The Infamous Coronary Stent Saga
Abstract
Introduction
History of coronary stenting
Clinical results of coronary stents and their many misinterpretations
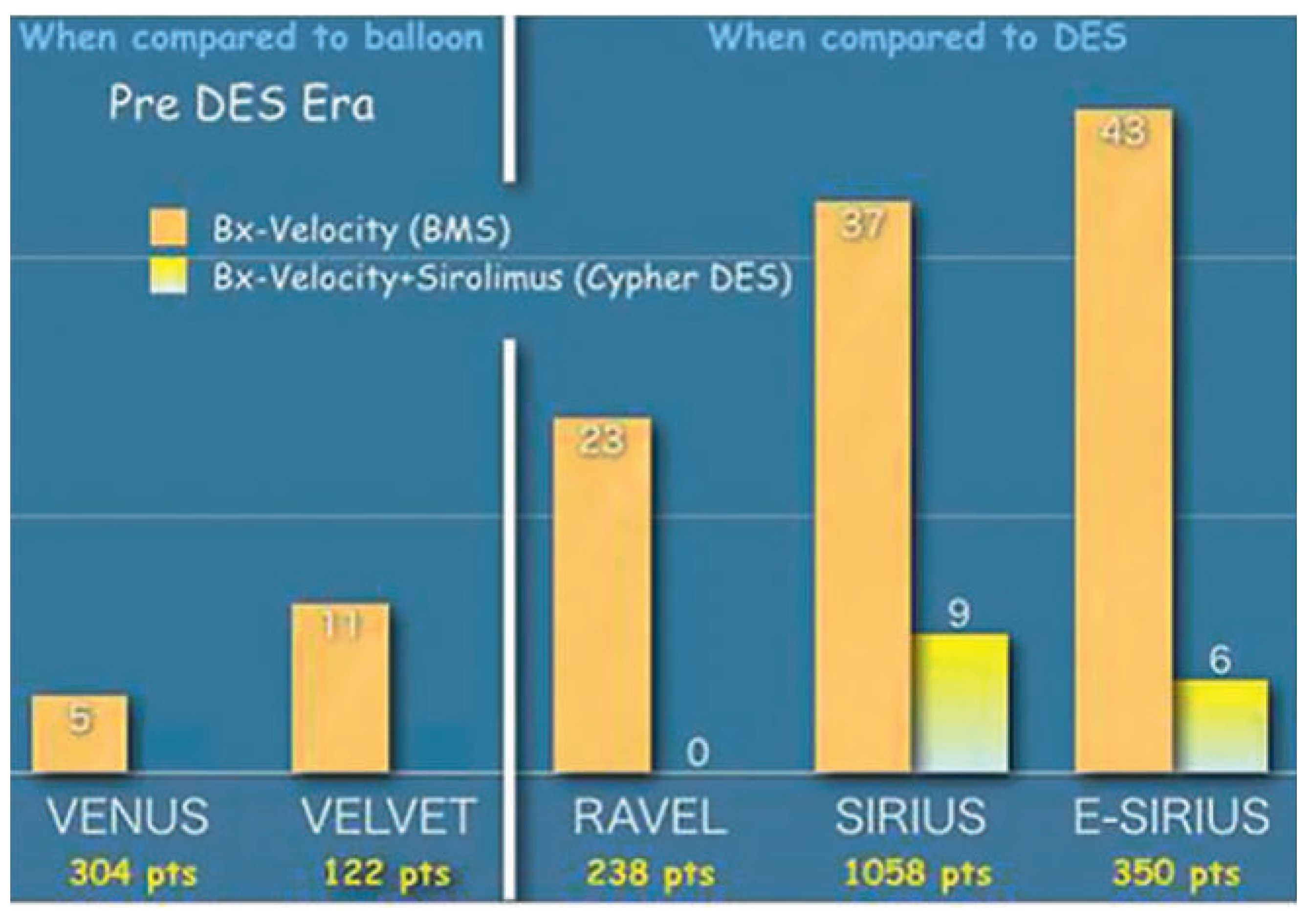
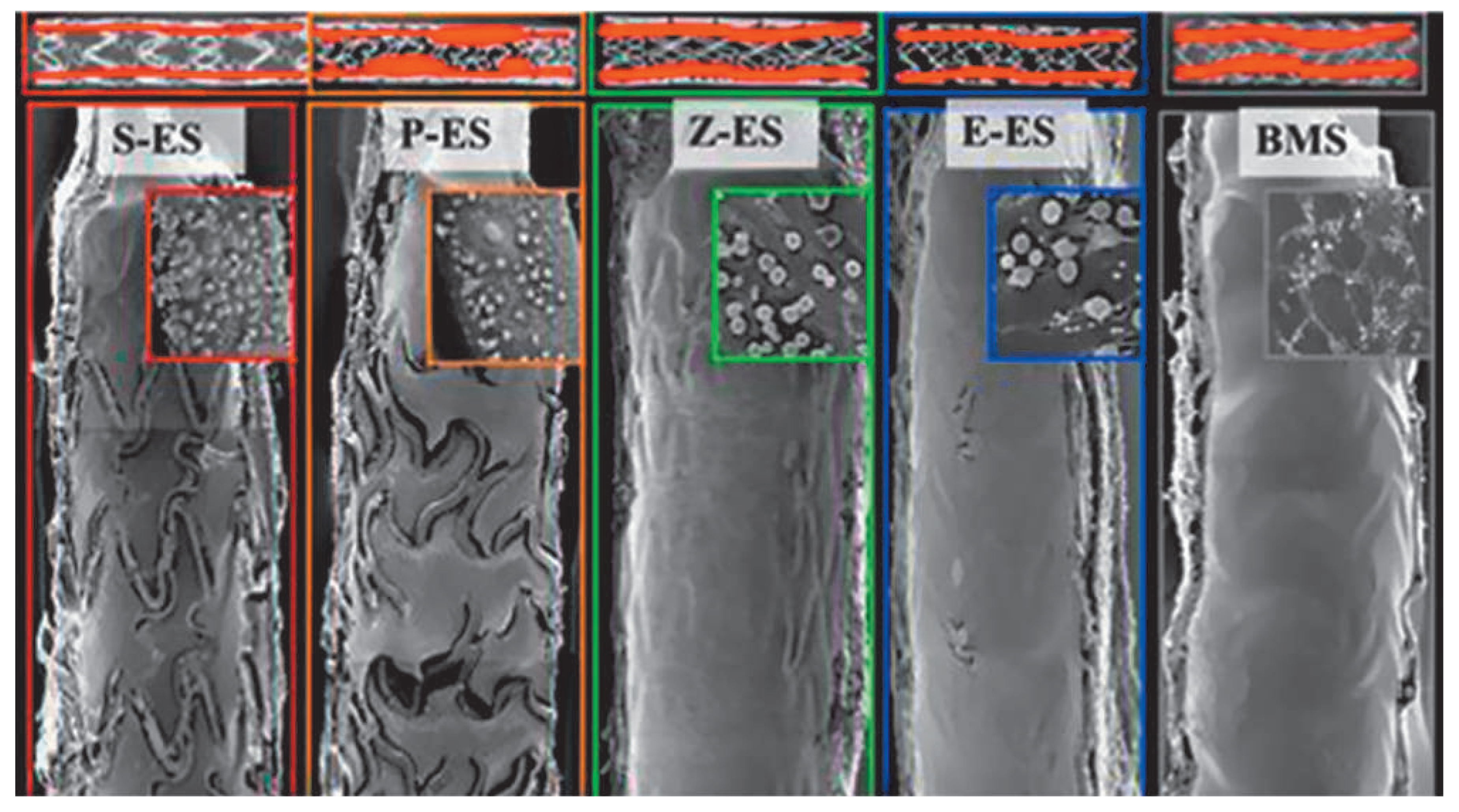
Drug-eluting stents and the restenosis bubble
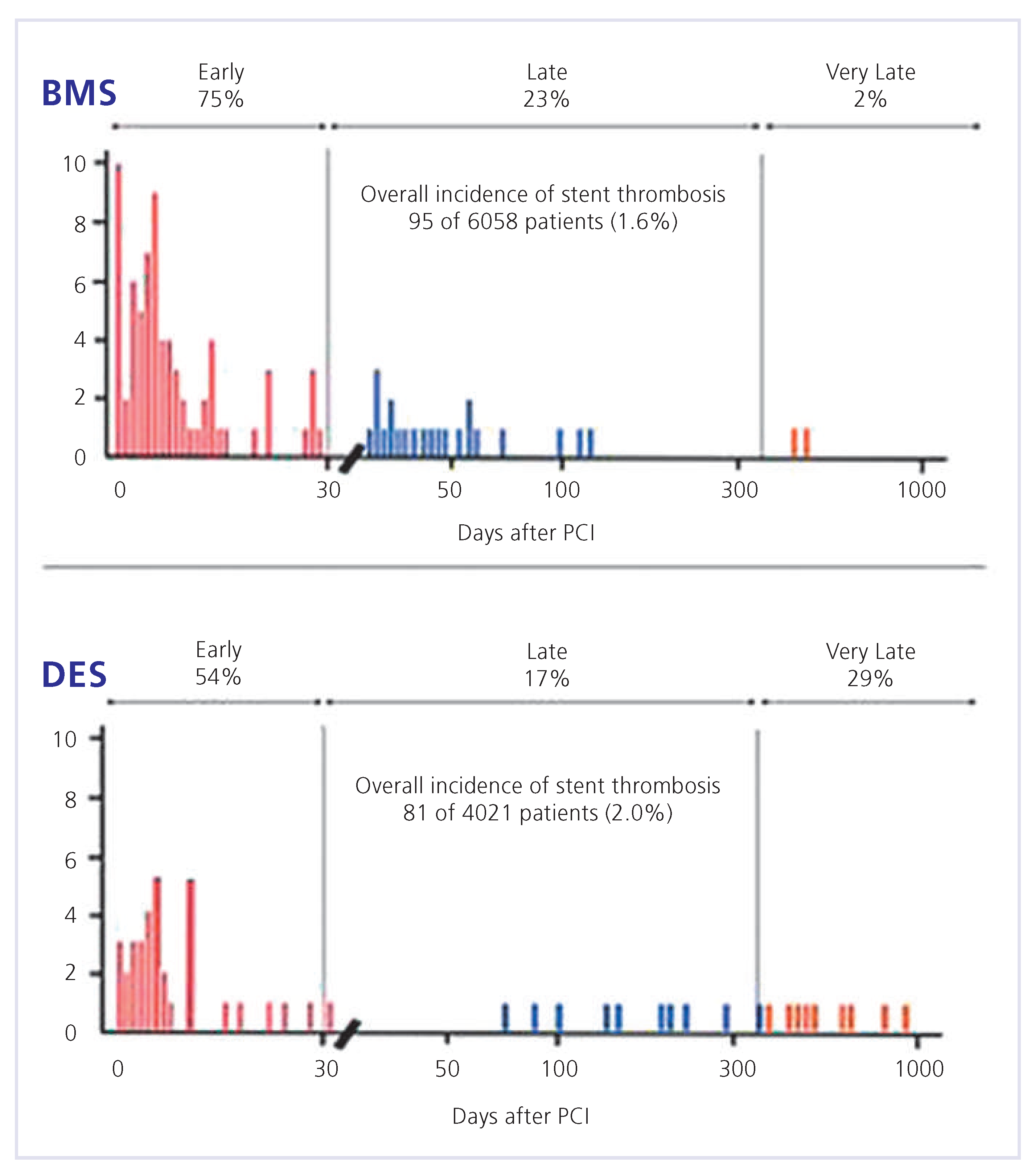
Drug-eluting stents and the double-antiplatelet therapy myth
Conclusion
Funding/potential competing interests:
References
- Roguin, A. Stent: The man and world behind the coronary metal prosthesis. Circ Cardiovasc Interv. 2011, 4, 206–209. [Google Scholar] [CrossRef]
- Puel, J.; Joffre, F.; Rousseau, H.; Guermonprez, B.; Lancelin, B.; Valeix, B.; et al. Endo-prothèses coronariennes auto-expansives dans la prévention des resténoses après angioplastie transluminale. Arch Mal Coeur. 1987, 8, 1311–1312. [Google Scholar]
- Detre, K.M.; Holmes, D.R.; Holubkov, R., Jr.; Cowley, M.J.; Bourassa, M.G.; Faxon, D.P.; et al. Incidence and consequences of periprocedural occlusion. The 1985–1986 National Heart, Lung, and Blood Institute Percutaneous Transluminal Coronary Angioplasty Registry. Circulation 1990, 82, 739–750. [Google Scholar] [CrossRef]
- Schömig, A.; Kastrati, A.; Mudra, H.; Blasini, R.; Schuhlen, H.; Klauss, V.; et al. Four-year experience with Palmaz-Schatz stenting in coronary angioplasty complicated by dissection with threatened or present vessel closure. Circulation 1994, 90, 2716–2724. [Google Scholar] [CrossRef]
