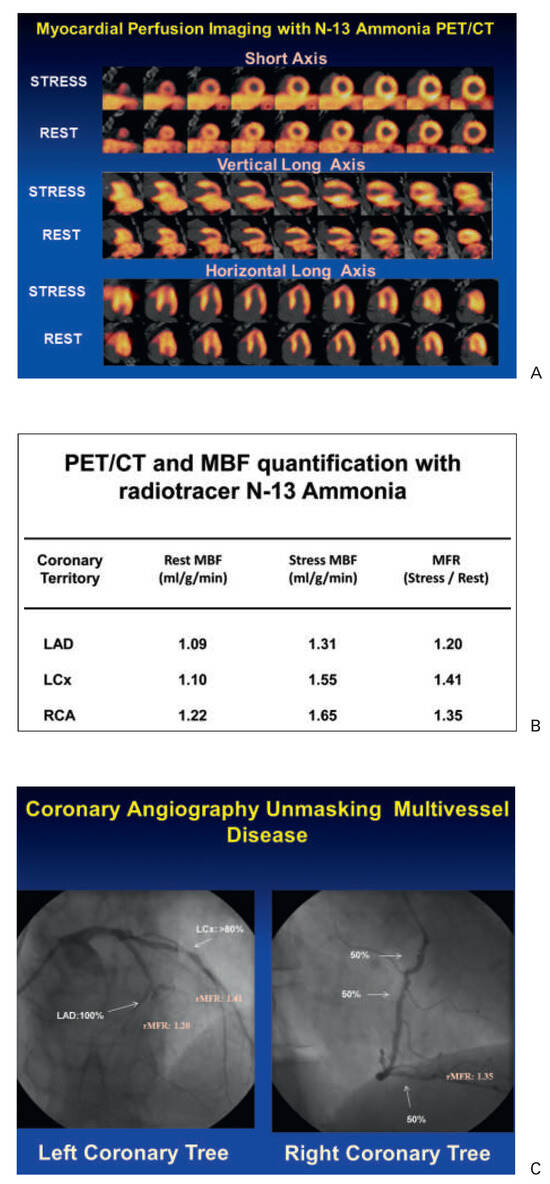
Figure 1.
13N-ammonia PET in the evaluation of multivessel CAD. (A) Myocardial perfusion study with 13N-ammonia PET/CT during dipyridamole stimulation and at rest in a 61-year-old patient with arterial hypertension and type 2 diabetes mellitus. On stress images, there is a moderately decreased perfusion defect involving the mid-to-distal anterior, anteroseptal, and apical regions of the left ventricle, which becomes reversible on the rest images. Uptake is preserved in the lateral and inferior regions. (B) Regional myocardial blood flow quantification (MBF) and myocardial flow reserve (MFR) calculation with 13N-ammonia PET/CT and tracer kinetic modelling. The summarised quantitative data suggest a distinct impairment of the MFR not only in the (LAD) territory, but also in the RCA and LCX vascular territories (regional MFR <2.0). (C) Invasive coronary angiography in this patient demonstrated a proximal occlusion of the LAD, 80% stenosis in the proximal segments of the LCX (left panel), and sequential 50% to 60% lesions in the RCA (right panel). Corresponding regional MFRs are demonstrated for each vascular territory. Reprinted from: Schindler TH, Schelbert HR, Quercioli A, Dilsizian V. Cardiac PET imaging for the detection and monitoring of coronary artery disease and microvascular health. JACC Cardiovasc Imaging. 2010;3:623–40, with permission from Elsevier. (MBF = myocardial blood flow; MFR = myocardial flow reserve; LAD = left anterior descending artery; LCx = left circumflex artery; RCA = right coronary artery.).
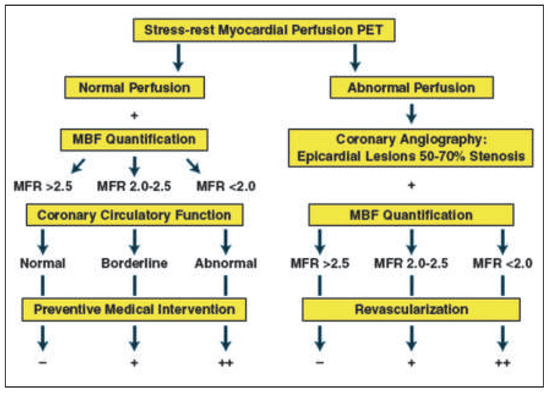
Figure 2.
Potential algorithm for the integration of PET/CT myocardial perfusion imaging and absolute myocardial blood flow (MBF) and flow reserve (MFR) quantification in individuals with suspected or an intermediate risk for developing CAD for clinical decision making towards revascularisation or preventive medical therapy. Reprinted from: Schindler TH, Schelbert HR, Quercioli A, Dilsizian V. Cardiac PET imaging for the detection and monitoring of coronary artery disease and microvascular health. JACC Cardiovasc Imaging. 2010;3:623–40, with permission from Elsevier.
Identification of diffuse ischaemia
In patients with stress induced diffuse myocardial ischaemia owing to significant left main stem lesion or three vessel diseases, the conventional approach of myocardial scintigraphic perfusion imaging to assess the “relative” distribution of the radiotracer uptake in the left ventricular myocardium may fail to signify “balanced” reduction of MBF in all vascular territories. Under such circumstances, the relative distribution of myocardial perfusion may be homogeneously reduced in the entire left ventricle without visually discernable regional perfusion defects during vasomotor stress [38]. For example, only in 10% (14/143) of patients with angiographically proven three vessel CAD stress induced regional perfusion defects could be identified [39]. Adding regional wall motion abnormalities on post-stress gated SPECT to myocardial perfusion imaging increased the detection for three vessels CAD but only to 25%. In another direction, Berman et al. [40] investigated 101 patients with left main CAD (≥50% stenosis) without prior myocardial infarction or coronary revascularisation who underwent gated exercise or adenosine stress 99mtechnetium sestamibi SPECT myocardial perfusion imaging. Interestingly, when evaluating myocardial perfusion visually and semi-quantitatively, a high-risk feature with moderate to severe perfusion defects (>10% myocardium at stress) was observed in 56% and 59% of patients, respectively. Conversely, no significant stress induced perfusion defect (≥5% myocardium) was noted in 13–15% of patients. Combining abnormal perfusion and wall motion on post-stress gated SPECT, however, 83% of patients were detected as high-risk individuals. In this direction, the concurrent assessment of hyperaemic MBF and MFR to myocardial perfusion with PET may be of further help and directly signify balanced ischaemia of the left ventricular myocardium in all three major coronary artery vascular territories of the LAD, LCx, and RCA. Diffuse reductions of MFR, however, may be nonspecific and, thus, stress induced ischaemia should always be confirmed by a “peak” stress transient ischaemic cavity dilation of the left ventricle associated with a global hypokinesis on gated PET images [41, 42].
