The Heart and Cirrhosis
Abstract
Clinical vignette
Introduction
Pathophysiology
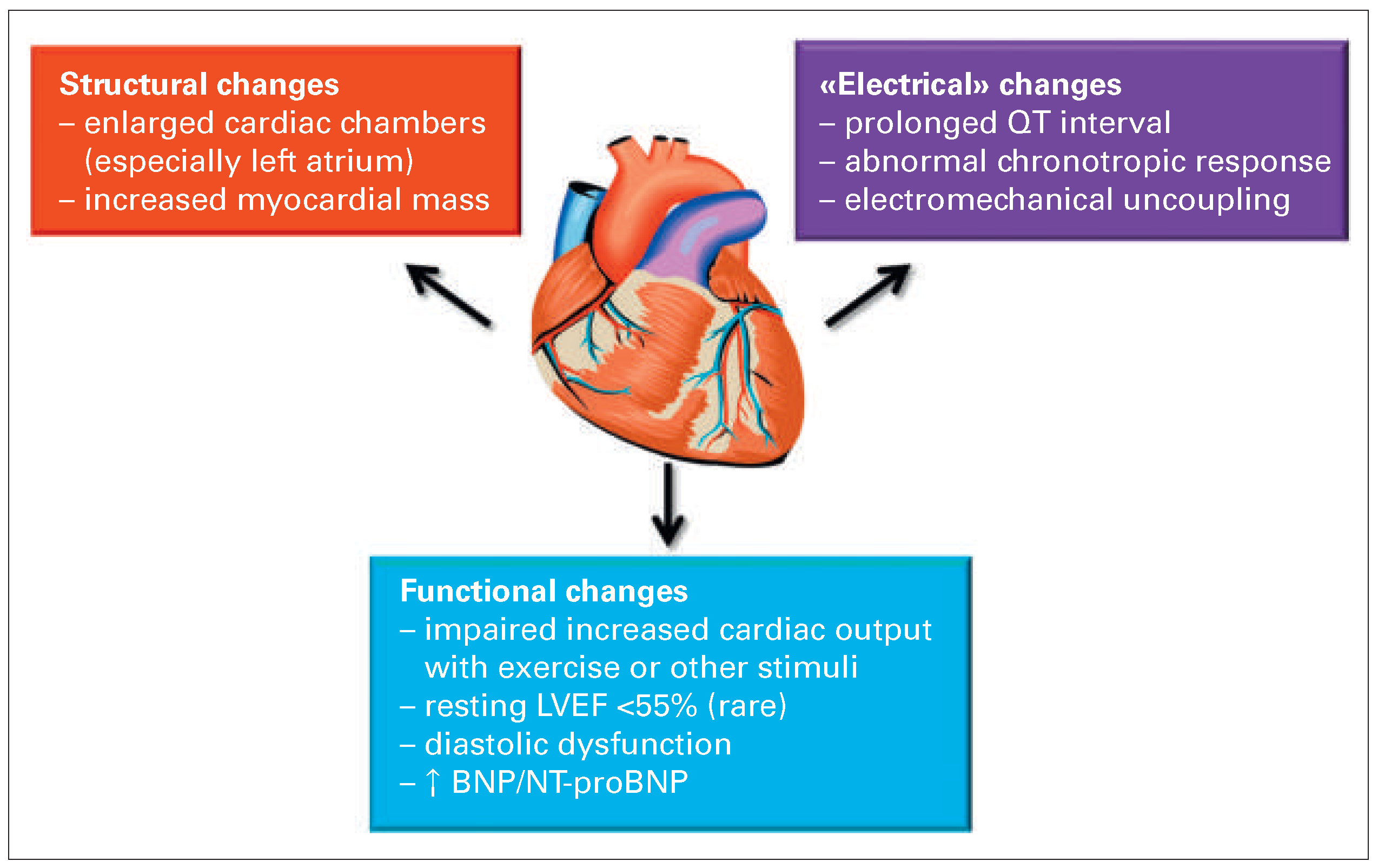
Features of cirrhotic cardiomyopathy
Structural changes
Impaired cardiac response to exercise
Diastolic dysfunction
Prolonged QT interval
Natriuretic peptides
Clinical relevance of cirrhotic cardiomyopathy
Treatment of cirrhotic cardiomyopathy
Conclusions
Funding/potential competing interests
References
- Moller, S.; Bernardi, M. Interactions of the heart and the liver. Eur Heart J. 2013, 34, 2804–2811. [Google Scholar] [CrossRef] [PubMed]
- Wiese, S.; Hove, J.D.; Bendtsen, F.; Moller, S. Cirrhotic cardiomyopathy: pathogenesis and clinical relevance. Nature reviews Gastroenterology & hepatology. 2014, 11, 177–186. [Google Scholar]
- Moller, S.; Henriksen, J.H. Cardiovascular complications of cirrhosis. Gut. 2008, 57, 268–278. [Google Scholar] [CrossRef] [PubMed]
- Timoh, T.; Protano, M.A.; Wagman, G.; Bloom, M.; Vittorio, T.J. A perspective on cirrhotic cardiomyopathy. Transplant Proc. 2011, 43, 1649–1653. [Google Scholar] [CrossRef]
- Kowalski, H.J.; Abelmann, W.H. The cardiac output at rest in Laennec’s cirrhosis. J Clin Invest. 1953, 32, 1025–1033. [Google Scholar] [CrossRef]
- Duffin, J.M. Why does cirrhosis belong to Laennec? CMAJ. 1987, 137, 393–396. [Google Scholar]
- Laleman, W.; Landeghem, L.; Wilmer, A.; Fevery, J.; Nevens, F. Portal hypertension: from pathophysiology to clinical practice. Liver Int. 2005, 25, 1079–1090. [Google Scholar] [CrossRef]
- Moller, S.; Iversen, J.S.; Henriksen, J.H.; Bendtsen, F. Reduced baroreflex sensitivity in alcoholic cirrhosis: relations to hemodynamics and humoral systems. Am J Physiol Heart Circ Physiol. 2007, 292, H2966–72. [Google Scholar] [CrossRef]
- Ortiz-Olvera, N.X.; Castellanos-Pallares, G.; Gomez-Jimenez, L.M.; Cabrera-Munoz, M.L.; Mendez-Navarro, J.; Moran-Villota, S.; et al. Anatomical cardiac alterations in liver cirrhosis: an autopsy study. Ann Hepato. 2011, 10, 321–326. [Google Scholar] [CrossRef]
- Abd-El-Aziz, T.A.; Abdou, M.; Fathy, A.; Wafaie, M. Evaluation of cardiac function in patients with liver cirrhosis. Int Med. 2010, 49, 2547–2552. [Google Scholar] [CrossRef]
