Left Atrial Appendage Device Closure as Non-Pharmacological Prevention of Thromboembolism in Atrial Fibrillation
Abstract
Introduction
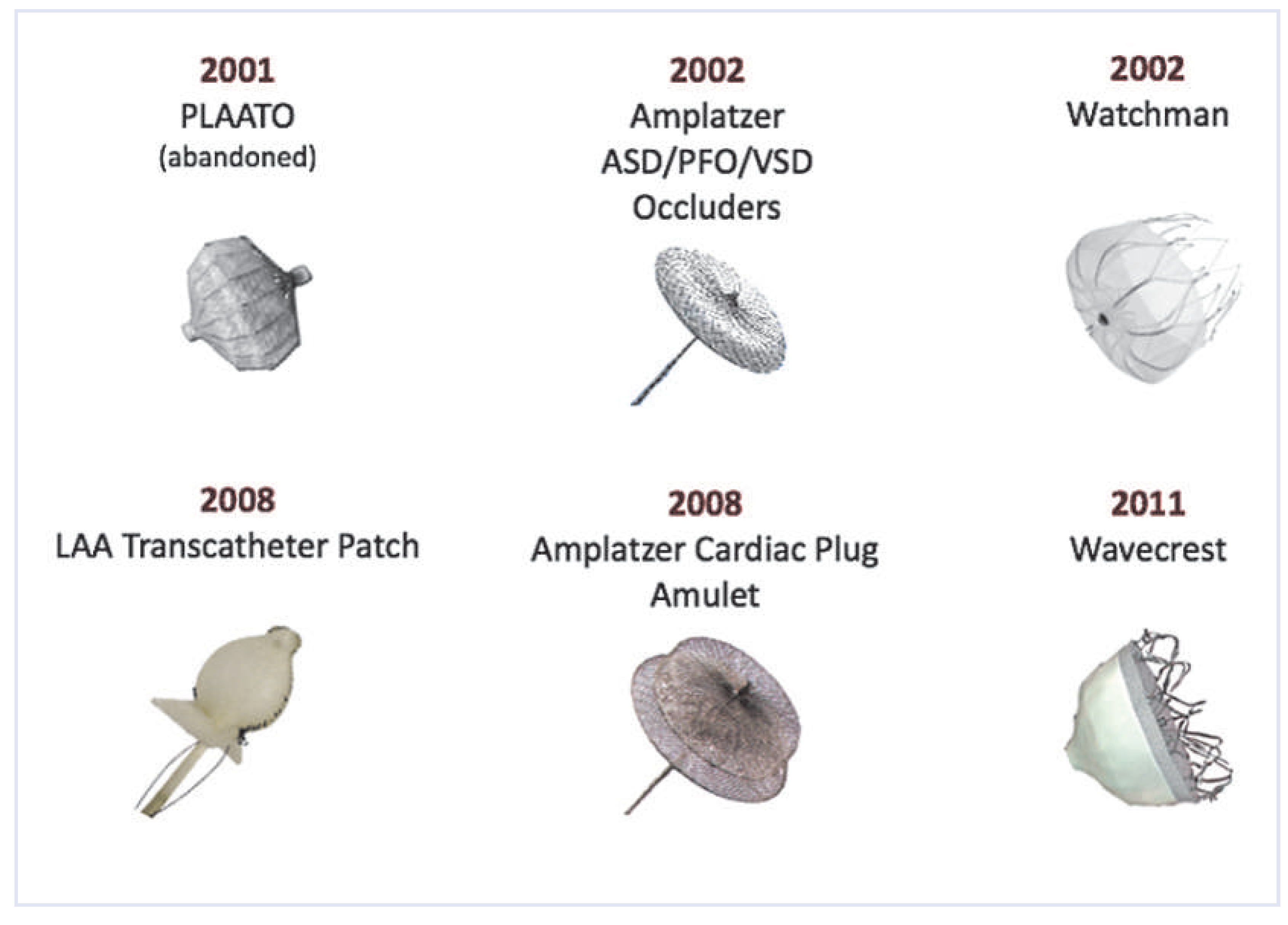
Techniques of left atrial appendage occlusion
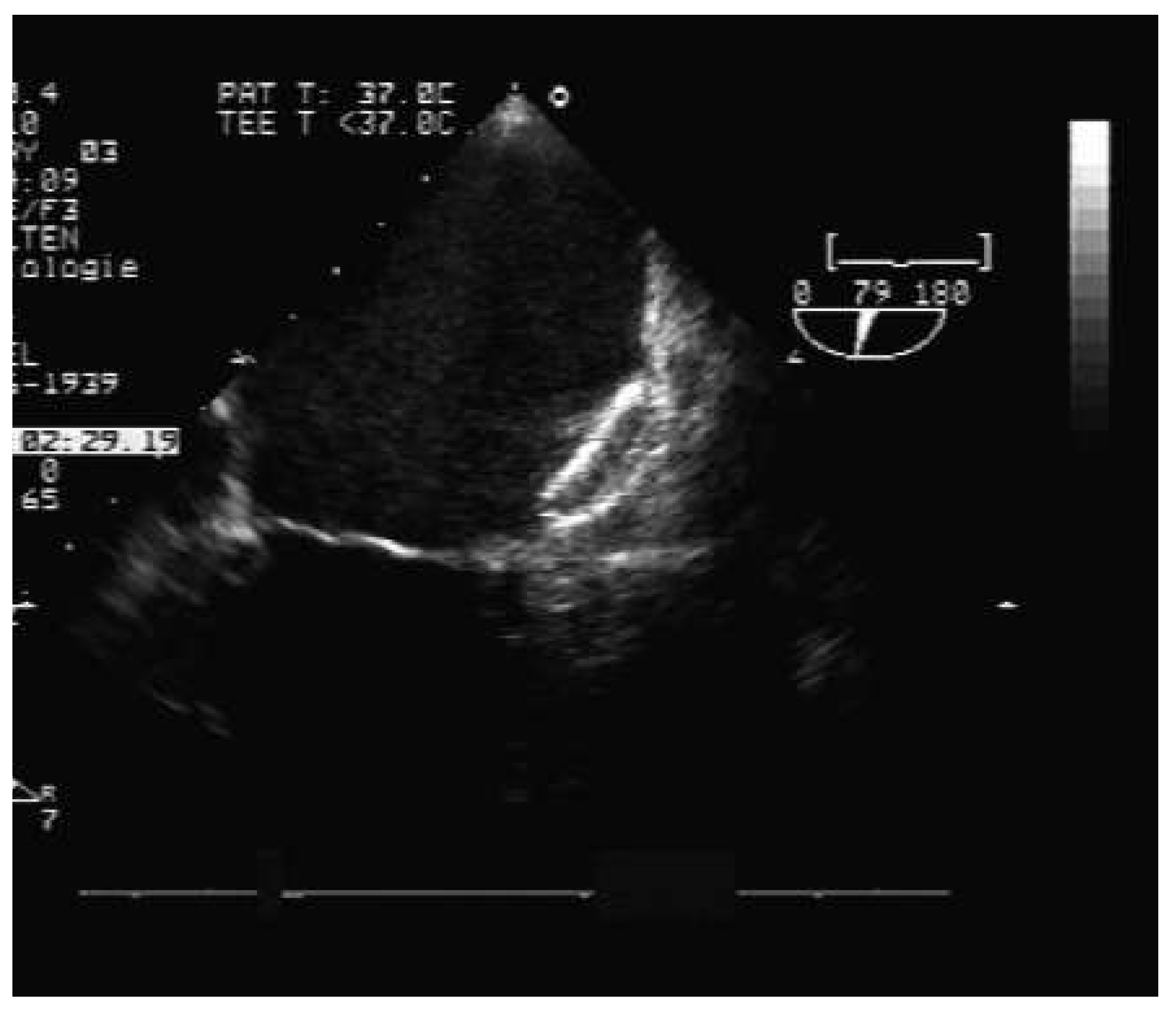
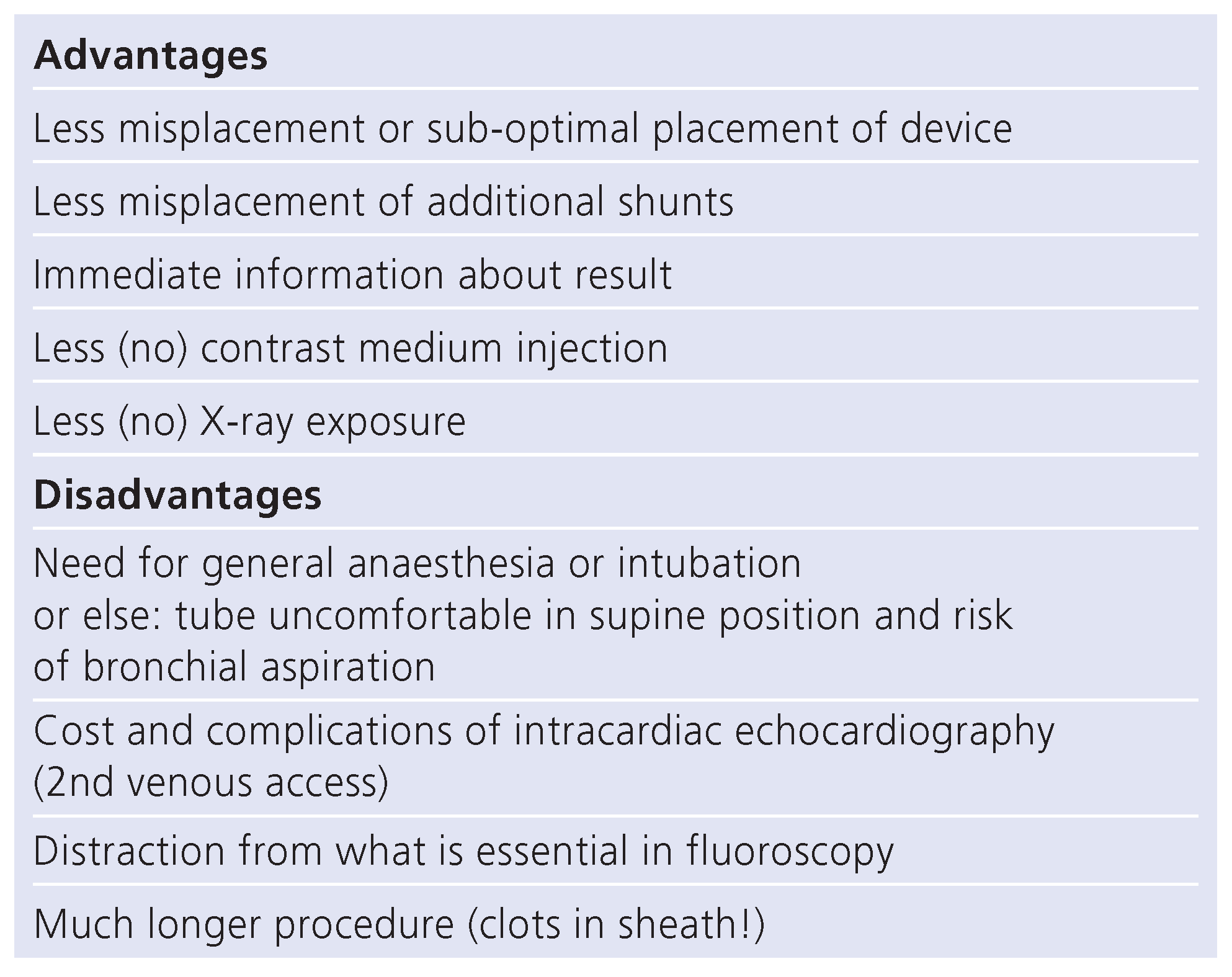
Percutaneous technique for left atrial appendage occlusion
Complications of left atrial appendage occlusion
Clinical results of left atrial appendage occlusion
Conclusion
Funding/potential competing interests:
References
- Blackshear, J.; Odell, J. Appendage obliteration to reduce stroke in cardiac surgical patients with atrial fibrillation. Ann Thorac Surg. 1996, 61, 755–759. [Google Scholar] [CrossRef] [PubMed]
- Madden, J. Resection of the left auricular appendix; a prophylaxis for recurrent arterial emboli. J Am Med Assoc. 1949, 140, 769–772. [Google Scholar] [CrossRef] [PubMed]
- Bonow, R.; Carabello, B.; Chatterjee, K.; de Leon, A.J.; Faxon, D.; Freed, M.; et al. ACC/AHA 2006 guidelines for the management of patients with valvular heart disease: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (writing Committee to Revise the 1998 guidelines for the management of patients with valvular heart disease) developed in collaboration with the Society of Cardiovascular Anesthesiologists endorsed by the Society for Cardiovascular Angiography and Interventions and the Society of Thoracic Surgeons. J Am Coll Cardiol. 2006, 48, e1–e148. [Google Scholar] [PubMed]