Identification of subclinical CAD and prognostic implications
In patients with known or suspected CAD, MPS accurately identifies haemodynamically obstructive CAD lesions [1, 2]. Conversely, cardiovascular risk factors induced dysfunction of the coronary arteriolar vessels, commonly regarded as functional precursor of the CAD process [43, 44], remain undetected with the conventional myocardial perfusion SPECT/CT or PET/ CT approach [45]. Individuals with subclinical stages of CAD may exhibit either subtle heterogeneity in relative myocardial uptake of the radiotracer or homogeneously impaired hyperaemic blood flow of the left ventricle [33]. By assessing also hyperaemic MBF and MFR, PET/CT can identify such early functional disturbances of the coronary circulation, which may favour the development of structural CAD [36, 44, 46, 47]. For example, in insulin-resistant population without other traditional cardiovascular risk factors and with normal stress-rest myocardial perfusion on PET images, concurrent MBF quantification has unravelled disturbances in endothelium-related MBF responses to cold pressor testing (CPT), while pharmacologically induced hyperaemic flow increase were still normal [48]. These observations outline that initial stages of the vascular damage may involve only the endothelium [49–51], while in more advanced stages of cardiovascular risk factors states, commonly associated with increases in oxidative stress burden, may also manifest in an impairment in smooth muscle cell vasodilator function of the coronary arteriolar vessels [47, 52, 53]. Notably, a dysfunction of the coronary endothelium in cardiovascular risk individuals but with normal coronary angiograms, as determined with PET-measured flow responses to sympathetic stimulation with CPT and its MFR, was associated with a higher risk for cardiovascular events when compared to those individuals with normal flow increases [43]. In particular, it was observed that the incidence of cardiovascular events increased with progressive worsening of coronary endothelial dysfunction (Figure 3). These observations were followed by more recent investigations with PET-measured hyperaemic MBFs during pharmacologic vasodilation [44, 54–57]. Herzog et al. [44] observed that adding the MFR information to the stress 13N-ammonia perfusion PET with normal perfusion enabled a further risk stratification, while a “warranty” period of event-free survival of three years was observed when the MFR was normal. On the other hand, when patients had stress-induced regional myocardial perfusion defects and abnormally reduced MFR, impaired MFR provided incremental information to the stress 13N-ammonia perfusion imaging for the prediction of adverse cardiovascular outcome (Figure 4). This was also confirmed by Ziadi et al. [54] in a more extended clinical investigation. In this study, 704 patients were prospectively enrolled for prognostic evaluation using 82rubidium-PET for assessment of myocardial perfusion and MBF. For those with a normal perfusion imaging and reduced MFR as compared with those with normal MFR, there was a higher incidence of cardiovascular events (Figure 5). Conversely, among patients with stress-induced perfusion defects, those individuals with a MFR <2.0 as compared with those with a normal MFR had also a significantly higher cardiovascular event rate (Figure 5). Overall, this investigation demonstrated the added and independent prognostic valve of hyperaemic and MFR as determined with 82Rb-PET in patients with and without stress-induced regional perfusion defects in a large cohort of patients with suspected or known CAD. These observations of an incremental predictive value of MFR were for cardiac death were also reported for specific risk populations like patients with diabetes mellitus [55], and chronic kidney disease [57], ischaemic or idiopathic cardiomyopathy [58, 59]. These cardiovascular outcome data strongly emphasise coronary circulatory dysfunction as an integrating index of the overall stress burden imposed by various coronary risk factors on the arterial wall [3, 60]. Beyond the described incremental prognostic value of reduced MFR in CAD patients or individuals without clinically manifest CAD, an impairment of hyperaemic MBF increase and MFR has also been demonstrated to predict future cardiovascular outcome in patients with idiopathic or hypertrophic cardiomyopathy [58, 59, 61].
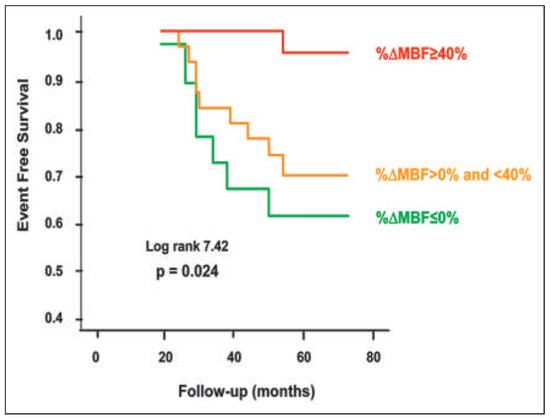
Figure 3.
PET determined coronary endothelial vasoreactivity and prognosis. Kaplan-Meier analyses in patients with cardiovascular risk factors and normal coronary angiograms undergoing assessment of myocardial blood flow (MBF) response to cold pressor test (CPT) with positron emission tomography (PET). Attenuation of PET-measured and endothelium related MBF responses to sympathetic stimulation with cold pressor testing are associated with a higher risk for cardiac events (during long-term follow-up) as compared with those with normal flow increases; normal (%ΔMBF ≥40%), impaired (%ΔMBF >0% and <40%), and decreased (%ΔMBF ≤0%). Reprinted from: Schindler TH, Nitzsche EU, Schelbert HR, Olschewski M, Sayre J, Mix M, et al. Positron emission tomography-measured abnormal responses of myocardial blood flow to sympathetic stimulation are associated with the risk of developing cardiovascular events. J Am Coll Cardiol. 2005;45:1505–12, with permission from Elsevier.
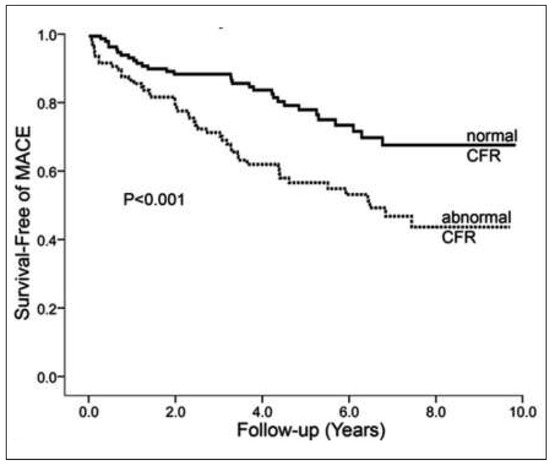
Figure 4.