- Moller, S.; Sondergaard, L.; Mogelvang, J.; Henriksen, O.; Henriksen, J.H. Decreased right heart blood volume determined by magnetic resonance imaging: evidence of central underfilling in cirrhosis. Hepatology. 1995, 22, 472–478. [Google Scholar]
- Wong, F.; Girgrah, N.; Graba, J.; Allidina, Y.; Liu, P.; Blendis, L. The cardiac response to exercise in cirrhosis. Gut. 2001, 49, 268–275. [Google Scholar] [CrossRef]
- Colombato, L.A.; Spahr, L.; Martinet, J.P.; Dufresne, M.P.; Lafortune, M.; Fenyves, D.; et al. Haemodynamic adaptation two months aher transjugular intrahepatic portosystemic shunt (TIPS) in cirrhotic patients. Gut. 1996, 39, 600–604. [Google Scholar] [CrossRef]
- Gerbes, A.L.; Remien, J.; Jungst, D.; Sauerbruch, T.; Paumgartner, G. Evidence for down-regulation of beta-2–adrenoceptors in cirrhotic patients with severe ascites. Lancet. 1986, 1, 1409–11. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.S.; Marty, J.; Mantz, J.; Samain, E.; Braillon, A.; Lebrec, D. Desensitization of myocardial beta-adrenergic receptors in cirrhotic rats. Hepatology. 1990, 12 (Pt 1), 481–485. [Google Scholar] [CrossRef] [PubMed]
- Ward, C.A.; Liu, H.; Lee, S.S. Altered cellular calcium regulatory systems in a rat model of cirrhotic cardiomyopathy. Gastroenterology. 2001, 121, 1209–1218. [Google Scholar]
- Liu, H.; Gaskari, S.A.; Lee, S.S. Cardiac and vascular changes in cirrhosis: pathogenic mechanisms. World J Gastroenterol. 2006, 12, 837–842. [Google Scholar] [CrossRef] [PubMed]
- Bernardi, M.; Calandra, S.; Colantoni, A.; Trevisani, F.; Raimondo, M.L.; Sica, G.; et al. Q-T interval prolongation in cirrhosis: prevalence, relationship with severity, and etiology of the disease and possible pathogenetic factors. Hepatology. 1998, 27, 28–34. [Google Scholar] [CrossRef]
- Zambruni, A.; Trevisani, F.; Di Micoli, A.; Savelli, F.; Berzigotti, A.; Bracci, E.; et al. Effect of chronic beta-blockade on QT interval in patients with liver cirrhosis. J Hepatol. 2008, 48, 415–421. [Google Scholar] [CrossRef]
- Henriksen, J.H.; Gotze, J.P.; Fuglsang, S.; Christensen, E.; Bendtsen, F.; Moller, S. Increased circulating pro-brain natriuretic peptide (proBNP) and brain natriuretic peptide (BNP) in patients with cirrhosis: relation to cardiovascular dysfunction and severity of disease. Gut. 2003, 52, 1511–1517. [Google Scholar] [CrossRef]