- Sievert, H.; Lesh, M.; Trepels, T.; Omran, H.; Bartorelli, A.; Della Bella, P.; et al. Percutaneous left atrial appendage transcatheter occlusion to prevent stroke in high-risk patients with atrial fibrillation: Early clinical experience. Circulation 2002, 105, 1887–1889. [Google Scholar] [CrossRef] [PubMed]
- Ostermayer, S.; Reisman, M.; Kramer, P.; Matthews, R.; Gray, W.; Block, P.; et al. Percutaneous left atrial appendage transcatheter occlusion (PLAATO system) to prevent stroke in high-risk patients with non-rheumatic atrial fibrillation: Results from the international multi-center feasibility trials. J Am Coll Cardiol. 2005, 46, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Bayard, Y.; Omran, H.; Neuzil, P.; Thuesen, L.; Pichler, M.; Rowland, E.; et al. PLAATO (Percutaneous Left Atrial Appendage Transcatheter Occlusion) for prevention of cardioembolic stroke in non-anticoagulation eligible atrial fibrillation patients: Results from the European PLAATO study. EuroIntervention 2010, 6, 220–226. [Google Scholar] [CrossRef] [PubMed]
- Meier, B.; Palacios, I.; Windecker, S.; Rotter, M.; Cao, Q.; Keane, D.; et al. Transcatheter left atrial appendage occlusion with Amplatzer devices to obviate anticoagulation in patients with atrial fibrillation. Catheter Cardiovasc Interv. 2003, 60, 417–422. [Google Scholar] [CrossRef] [PubMed]
- Sick, P.; Schuler, G.; Hauptmann, K.; Grube, E.; Yakubov, S.; Turi, Z.; et al. Initial worldwide experience with the Watchman left atrial appendage system for stroke prevention in atrial fibrillation. J Am Coll Cardiol. 2007, 49, 1490–1495. [Google Scholar] [CrossRef] [PubMed]