Myocardial perfusion, coronary flow reserve and prognosis. Coronary flow reserve (CFR) predicts major cardiovascular events (MACE) such as cardiac death, nonfatal myocardial infarction, and hospitalisation for any cardiac reasons including late percutaneous coronary intervention (PCI) or late coronary artery bypass grafting (CABG) in the entire study population. Reprinted from: Herzog BA, Husmann L, Valenta I, Gaemperli O, Siegrist PT, Tay FM, et al. Long-term prognostic value of 13N-ammonia myocardial perfusion positron emission tomography added value of coronary flow reserve. J Am Coll Cardiol. 2009;54:150–6, with permission from Elsevier.
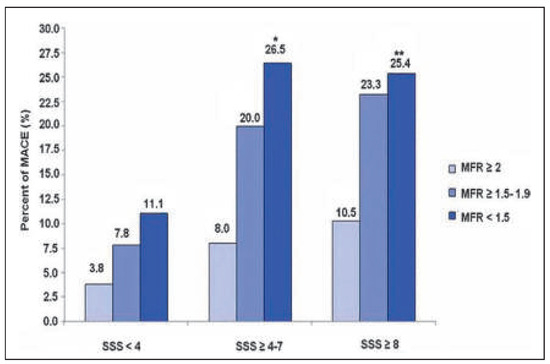
Figure 5.
Prognostic value of PET-determined myocardial flow reserve (MFR) in CAD patients. Within subgroups of summed stress score (SSS) for different levels of MFR, at any level of SSS, the prevalence of major cardiac events (MACE) is higher in patients with the lowest MFR (<1.5) and statistically significant different compared with MFR ≥2 among patients with ischaemia. Reprinted from: Ziadi MC, Dekemp RA, Williams KA, Guo A, Chow BJ, Renaud JM, et al. Impaired myocardial flow reserve on rubidium-82 positron emission tomography imaging predicts adverse outcomes in patients assessed for myocardial ischaemia. J Am Coll Cardiol. 2011;58:740–8, with permission from Elsevier. (*p = 0.028 for SSS ≥4–7 and MFR <1.5 vs MFR ≥2. **p = 0.002 for SSS ≥8 and MFR <1.5 vs MFR ≥2.).
Monitoring reductions in cardiovascular risk with PET
PET assessment of MBF at rest and MBFs during pharmacologic vasodilation can be applied to verify and follow-up the effects alterations in lifestyle, loss of body weight, and/or preventive medical care on coronary circulatory function in cardiovascular risk individuals with subclinical or with clinically manifest CAD [3, 4, 62]. Several PET flow studies have demonstrated an improvement in coronary circulatory dysfunction with antioxidant challenges, regular exercise, preventive medical care, and euglycaemic control with antidiabetic medication in cardiovascular risk individuals [63–67]. For example, angiotensin II receptor blocker (ARB) with olmesartan normalised endothelium-related MBF responses to sympathetic stimulation with cold pressor testing (CPT) in patients with essential hypertension [68]. The observed improvement in endothelium related flow responses to CPT could be assigned to ARB-induced elevations in superoxide dismutase levels. Thus, specific antioxidative effects of ARB inhibition, potentially conferred by inhibition of endothelial NADPH oxidase activation accompanied by a decrease in reactive oxygen species or increases in antioxidative superoxide dismutase levels, are likely to account for the beneficial effects on the function of the coronary endothelium in hypertensive patients. In recent years, it has been realised that increases in body weight may stimulate the release of mediators from the adipose tissue, so-called adipocytokines, such as leptin, adiponectine and endocannabinoid [47, 53, 69–72]. While adiponectine may stimulate the endothelial production and release of nitric-oxide and, thus, is deemed to exert vasoprotective effects against the initiation and development of the CAD process, the influence of increased plasma levels of leptin on the vascular wall remains controversial [47, 70, 73]. More recently, increases in endocannabinoid plasma levels could be identified to cause a dysfunction of the coronary circulation in obesity and, thus, they may favour the initiation and/or progression of the CAD process. In this respect, the effects of surgical bypass induced weight loss in morbidly obese individuals (BMI ≥40 kg/m2) without other traditional cardiovascular risk factors on coronary circulatory dysfunction were investigated [69]. After a median follow-up period of 22 months, gastric bypass induced a decrease in BMI from a median of 44.8 to 30.8 kg/m2. The gastric bypass related weight loss in initial morbidly obese individuals, which was accompanied by beneficial alterations in lipid profile, increase in insulin sensitivity, decrease in chronic inflammation and endocannabinoid plasma levels, strikingly improved both endothelium-related MBF responses to CPT and hyperaemic MBFs during pharmacologic vasodilation, respectively (Figure 6). The decrease in endocannabinoid plasma levels, such as anandamide, induced by the weight loss, correlated with the observed restoration of MBF-responses to CPT (Figure 7). This association between the decrease in anadamide plasma levels and improvement in coronary endothelial function emphasises potential adverse effects of elevated endocannabinoid plasma levels on coronary vasculopathy in obese individuals [53, 70]. Conversely, the gastric bypass-induced increase in adiponectine plasma levels was paralleled by a normalisation in hyperaemic flow increases during pharmacologic vasodilation (Figure 8). This association strongly outlines a beneficial effect of increases in adiponectine plasma levels in concert with metabolic alterations and decreases in systemic inflammation on coronary circulatory function [69]. The effects of glucose lowering intervention with glyburide and/or metformin on coronary artery calcifications (CAC) and coronary circulatory function, as determined with 13N-ammonia PET, over a mean follow-up period of 14 months in 22 individuals with type 2 diabetes mellitus were also investigated [74]. After the follow-up, plasma glucose levels had markedly decreased to 160 ± 44 mg/dl from initial 205 ± 72 mg/dl after glucose-lowering treatment. The noted decrease in plasma glucose levels after a one year follow-up was associated with a lower progression of CAC and increases in endothelium-related MBF-responses to CPT and during pharmacologic vasodilation with adenosine, respectively (r = 0.46, p = 0.038 and r = 0,36, p = 0.056), signifying beneficial effects of euglycaemic control on structure and function of the arterial wall. Of particular interest, the magnitude of increases in endothelium-related flow responses to CPT and slowed progression of CAC closely correlated (Figure 9). When adjusting for metabolic parameters by multivariate analysis, the improvement of coronary endothelial dysfunction after glucose-lowering medical intervention remained an independent predictor of a slowed progression of CAC in these individuals with type 2 diabetes mellitus [74]. These observations are unique by demonstrating that an improvement in coronary endothelial dysfunction in type 2 diabetes mellitus may mediate, at least in part, direct preventive effects on the progression of diabetic vasculopathy. Also of interest, PET flow measurements contributed to identify and monitor effects of hormone replacement therapy (HRT) on coronary circulatory dysfunction in postmenopausal women [46, 50, 75]. HRT applying oestrogen alone or in concert with progesterone in postmenopausal women, in addition to standard preventive medical treatment of traditional cardiovascular risk factors, contributed to maintain a proper function of the coronary endothelium (Figure 10) [46]. As an impairment of coronary circulatory function has been widely appreciated as functional precursor of the CAD process, its normalisation after life-style intervention and/or preventive medical care, for example by statin and/or ACE inhibitors, is likely to result into an improved cardiovascular outcome, which, however, needs to be tested clinically.