- Brophy, J.M.; Belisle, P.; Joseph, L. Evidence for use of coronary stents. A hierarchical bayesian meta-analysis. Ann Intern Med. 2003, 138, 777–786. [Google Scholar] [CrossRef]
- Kiemeneij, F.; Serruys, P.W.; Macaya, C.; Rutsch, W.; Heyndrickx, G.; Albertsson, P.; et al. Continued benefit of coronary stenting versus balloon angioplasty: Five-year clinical follow-up of Benestent-I trial. J Am Coll Cardiol. 2001, 37, 1598–1603. [Google Scholar] [CrossRef]
- Zidar, J.P.; Fry, E.; Lambert, C.; Rubinstein, R.; Raizner, A.E.; Fischell, T.A.; et al. The VENUS trial: A multi-center registry of the Cordis Bx VELOCITY Stent. Am J Cardiol 2000, 86 (Suppl. 1), 17i. [Google Scholar]
- Serruys, P.; Ijsselmuiden, S.; Hout, B.; Vermeersch, P.; Bramucci, E.; Legrand, V.; et al. Direct stenting with the Bx Velocity balloon-expandable stent mounted on the raptor rapid exchange delivery system versus predilatation in a European randomized trial (VELVET). Int J Cardiovasc Intervent. 2003, 5, 17–26. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Morice, M.C.; Serruys, P.W.; Sousa, J.E.; Fajadet, J.; Ban Hayashi, E.; Perin, M.; et al. A randomized comparison of a sirolimus-eluting stent with a standard stent for coronary revascularization. N Engl J Med. 2002, 346, 1773–1780. [Google Scholar] [CrossRef]
- Moses, J.W.; Leon, M.B.; Popma, J.J.; Fitzgerald, P.J.; Holmes, D.R.; O’Shaughnessy, C.; et al. Sirolimus-eluting stents versus standard stents in patients with stenosis in a native coronary artery. N Engl J Med. 2003, 349, 1315–1323. [Google Scholar] [CrossRef]