- Freixa, X.; Chan, J.L.; Tzikas, A.; Garceau, P.; Basmadjian, A.; Ibrahim, R. The Amplatzer Cardiac Plug 2 for left atrial appendage occlusion: Novel features and first-in-man experience. EuroIntervention 2013, 8, 1094–1098. [Google Scholar] [CrossRef] [PubMed]
- Nietlispach, F.; Gloekler, S.; Krause, R.; Shakir, S.; Schmid, M.; Khattab, A.A.; et al. Amplatzer left atrial appendage occlusion: Single center 10-year experience. Catheter Cardiovasc Interv. 2013, 82, 283–289. [Google Scholar] [CrossRef] [PubMed]
- Lee, R.; Bartus, K.; Yakubov, S. Catheter-based left atrial appendage (LAA) ligation for the prevention of embolic events arising from the LAA: Initial experience in a canine model. Circ Cardiovasc Interv. 2010, 3, 224–229. [Google Scholar] [CrossRef] [PubMed]
- Bartus, K.; Bednarek, J.; Myc, J.; Kapelak, B.; Sadowski, J.; Lelakowski, J.; et al. Feasibility of closed-chest ligation of the left atrial appendage in humans. Heart Rhythm. 2011, 8, 188–193. [Google Scholar] [CrossRef] [PubMed]
- Shetty, R.; Leitner, J.; Zhang, M. Percutaneous catheter-based left atrial appendage ligation and management of periprocedural left atrial appendage perforation with the LARIAT suture delivery system. J Invasive Cardiol. 2012, 24, E289–93. [Google Scholar] [PubMed]
- Friedman, P.A.; Asirvatham, S.J.; Dalegrave, C.; Kinoshita, M.; Danielsen, A.J.; Johnson, S.B.; et al. Percutaneous epicardial left atrial appendage closure: Preliminary results of an electrogram guided approach. J Cardiovasc Electrophysiol. 2009, 20, 908–915. [Google Scholar] [CrossRef] [PubMed]