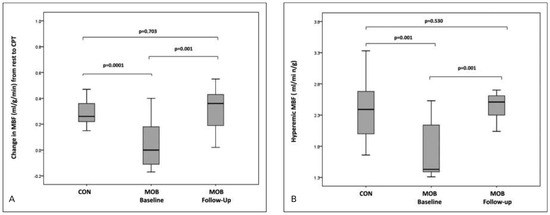
Figure 6.
Effects of gastric bypass induced weight loss on coronary circulatory function. Myocardial blood flows (MBF) during vasomotor stress in controls (CON) and morbidly obese (MOB) individuals. (A) Change in MBF to cold pressor testing from rest and (B) in hyperaemic MBF in CON and in MOB individuals at baseline and at the follow-up. Reprinted from: Quercioli A, Montecucco F, Pataky Z, Thomas A, Ambrosio G, Staub C, et al. Improvement in coronary circulatory function in morbidly obese individuals after gastric bypass-induced weight loss: relation to alterations in endocannabinoids and adipocytokines. Eur Heart J. 2013;34:2063–73, with permission from Oxford University Press.
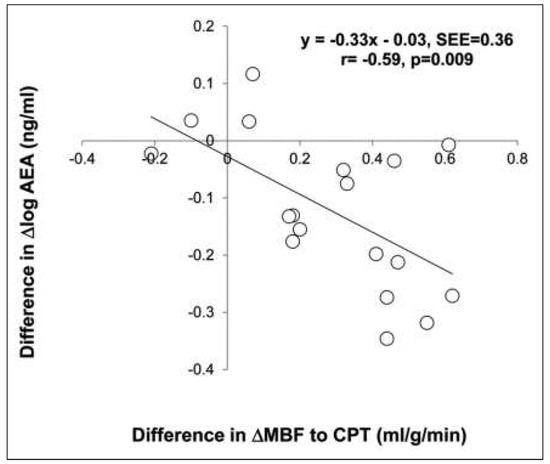
Figure 7.
Relationship between the decrease in anandamide (AEA) plasma levels and coronary circulatory function after gastric bypass induced weight loss. Association of the differences in Δlog AEA and endothelium related ΔMBF with CPT between baseline and follow-up. Reprinted from: Quercioli A, Montecucco F, Pataky Z, Thomas A, Ambrosio G, Staub C, et al. Improvement in coronary circulatory function in morbidly obese individuals after gastric bypass-induced weight loss: relation to alterations in endocannabinoids and adipocytokines. Eur Heart J. 2013;34:2063–73, with permission from Oxford University Press.
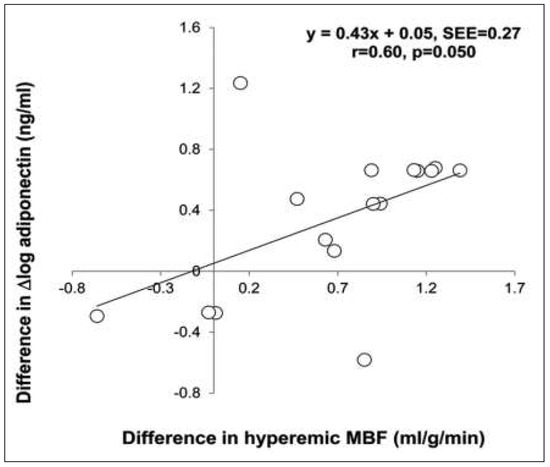
Figure 8.
Relationship between the increase in adiponectin plasma levels and coronary circulatory function after gastric bypass induced weight loss. Association between the differences in Δlog adiponectin and in hyperaemic MBF between baseline and follow-up. Reprinted from: Quercioli A, Montecucco F, Pataky Z, Thomas A, Ambrosio G, Staub C, et al. Improvement in coronary circulatory function in morbidly obese individuals after gastric bypass-induced weight loss: relation to alterations in endocannabinoids and adipocytokines. Eur Heart J. 2013;34:2063–73, with permission from Oxford University Press.
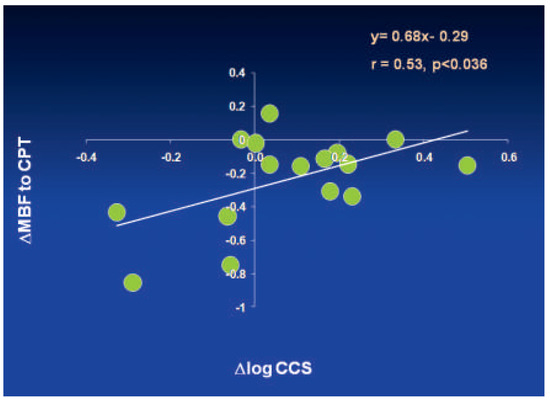
Figure 9.
Relationship between improvement in coronary endothelial dysfunction and slowed CAD progression in response to glucose lowering therapy in type 2 diabetes mellitus after one year follow-up. Association between the differences in ΔMBF with CPT and in Δlog- CCS (coronary calcium score) between baseline and follow-up (negative values on the x-ordinate are indicative for a progression of coronary artery calcification). Reprinted from: Schindler TH, Cadenas J, Facta AD, Li Y, Olschewski M, Sayre J, et al. Improvement in coronary endothelial function is independently associated with a slowed progression of coronary artery calcification in type 2 diabetes mellitus. Eur Heart J. 2009;30:3064–73, with permission from Oxford University Press.