- Krag, A.; Bendtsen, F.; Henriksen, J.H.; Moller, S. Low cardiac output predicts development of hepatorenal syndrome and survival in patients with cirrhosis and ascites. Gut. 2010, 59, 105–110. [Google Scholar] [CrossRef]
- Rabie, R.N.; Cazzaniga, M.; Salerno, F.; Wong, F. The use of E/A ratio as a predictor of outcome in cirrhotic patients treated with transjugular intrahepatic portosystemic shunt. Am J Gastroenterol. 2009, 104, 2458–2466. [Google Scholar] [CrossRef] [PubMed]
- Henriksen, J.H.; Bendtsen, F.; Hansen, E.F.; Moller, S. Acute non-selective beta-adrenergic blockade reduces prolonged frequencyadjusted Q-T interval (QTc) in patients with cirrhosis. J Hepatol. 2004, 40, 239–246. [Google Scholar] [CrossRef] [PubMed]
- Serste, T.; Melot, C.; Francoz, C.; Durand, F.; Rautou, P.E.; Valla, D.; et al. Deleterious effects of beta-blockers on survival in patients with cirrhosis and refractory ascites. Hepatology. 2010, 52, 1017–1022. [Google Scholar] [CrossRef] [PubMed]
- Pozzi, M.; Grassi, G.; Ratti, L.; Favini, G.; Dell’Oro, R.; Redaelli, E.; et al. Cardiac, neuroadrenergic, and portal hemodynamic effects of prolonged aldosterone blockade in postviral child A cirrhosis. Am J Gastroenterol. 2005, 100, 1110–1116. [Google Scholar] [CrossRef]
- Fouad, T.R.; Abdel-Razek, W.M.; Burak, K.W.; Bain, V.G.; Lee, S.S. Prediction of cardiac complications aher liver transplantation. Transplantation. 2009, 87, 763–770. [Google Scholar] [CrossRef]


© 2015 by the author. Attribution-Non-Commercial-NoDerivatives 4.0.
Share and Cite
Meyer, P.; Spahr, L. The Heart and Cirrhosis. Cardiovasc. Med. 2015, 18, 20. https://doi.org/10.4414/cvm.2015.00299
Meyer P, Spahr L. The Heart and Cirrhosis. Cardiovascular Medicine. 2015; 18(1):20. https://doi.org/10.4414/cvm.2015.00299
Chicago/Turabian StyleMeyer, Philippe, and Laurent Spahr. 2015. "The Heart and Cirrhosis" Cardiovascular Medicine 18, no. 1: 20. https://doi.org/10.4414/cvm.2015.00299
APA StyleMeyer, P., & Spahr, L. (2015). The Heart and Cirrhosis. Cardiovascular Medicine, 18(1), 20. https://doi.org/10.4414/cvm.2015.00299