- Meier, B.; Blaauw, Y.; Khattab, A.; Lewalter, T.; Sievert, H.; Tondo, C.; et al. Catheter Based Left Atrial Appendage Occlusion, Scientific document supported by the European Association of Percutaneous Coronary Interventions (EAPCI) and the European Heart Rhythm Association (EHRA). EuroInterv, 2014; in print. [Google Scholar]
- Santoro, G.; Meucci, F.; Stolcova, M.; Rezzaghi, M.; Mori, F.; Balmieri, C.; et al. Percutaneous left atrial appendage occlusion with patients with nonvalvular atial fibrillation: Implantation and op to four-years follow-up of the Amplatzer cardiac Plug. EuroInterv, 2014; in print. [Google Scholar]
- Nietlispach, F.; Krause, R.; Khattab, A.; Gloekler, S.; Schmid, M.; Wenaweser, P.; et al. Ad hoc percutaneous left atrial appendage closure. J Invasive Cardiol. 2013, 25, 683–686. [Google Scholar] [PubMed]
- Hannazawa, K.; Brunelli, M.; Saenger, J.; Grosse, A.; Santi, R.; Lauer, B.; et al. Clouse proximity between pulmonary artery and left atrial appendage leading to perforation of the artery tamponade and death after appendage closure you using cardiac plug device. Interv J Cardiol. 2014; in print. [Google Scholar]
- Park, J.; Bethencourt, A.; Sievert, H.; Santoro, G.; Meier, B.; Walsh, K.; et al. Leithauser B. Left atrial appendage closure with Amplatzer Cardiac Plug in atrial fibrillation: Initial European experience. Catheter Cardiovasc Interv. 2011, 77, 700–706. [Google Scholar] [PubMed]
- Reddy, V. Watchman LAA closure device reduces the risk of ischemic stroke in patients with AF entirely without anticoagulation. The ASAPlavix (ASAP) Registry. In Proceedings of the Presentation of Transcatheter Therapeutics (TCT), Miami, Florida, USA, November 2012. [Google Scholar]