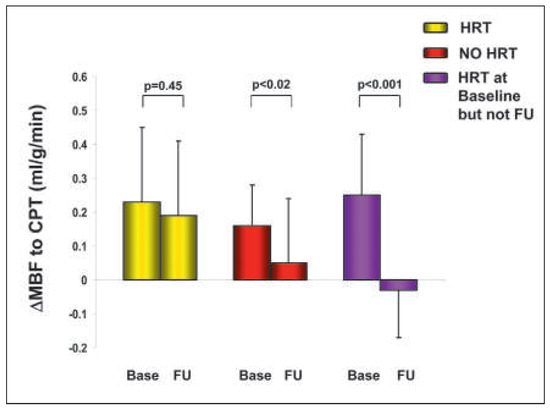
Figure 10.
Hormone replacement and PET determined coronary endothelial vasoreactivity in postmenopausal women. Effect of hormone replacement therapy (HRT) on coronary endothelial dysfunction in postmenopausal women with medically treated cardiovascular risk factors. The endothelium related change in ΔMBF from rest to CPT at baseline (base) and follow-up (FU) among different groups are shown. In postmenopausal women with HRT at baseline and follow-up,the ΔMBF response to CPT was generally maintained, whereas in the women without HRT, there was a significant decrease in ΔMBF to CPT. Interestingly, the ΔMBF to CPT in postmenopausal women who had discontinued HRT during follow-up was even worse than in those women who had never been on HRT, suggesting perhaps a “rebound” phenomenon on coronary endothelial (dys)function. Reprinted from: Schindler TH, Campisi R, Dorsey D, Prior JO, Olschewski M, Sayre J, et al. Effect of hormone replacement therapy on vasomotor function of the coronary microcirculation in post-menopausal women with medically treated cardiovascular risk factors. Eur Heart J. 2009;30:978–86, with permission from Oxford University Press.
Cardiac sarcoid involvement
Sarcoidosis is a multisystem granulomatous disease of unknown aetiology that characterised by the formation of noncaseating granuloma in affected organs [76, 77]. Clinically, cardiac sarcoid involvement may manifest through conduction abnormalities, arrhythmias, congestive heart failure and sudden cardiac death. An involvement of the heart in sarcoid disease portends a worse prognosis, if not detected and treated in a timely manner with corticosteroids and/or other immunosuppressive agents [78]. The diagnosis of cardiac sarcoidosis is frequently difficult and is contingent on the integration of both clinical and imaging findings. As regards the clinical diagnosis for cardiac sarcoidosis, it is based upon the Japanese Ministry of Health and Welfare Diagnostic Guidelines [79]. According to these guidelines, either a direct biopsy proven confirmation of cardiac sarcoidosis or direct histologically proven extra cardiac sarcoidosis combined with indirect evidence of an inflammatory myocardial lesion is needed.
18F-fluorodeoxyglucose (18F-FDG), a glucose analogue, in concert with PET/CT is increasingly applied to identify and characterise systemic sarcoid disease [78–80] (Figure 11). 18F-FDG-PET/CT is most suitable for the detection of sarcoid disease as upregulation of glucose metabolism is observed at sites of macrophage-mediated inflammation, like the one in an active sarcoid disease state. In a meta-analysis of 7 studies involving 164 patients of whom 50% had diagnosed systemic sarcoidosis, the sensitivity and specificity of 18F-FDG-PET in the detection of cardiac sarcoid involvement was reported to be as high as 89% and 78%, respectively [81]. “Inflammation” imaging with cardiac 18F-FDG-PET/CT aims to identify the presence of 18F-FDG uptake as an “abnormal” finding. A normal 18F-FDG-PET/CT study, therefore, is considered when a complete absence of myocardial 18F-FDG uptake is noted under fasting conditions in order to avoid some physiologic uptake of about 20%–30% due to glucose metabolism of the heart. Yet, the physiologic FDG uptake of the heart can be quite variable and avid. Dietary modifications and intravenous heparin administration have been implemented in the protocols to ascertain sufficient suppression of physiologic 18F-FDG uptake in the heart by fostering the free fatty acid (FFA) metabolism. In this direction, a low carbohydrate meal the evening prior to scanning with at least an overnight fast is recommended [79]. In addition, it is advocated to administer unfractionated heparin intravenously, that promotes the increase in FFA plasma levels and its utilisation in the myocardium instead of glucose. Unfractionated heparin, in fact, activates the lipoprotein lipase with lipolytic effects leading to a substantial increase in FFA in the circulation. While the use of intravenous heparin application to stimulate a FFA loading prior to the PET scanning is variable between institutions, one possibility is to administer 700 to 1000 IU intravenously in divided doses 30 and 15 minutes prior to scanning in patients where there is no contraindication.
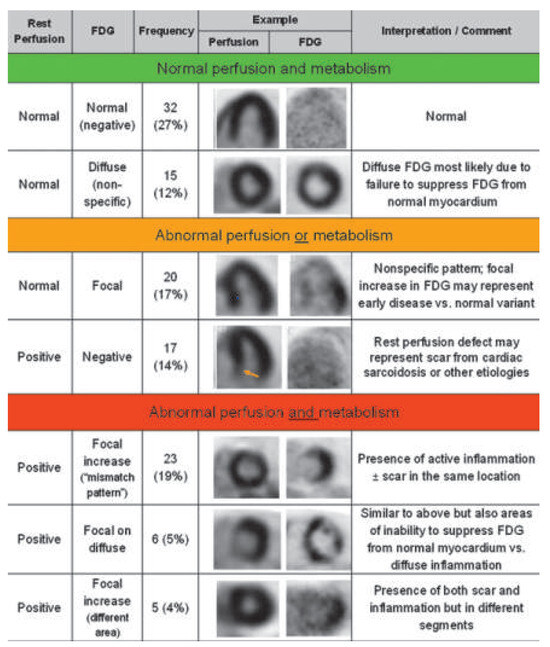
Figure 11.
Classification of cardiac PET/CT perfusion and metabolism imaging for the detection of cardiac sarcoid involvement. Normal perfusion and metabolism (category 1), abnormal perfusion or metabolism (category 2), abnormal perfusion and metabolism (category 3). Reprinted from: Blankstein R, Osborne M, Naya M, Waller A, Kim CK, Murthy VL, et al. Cardiac positron emission tomography enhances prognostic assessments of patients with suspected cardiac sarcoidosis. J Am Coll Cardiol. 2014;63:329–36, with permission from Elsevier. (FDG = fluorodeoxyglucose.).
