Duration of Dual Antiplatelet Therapy After Coronary Artery Stenting
Funding/potential competing interests
References
- Serruys, P.W.; Strauss, B.H.; Beatt, K.J.; et al. Angiographic follow-up aher placement of a self-expanding coronary-artery stent. The New England journal of medicine 1991, 324, 13–17. [Google Scholar] [CrossRef] [PubMed]
- Sigwart, U.; Puel, J.; Mirkovitch, V.; Joffre, F.; Kappenberger, L. Intravascular stents to prevent occlusion and restenosis aher transluminal angioplasty. The New England journal of medicine 1987, 316, 701–706. [Google Scholar] [CrossRef] [PubMed]
- Cassese, S.; Byrne, R.A.; Schulz, S.; et al. Prognostic role of restenosis in 10 004 patients undergoing routine control angiography aher coronary stenting. European heart journal 2014. [Google Scholar]
- Morice, M.C.; Serruys, P.W.; Sousa, J.E.; et al. A randomized comparison of a sirolimus-eluting stent with a standard stent for coronary revascularization. The New England journal of medicine 2002, 346, 1773–1780. [Google Scholar] [CrossRef] [PubMed]
- Yusuf, S.; Zhao, F.; Mehta SRet, a.l. Effects of clopidogrel in addition to aspirin in patients with acute coronary syndromes without ST-segment elevation. The New England journal of medicine 2001, 345, 494–502. [Google Scholar] [PubMed]
- Wiviott, S.D.; Braunwald, E.; McCabe, C.H.; et al. Prasugrel versus clopidogrel in patients with acute coronary syndromes. The New England journal of medicine 2007, 357, 2001–2015. [Google Scholar] [CrossRef] [PubMed]
- Wallentin, L.; Becker, R.C.; Budaj, A.; et al. Ticagrelor versus clopidogrel in patients with acute coronary syndromes. The New England journal of medicine 2009, 361, 1045–1057. [Google Scholar] [CrossRef] [PubMed]
- Windecker, S.; Serruys, P.W.; Wandel, S.; et al. Biolimus-eluting stent with biodegradable polymer versus sirolimus-eluting stent with durable polymer for coronary revascularisation (LEADERS): a randomised non-inferiority trial. Lancet 2008, 372, 1163–1173. [Google Scholar] [CrossRef] [PubMed]