- Schofer, J.; Schluter, M.; Gershlick, A.H.; Wijns, W.; Garcia, E.; Schampaert, E.; et al. Sirolimus-eluting stents for treatment of patients with long atherosclerotic lesions in small coronary arteries: Double-blind, randomised controlled trial (E-SIRIUS). Lancet. 2003, 362, 1093–1099. [Google Scholar]
- Meier, B. New devices for coronary angioplasty: The Emperor’s new clothes revisited. Am J Med. 1995, 98, 429–431. [Google Scholar] [CrossRef]
- Togni, M.; Windecker, S.; Meier, B. Treatment of Restenosis. Curr Interv Cardiol Rep. 2001, 3, 306–310. [Google Scholar]
- Wenaweser, P.; Rey, C.; Eberli, F.R.; Togni, M.; Tuller, D.; Locher, S.; et al. Stent thrombosis following bare-metal stent implantation: Success of emergency percutaneous coronary intervention and predictors of adverse outcome. Eur Heart J. 2005, 26, 1180–1187. [Google Scholar] [CrossRef]
- Wenaweser, P.; Daemen, J.; Zwahlen, M.; Van Domburg, R.; Jüni, P.; Vaina, S.; et al. Incidence and correlates of drug-eluting stent thrombosis in routine clinical practice: 4-year results from a large 2-institutional cohort study. JACC 2008, 52, 1134–1140. [Google Scholar] [CrossRef] [PubMed]
- Camenzind, E. Treatment of in-stent restenosis–back to the future? N Engl J Med. 2006, 16, 2149–2151. [Google Scholar] [CrossRef]
- Jensen, L.O.; Maeng, M.; Kaltoft, A.; Thayssen, P.; Hansen, H.H.; Bottcher; et al. Stent thrombosis, myocardial infarction, and death after drug-eluting and bare-metal stent coronary interventions. J Am Coll Cardiol. 2007, 50, 463–470. [Google Scholar] [CrossRef] [PubMed]