While “fasting” 18F-FDG-PET/CT provides important information of an inflammatory active state of cardiac sarcoid involvement, the assessment of myocardial perfusion at rest strives to identify regional perfusion defects suggestive of myocardial areas with fibrosis or due to inflammation induced oedema associated with a compression of the coronary arteriolar vessels [78]. Abnormalities in myocardial perfusion and myocardial inflammation are not only most suitable in establishing the diagnosis of cardiac sarcoid involvement (Figure 11) but they carry also important prognostic information (Figure 12). Blankstein et al. [78] investigated 118 consecutive patients with no history of coronary artery disease, who were referred for PET, using 18F-FDG as radiotracer to assess for inflammation and 82rubidium to evaluate for myocardial perfusion defects, following a high-fat/low-carbohydrate diet to suppress normal myocardial glucose uptake. Among these patients with suspicious for cardiac sarcoid involvement, 40% had normal and 60% had abnormal cardiac PET findings, respectively. Thirty-one (26%) adverse events (27 ventricular tachycardia and 8 deaths) occurred over a median follow-up of 1.5 years. Cardiac PET findings were predictive of adverse events, and the presence of both a regional myocardial perfusion defect and abnormal 18F-FDG uptake (29% of patients) was associated with a hazard ratio of 3.9 (p <0.01). In particular, perfusion abnormalities and/or abnormal 18F-FDG uptake on cardiac PET images remained significant for the occurrence of ventricular tachycardia and cardiac death after adjusting for left ventricular ejection fraction and clinical criteria [78]. Conversely, extra cardiac 18F-FDG uptake observed in 26% of patients was not associated with an adverse outcome. Notably, it appears that abnormal cardiac PET findings in patients with sarcoid disease afford a prognostic value, which goes beyond the Japanese Ministry of Health and Welfare clinical criteria, the presence of extracardiac sarcoidosis and left ventricular ejection fraction. Importantly, initial results of monitoring the success of immunosuppressive treatment in concert with standard heart failure therapy signifies that the reduction in the intensity and extent of myocardial inflammation on FDG-PET is indeed associated with an improvement in left ventricular ejection fraction [80]. A longitudinal regression analysis demonstrated a significant inverse linear relationship between maximum SUV and EF with an expected increase in EF of 7.9% per SUV reduction of 10 g/ml (p = 0.008). These initial results emphasise that serial PET scanning may indeed help to identify the success of immunosuppressive treatment in the prevention and treatment of heart failure due to cardiac sarcoid involvement. Although this PET-guided approach to identify and guide treatment success in cardiac sarcoidosis is likely to improve the clinical outcome in these patients, further clinical evaluation is needed.
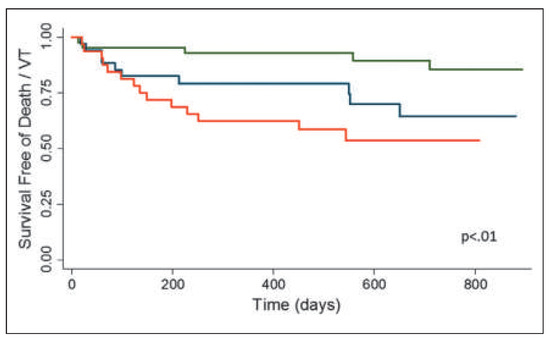
Figure 12.
Survival free of death or ventricular tachycardia stratified by cardiac PET examination results. Myocardial perfusion defects at rest, as indicative for structural alterations such as interstitial fibrosis and/or oedema, as well as an inflammatory active myocardial state on FDG-PET were associated with a pronounced lower survival free of death or ventricular tachycardia as compared to normal cardiac PET findings. Normal perfusion and FDG (green line), abnormal perfusion or FDG (blue line), and abnormal perfusion and FDG (red line). Reprinted from: Blankstein R, Osborne M, Naya M, Waller A, Kim CK, Murthy VL, et al. Cardiac positron emission tomography enhances prognostic assessments of patients with suspected cardiac sarcoidosis. J Am Coll Cardiol. 2014;63:329–36, with permission from Elsevier.
Plaque imaging
A more recent epidemiologic investigation in Sweden [82] indicates that of all deaths related to a first-ever manifestation of a major coronary event, almost 80% occurred as an out-of-hospital death. Thus, there is an increasing proportion of fatal coronary events that do not reach advanced hospital care. The vast majority of myocardial infarctions (86%) is deemed to originate from haemodynamically nonobstructive CAD burden that cannot be identified with conventional stress-rest myocardial perfusion imaging. In this regard, PET/CT imaging with different radiotracers may contribute in the identification of high risk coronary plaque burden prone to rupture [83]. Inflammation is assumed to play a central role in plaque rupture. Histologically, vulnerable coronary plaque burden is commonly described by a lipid rich pool, infiltration of inflammatory cells, and a thin fibrous cap [84]. Vascular inflammation can be determined noninvasively with 18F-FDG-PET/CT as it had been demonstrated first for the carotid artery by Rudd et al. [85], but also for the aorta, iliac, and femoral arteries [86]. As regards the coronary arteries, increased 18F-FDG was reported in patients with coexisting malignancy [87–89]. Following, two studies have demonstrated the feasibility of 18F-FDG PET in the detection of inflammatory plaques in coronary vessels with recent myocardial infarction [90, 91]. Yet, in 50% of patients with acute myocardial infarction no increased 18F-FDG was noted, which might suggest that non-inflammatory plaques may also be prone to rupture [90], potentially by increased activity of metalloproteinases leading to soft coronary plaque burden [92]. Such observations also emphasise the complexity of coronary plaque composition, susceptibility to rupture, and plaque metabolic imaging for risk assessment. The 18F-FDG uptake in the arterial wall as non-invasive surrogate marker for inflammation has been demonstrated to correlate with macrophage burden in the plaque [93], symptoms [85], and conventional Framingham Risk Score [94]. In particular, increases in arterial 18F-FDG-uptake in the noncoronary arteries can be attenuated with statin and dalcetrapib medication, respectively [95, 96]. As regards the assessment of 18F-FDG uptake in the coronary arteries with PET/CT, it remains an ongoing challenge as cardiac and respiratory motion and concurrent 18F-FDG in the myocardium hamper an accurate visualisation of any plaque signal [91, 97]. 64- or 128-slice CT from the cardiac PET/CT systems is commonly used to assess coronary artery calcifications (CAC) or, with intravenous contrast CT coronary morphology, respectively [7, 98]. CAC scoring, as a surrogate marker of coronary atherosclerotic burden, has been established as a powerful cardiovascular risk predictor, which is increasingly applied to enhance primary risk stratification and justification of preventive medical care of the CAD process [99, 100]. CAC may be combined with measurements of high-sensitive CRP, as a marker of systemic micro-inflammation, to improve and refine the prediction of cardiovascular risk [100]. Although measure of CAC and CT-coronary angiography provide important information on coronary morphology and risk prediction, they cannot identify active inflammation of coronary plaque burden, commonly seen as vulnerable plaque prone to rupture with its atherothrombotic sequelae. As mentioned before, measurements of 18F-FDG uptake in coronary arteries with PET/CT is still challenging [91].