- Daemen, J.; Wenaweser, P.; Tsuchida, K.; et al. Early and late coronary stent thrombosis of sirolimus-eluting and paclitaxel-eluting stents in routine clinical practice: data from a large two-institutional cohort study. Lancet 2007, 369, 667–678. [Google Scholar] [CrossRef] [PubMed]
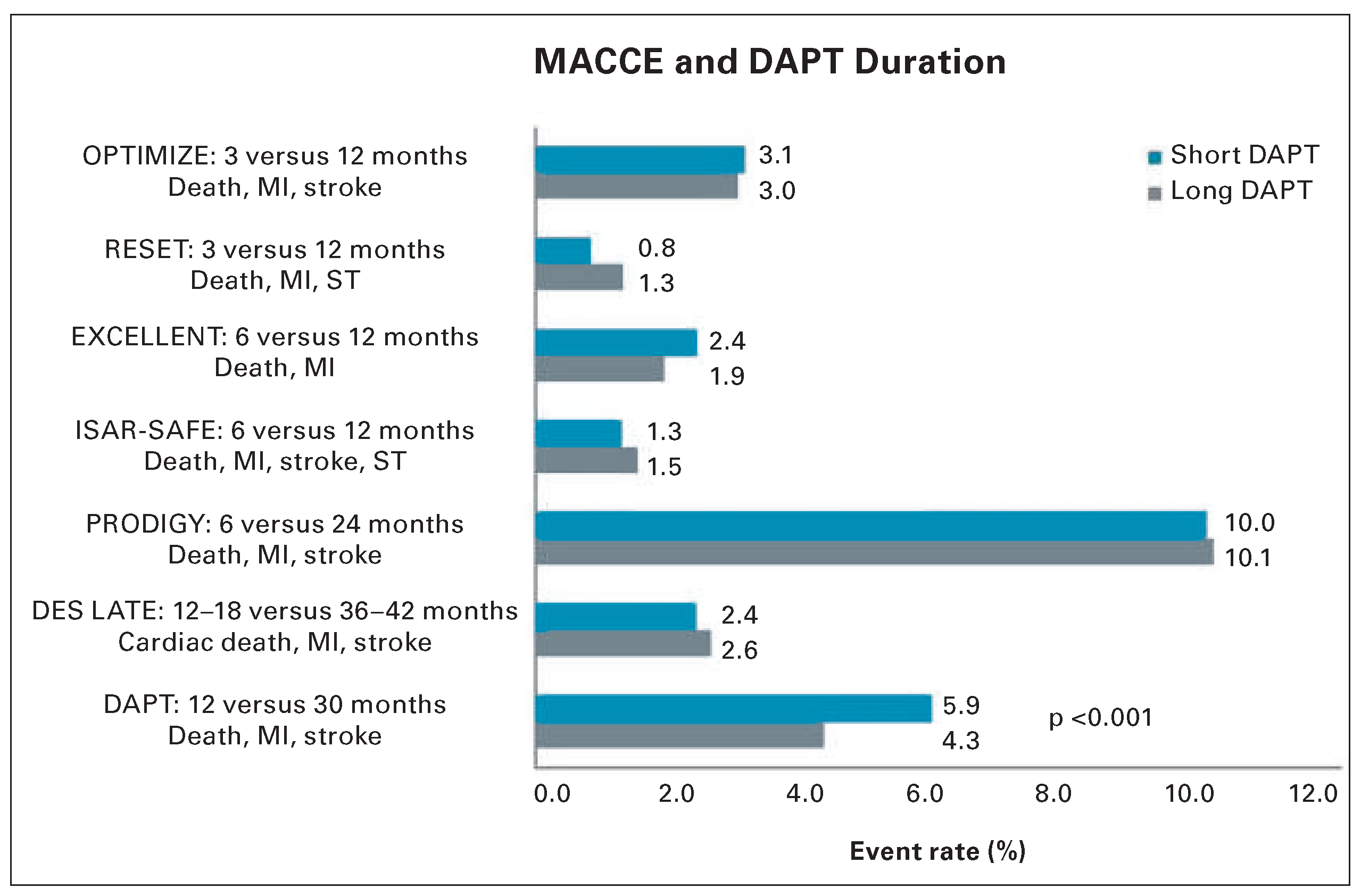
- Feres, F.; Costa, R.A.; Abizaid, A.; et al. Three vs twelve months of dual antiplatelet therapy aher zotarolimus-eluting stents: the OPTIMIZE randomized trial. Jama 2013, 310, 2510–2522. [Google Scholar] [PubMed]
- Gwon, H.C.; Hahn, J.Y.; Park, K.W.; et al. Six-month versus 12–month dual antiplatelet therapy aher implantation of drug-eluting stents: the Efficacy of Xience/Promus Versus Cypher to Reduce Late Loss Aher Stenting (EXCELLENT) randomized, multicenter study. Circulation 2012, 125, 505–513. [Google Scholar] [CrossRef] [PubMed]
- Valgimigli, M.; Campo, G.; Monti, M.; et al. Shortversus long-term duration of dual-antiplatelet therapy aher coronary stenting: a randomized multicenter trial. Circulation 2012, 125, 2015–2026. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.W.; Ahn, J.M.; Park, D.W.; et al. Optimal duration of dual antiplatelet therapy aher drug-eluting stent implantation: a randomized, controlled trial. Circulation 2014, 129, 304–312. [Google Scholar] [CrossRef] [PubMed]
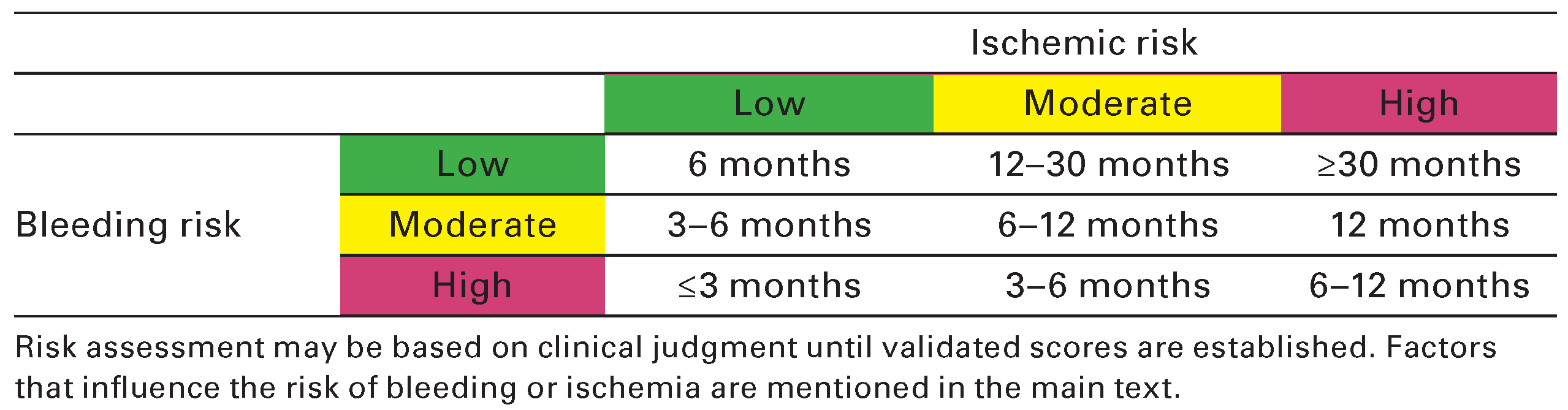
- Authors/Task Force m Windecker, S.; Kolh, P.; et al. 2014 ESC/EACTS Guidelines on myocardial revascularization: The Task Force on Myocardial Revascularization of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS)Developed with the special contribution of the European Association of Percutaneous Cardiovascular Interventions (EAPCI). European heart journal 2014, 35, 2541–2619. [Google Scholar]
- Mauri, L.; Kereiakes, D.J.; Yeh, R.W.; et al. Twelve or 30 months of dual antiplatelet therapy aher drug-eluting stents. The New England journal of medicine 2014, 371, 2155–2166. [Google Scholar] [CrossRef] [PubMed]