- Tzikas, A.; Shakir, S.; Sievert, H.; Omran, H.; Berti, S.; Santoro, G.; et al. Left atrial appendage occlusion for stroke prevention in atrial fibrillation: Multicenter experience with the Amplatzer Cardiac Plug. EuroInterv, 2014; in print. [Google Scholar]
- Singh, S.M.; Micieli, A.; Wijeysundera, H.C. Economic evaluation of percutaneous left atrial appendage occlusion, dabigatran, and warfarin for stroke prevention in patients with nonvalvular atrial fibrillation. Circulation 2013, 127, 2414–2423. [Google Scholar] [CrossRef] [PubMed]

 |
© 2014 by the author. Attribution - Non-Commercial - NoDerivatives 4.0.
Share and Cite
Meier, B. Left Atrial Appendage Device Closure as Non-Pharmacological Prevention of Thromboembolism in Atrial Fibrillation. Cardiovasc. Med. 2014, 17, 252. https://doi.org/10.4414/cvm.2014.00277
Meier B. Left Atrial Appendage Device Closure as Non-Pharmacological Prevention of Thromboembolism in Atrial Fibrillation. Cardiovascular Medicine. 2014; 17(9):252. https://doi.org/10.4414/cvm.2014.00277
Chicago/Turabian StyleMeier, Bernhard. 2014. "Left Atrial Appendage Device Closure as Non-Pharmacological Prevention of Thromboembolism in Atrial Fibrillation" Cardiovascular Medicine 17, no. 9: 252. https://doi.org/10.4414/cvm.2014.00277
APA StyleMeier, B. (2014). Left Atrial Appendage Device Closure as Non-Pharmacological Prevention of Thromboembolism in Atrial Fibrillation. Cardiovascular Medicine, 17(9), 252. https://doi.org/10.4414/cvm.2014.00277