Another interesting approach has been proposed more recently [101, 102] with the use of 18F-sodium fluoride (18F-NaF) as PET radiotracer, which identifies novel regions of bone formation and remodelling. Such a diagnostic approach is assumed to identify active calcification and/or micro-calcifications as potential source of microfractures and acute thrombosis [103–105]. Dweck MR et al. [102] investigated the potential of 18F-NaF and FDG uptake in the coronary arterial wall as marker for active calcification and inflammation, respectively. In 119 volunteers with and without aortic valve disease, coronary CAC score and 18F-NaF and FDG were determined with PET/CT. In individuals with a CAC score of 0 served as control individuals as compared to those with CAC (calcium score>0). As it turned out, the 18F-NaF strongly correlated with CAC, while in 41% of individuals with CAC >1000 had no significant 18F-NaF uptake in the arterial wall. This outlines that 18F-NaF uptake signifies different information reflecting metabolically active calcific plaque and developing microcalcification. 18F-NaF uptake, therefore, may differentiate between individuals with “dormant” coronary atherosclerotic plaque burden, developed many months or years previously, and individuals with metabolically active CAD and ongoing calcification process. This is substantiated by the observation that individual with increased coronary 18F-NaF activity (n = 40) had higher rates of prior cardiovascular events (p = 0.016), angina pectoris (p = 0.023) and higher Framingham risk scores (p = 0.011) (Figure 13). Somehow surprising, however, 18F-FDG activity was not increased in CAD individuals as compared to controls without CAC. This might suggest that 18F-FDG uptake in the coronary arterial wall is of little use in individuals with stable CAD. Someone could argue that inflammation, as reflected by increased arterial 18F-FDG uptake, is more prevalent in acute coronary syndrome than in stable CAD. The clinical observations of increases in 18F-NaF activity in the coronary arterial wall in CAD patients associated with angina symptoms, prior cardiovascular events, and cardiovascular risk scores may give raise to prospective investigations to assess its predictive value for future cardiovascular events in individuals with stable CAD. Subsequently, Joshi et al. [106] performed a prospective clinical trial in patients with myocardial infarction (n = 40) or stable angina pectoris (n = 40), respectively, undergoing 18F-NaF and 18F-FDG-PET/CT, and invasive coronary angiography (Figure 14). In addition, 18F-NaF uptake was compared with histology in carotid endarterectomy specimens from patients with symptomatic carotid disease, and with intravascular ultrasound in patients with stable angina. Overall, in 93% of patients with myocardial infarction, 18F-NaF was commonly highest in the culprit lesion than in the highest nonculprit lesion (median maximum tissue-to-background ratio: 1.66 versus 1.24, p <0.0001) (Figure 15A). Conversely, the 18F-FDG uptake in the coronary arterial wall was mostly masked, while when being differentiable no significant difference in 18F-FDG uptake between culprit and non-culprit lesion was noted (1.71 versus 1.58, p = 0.34) (Figure 15B). Furthermore, a distinct uptake of 18F-NaF at the site of all carotid plaque ruptures was reported that was accompanied by histological features of active calcification, macrophage infiltration, apoptosis, and necrosis. In addition, focal 18F-NaF in the coronary arteries was analysed with intravascular ultrasound for morphologic correlates. In 45% patients with stable angina had plaques with focal 18F-NaF uptake that was paralleled with highrisk features such as with microcalcifications, necrotic core, and positive remodelling index, while this was not observed in patients without 18F-NaF uptake in the arterial wall. The results of the current study may be seen more or less as a “proof of principle”, while it may provide an important framework to give raise to further large-scale clinical trials addressing the 18F-NaF uptake in coronary plaque burden, as determined with PET, and its potential predictive value for cardiovascular outcome in patients with subclinical CAD. There is also first evidence that hypoxia but not inflammation alone may trigger the 18F-FDG uptake in human macrophages within the arterial plaque burden [107], which would also accord, at least in part, with previous report that could not find increases in 18F-FDG in culprit plaque burden in 50% of patients with acute myocardial infarction [90]. Hypoxia is known to play a central role in stimulating atherosclerotic burden progression by stimulation foam cell formation, metabolic adaption of infiltrated macrophages, and plaque neovascularisation [108]. Hypoxaemic conditions may activate plaque growth by signalling of the hypoxia-inducible factor (HIF-1) leading to the formation of lipid droplets, the activation of a metabolic switch to anaerobic glycolysis, and increasing the secretion of proinflammatory and angiogenic mediators [109–111]. Imaging of hypoxia has been performed with PET and the radiotracer 18F-fluoromisonidazole (18F-FMISO) in patients with ischaemic stroke [112], myocardial ischaemia [113], and various malignancies [114]. 18F-FMISO is a cell-permeable 2-nitroimidazole derivative that is reduced in vivo by nitroreductases independent of the levels of intracellular oxygen. When there is a normal oxygenation environment, 18F-FMISO is rapidly reoxidised and diffuses out of the cells. Conversely, in hypoxic viable cells, 18F-FMISO is further reduced to a more reactive form that binds covalently to intracellular macromolecules and remains in the cells. In this direction, recent study evaluated the feasibility of 18F-FMISO PET imaging for in vivo detection of hypoxia in advanced lesions in a rabbit atherosclerosis model [115]. A significant 18F-FMISO accumulation in the aortas of atherosclerotic animals compared with healthy controls, and the uptake increased over time with atherogenic diet, suggesting hypoxia as a good biomarker of disease progression. In addition, the in-vivo detection of hypoxia was translated by ex-vivo PET imaging of the excised aorta that demonstrated regions of strong 18F-FMISO accumulation in atherosclerotic aortas when compared nearly absent uptake in the normal aorta. Thus, hypoxia in atherosclerotic plaque increases with disease progression and is present in macrophage-rich areas associated with neovascularisation, which can be determined noninvasively with 18F-FMISO. This novel imaging approach holds promise to further improve the cardiovascular risk prediction, which should be further tested in clinical investigations in CAD patients.