- Serruys, P.W.; Farooq, V.; Kalesan, B.; et al. Improved safety and reduction in stent thrombosis associated with biodegradable polymer-based biolimus-eluting stents versus durable polymer-based sirolimus-eluting stents in patients with coronary artery disease: final 5–year report of the LEADERS (Limus Eluted From A Durable Versus ERodable Stent Coating) randomized, noninferiority trial. JACC Cardiovascular interventions 2013, 6, 777–789. [Google Scholar] [PubMed]
- Rao, S.V.; McCoy, L.A.; Spertus, J.A.; et al. An updated bleeding model to predict the risk of post-procedure bleeding among patients undergoing percutaneous coronary intervention: a report using an expanded bleeding definition from the National Cardiovascular Data Registry CathPCI Registry. JACC Cardiovascular interventions 2013, 6, 897–904. [Google Scholar] [CrossRef] [PubMed]
- Jeger, R.V.; Pfisterer, M.E.; Sorensen, R.; et al. Tradeoff between bleeding and stent thrombosis in different dual antiplatelet therapy regimes: Importance of case fatality rates and effective treatment durations. American heart journal 2014, 168, 698–705. [Google Scholar] [CrossRef] [PubMed]

© 2015 by the author. Attribution-Non-Commercial-NoDerivatives 4.0.
Share and Cite
Binder, R.K.; Lüscher, T.F. Duration of Dual Antiplatelet Therapy After Coronary Artery Stenting. Cardiovasc. Med. 2015, 18, 3. https://doi.org/10.4414/cvm.2015.00308
Binder RK, Lüscher TF. Duration of Dual Antiplatelet Therapy After Coronary Artery Stenting. Cardiovascular Medicine. 2015; 18(1):3. https://doi.org/10.4414/cvm.2015.00308
Chicago/Turabian StyleBinder, Ronald K., and Thomas F. Lüscher. 2015. "Duration of Dual Antiplatelet Therapy After Coronary Artery Stenting" Cardiovascular Medicine 18, no. 1: 3. https://doi.org/10.4414/cvm.2015.00308
APA StyleBinder, R. K., & Lüscher, T. F. (2015). Duration of Dual Antiplatelet Therapy After Coronary Artery Stenting. Cardiovascular Medicine, 18(1), 3. https://doi.org/10.4414/cvm.2015.00308