- Sarno, G.; Lagerqvist, B.; Fröbert, O.; Nilsson, J.; Olivecrona, G.; Omerovic, E.; et al. Lower risk of stent thrombosis and restenosis with unrestricted use of “new-generation” drug-eluting stents: A report from the nationwide Swedish Coronary Angiography Registry (SCAAR). Eur Heart J. 2012, 33, 606–613. [Google Scholar] [CrossRef] [PubMed]
- Natsuaki, M.; Morimoto, T.; Furukawa, Y.; Nakagawa, Y.; Kadota, K.; Yamaji, K.; et al. Late adverse events after implantation of sirolimus-eluting stent and bare-metal stent: Long-term (5–7 years) follow-up of the coronary revascularization demonstrating outcome study-kyoto registry cohort-2. Circ Cardiovasc Interv. 2014, 7, 168–179. [Google Scholar] [CrossRef]
- Valgimigli, M.; Borghesi, M.; Tebaldi, M.; Vranckx, P.; Parrinello, G.; Ferrari, R. Should duration of dual antiplatelet therapy depend on the type and/ or potency of implanted stent? A prespecified analysis from the PROlonging Dual antiplatelet treatment after Granding stent-induced Intimal hyperplasia studY (PRODIGY). Eur Heart J. 2013, 34, 909–919. [Google Scholar] [CrossRef]
- Kolandaivelu, K.; Swaminathan, R.; Gibson, W.; Kolachalama, V.; NguyenEhrenreich, K.; Giddings, V.; et al. Stent thrombogenicity early in highrisk interventional settings is driven by stent design and deployment and protected by polymer-drug coatings. Circulation 2011, 123, 1400–1409. [Google Scholar] [CrossRef]
- Van Kuijk, J.; Flu, W.; Schouten, O.; Hoeks, S.; Schenkeveld, L.; de Jaegere, P.; et al. Timing of noncardiac surgery after coronary artery stenting with bare metal or drug-eluting stents. Am J Cardiol. 2009, 104, 1229–1234. [Google Scholar] [CrossRef]