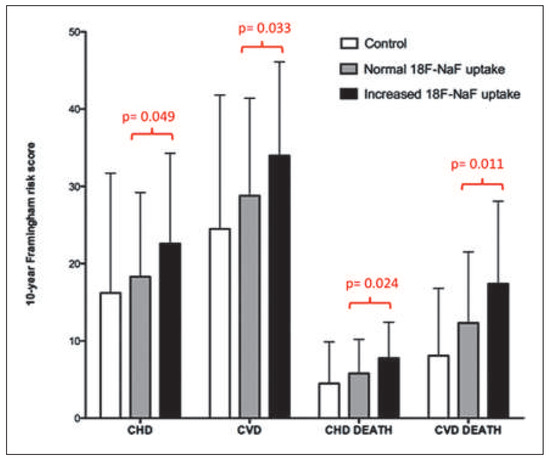
Figure 13.
10-year Framingham Risk Scores for control individuals and patients with atherosclerosis who did and did not have increased 18F-NaF uptake. Reprinted from: Dweck MR, Chow MW, Joshi NV, Williams MC, Jones C, Fletcher AM, et al. Coronary arterial 18F-sodium fluoride uptake: a novel marker of plaque biology. J Am Coll Cardiol. 2012;59:1539– 48, with permission from Elsevier. (CHD = coronary heart disease; CVD = cardiovascular disease).
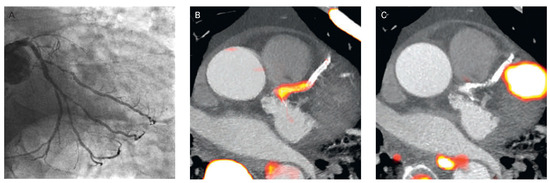
Figure 14.
Focal 18F-fluoride and 18F-fluorodeoxyglucose uptake in a patient with acute ST-segment elevation myocardial infarction. (A) Proximal occlusion (red arrow) of the left anterior descending artery on invasive coronary angiography and (B) intense focal 18F-fluoride (18F-NaF, tissue-to-background ratios, culprit 2.27 versus reference segment 1.09 uptake (yellow-red) at the site of the culprit plaque (red arrow) on the combined positron PET/CT). (C) Corresponding 18F-fluorodeoxyglucose PET-CT image signifying no uptake at the site of the culprit plaque (18F-FDG, tissue-to-background ratios, 1.63 versus reference segment 1.91. Of note, the significant myocardial uptake overlapping with the coronary artery (yellow arrow) and uptake within the oesophagus (blue arrow). Reprinted from: Joshi NV, Vesey AT, Williams MC, Shah AS, Calvert PA, Craighead FH, et al. 18F-fluoride positron emission tomography for identification of ruptured and high-risk coronary atherosclerotic plaques: a prospective clinical trial. Lancet. 2014;383:705–13, with kind permission.
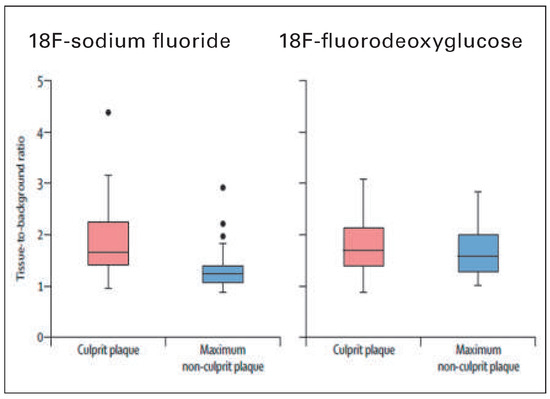
Figure 15.
18F-fluoride and 18F-fluorodeoxyglucose uptake in patients with myocardial infarction. 18F-fluoride activity (maximum tissue-to-background ratio) was increased in the culprit plaque (red) compared with the maximum uptake in any of the non-culprit plaques (blue). By contrast, there was no difference in the activity of 18F-fluorodeoxyglucose between these regions. Reprinted from: Joshi NV, Vesey AT, Williams MC, Shah AS, Calvert PA, Craighead FH, et al. 18F-fluoride positron emission tomography for identification of ruptured and high-risk coronary atherosclerotic plaques: a prospective clinical trial. Lancet. 2014;383:705–13, with kind permission.
Recapitulation
In the last decade, the assessment of hibernating stunning myocardium in ischaemic cardiomyopathy with PET/CT has evolved as mainstay in clinical practice for the decision making process for coronary revascularisation procedures. In addition, the assessment of myocardial perfusion and vasodilator capacity of the coronary circulation with PET/CT imaging is increasingly applied clinically for the detection and characterisation of CAD burden in subclinical and clinically manifest CAD, respectively, and risk stratification in patients with hypertrophic cardiomyopathy. An important role of PET/CT imaging for the detection of cardiac sarcoid involvement and the monitoring of successful immunosuppressive treatment has also been appreciated more recently. While the role of PET/ CT imaging with various radiotracers emerges as a promising tool for the identification of “vulnerable” coronary plaque burden, its clinical value remains to be tested. It is anticipated that with the advent of PET/ MRI (magnetic resonance imaging) further advances and refinement in the comprehensive assessment of cardiovascular pathology will ensue.
Funding / potential competing interests
No potential conflict of interest exists and all sources of funding for the work are acknowledged as follows. This work was supported by a departemental fund of Johns Hopkins University, Baltimore, MD, USA; and a Research Grant of the Swiss National Science Foundation (SNF: 3200B0–122237, Dr Schindler), with contributions of the Clinical Research Centre, University Hospital and Faculty of Medicine, Geneva, and the Louis-Jeantet Foundation, Gustave and Simone Prevot, and Swiss Heart Foundation.
Acknowledgments
Some sections of the manuscript are similar to sections of extensive reviews of cardiac PET by Schindler et al. [1, 3].
References
The full list of references is attached to the online version at www.cardiovascmed.ch.
© 2015 by the author. Attribution - Non-Commercial - NoDerivatives 4.0.