- Tokushige, A.; Shimoni, H.; Morimoto, T.; Furukawa, Y.; Nakagawa, Y.; Kadota, K.; et al. Incidence and outcome of surgical procedures after coronary bare-metal and drug-eluting stent implantation: A report from the CREDO-Kyoto PCI/CABG registry cohort-2. Circ Cardiovasc Interv. 2012, 5, 237–246. [Google Scholar] [CrossRef] [PubMed]
- Cruden, N.; Harding, S.; Flapan, A.; Graham, C.; Wild, S.; Slack, R.; et al. Previous coronary stent implantation and cardiac events in patients undergoing noncardiac surgery. Circ Cardiovasc Interv. 2010, 3, 236–242. [Google Scholar] [CrossRef] [PubMed]
- Hawn, M.; Graham, L.; Richman, J.; Itani, K.; Henderson, W.; Maddox, T. Risk of major adverse cardiac events following noncardiac surgery in patients with coronary stents. JAMA 2013, 310, 1462–1472. [Google Scholar] [CrossRef] [PubMed]

© 2014 by the author. Attribution - Non-Commercial - NoDerivatives 4.0.
Share and Cite
Meier, B. The Infamous Coronary Stent Saga. Cardiovasc. Med. 2014, 17, 239. https://doi.org/10.4414/cvm.2014.00274
Meier B. The Infamous Coronary Stent Saga. Cardiovascular Medicine. 2014; 17(9):239. https://doi.org/10.4414/cvm.2014.00274
Chicago/Turabian StyleMeier, Bernhard. 2014. "The Infamous Coronary Stent Saga" Cardiovascular Medicine 17, no. 9: 239. https://doi.org/10.4414/cvm.2014.00274
APA StyleMeier, B. (2014). The Infamous Coronary Stent Saga. Cardiovascular Medicine, 17(9), 239. https://doi.org/10.4414/cvm.2014.00274



