Introduction
Inflammation plays an important role in the pathogenesis of an ischaemic stroke and other forms of ischaemic brain injury. The evidence available show that the inflammatory response has a double beneficial effect: it does not exacerbate a secondary brain injury in the acute stroke stage, and, which is more important, it contributes to brain recovery after stroke [
1,
2]. Irrespective of a number of recent clinical studies, which demonstrated an indirect interrelation between circulating proinflammatory cytokines and a cardiovascular risk in hypertensive patients after an ischaemic stroke, the effect of low intensity proinflammatory activation on modulation of recurrent cardiovascular events is still understood and controversial [
3,
4,
5]. Proinflammatory cytokines were postulated to be able to modulate the activity of endothelial cells via induction of synthesis of vascular endothelial growth factor (VEGF) [
6,
7]. VEGF-1 is a heterodimer with a glycoprotein structure; as a member of superfamily of endothelial factors, it is synthesised by a wide spectrum of cells and it possesses a pronounced angiopoetic capacity [
6]. VEGF-1 has its biological effect through cooperation with the tirosinkinase receptors located on the endothelial cells surface, which causes cell growth, proliferation, and migration, as well as neovascularisation and angiogenesis [
8,
9]. VEGF-1, being a ligand for alpha-5 / beta-1 integrin, was found to be able to activate the migration of mononuclears and endothelial cells, to potentiate vasodilation, and to increase an inflammatory response [
7,
10]. A paracrine regulation of the VEGF-1 activity is mediated by a specific solubilised receptor that plays a key role in the modulation of the processes above mentioned [
11]. Recent studies revealed that some biological markers of endothelial dysfunction, such as VEGF-1, and some indicators of proinflammatory activation had a predictive value for clinical outcomes in patients at high cardiovascular risk only [
12,
13,
14,
15,
16]. The study aim was to investigate the predictive value of the repeatedly measured circulating VEGF-1 level for recurrent cardiovascular events in hypertensive patients after an ischaemic stroke.
Methods
Study population
The study included 102 patients with mild to moderate arterial hypertension within a 3-week post ischaemic stroke period. Neurological impairment at presentation was assessed by the National Institute of Health Stroke Scale (NIHSS) [
17]. The acute ischaemic stroke type was classified according to the TOAST classification: 1) large artery atherosclerosis (LAAS); 2) cardioembolic infarct (CEI); 3) lacunar infarct (LAC); 4) stroke of other determined aetiology (ODE); 5) stroke of undetermined aetiology (UDE) [
18]. The Barthel Index [
19] and the modified Rankin Scale score [
20] were used to assess functional disability. The functional outcome was evaluated by using these scales upon hospital admission and on the 21st day of the acute stroke period prior to including patients into the study.
Contrast-enhanced computer spiral tomography (CT) was performed on Somatom Spirit scanner manufactured by Siemens, Germany, with two rows of detectors. Omnipak nonionic contrast manufactured by Amersham Health, Ireland, was used. Scanning began at the cranial base and continued upwards, with the scanning pitch being 80 mm. The total average acquisition time was 26 seconds.
Blood sample, VEGF and high-sensitive C-reactive protein assay
The blood samples were collected in a cooling vacutainer that was centrifuged immediately (at 6000 rpm and 4 °C for 15 min). The centrifuged serum samples were coded blindly and stored at –70 °C until used. VEGF-1 concentrations were measured by ELISA at baseline and after a 6-month follow-up by using laboratory kits manufactured by Bioscience, USA. Each assay was done twice. The mean intra-assay coefficients of variation were <10% for all the assays. The high-sensitivity C-RP (hs-CRP) levels were measured by using nephelometric technique on AU640 analyser manufactured by Diagnostic Systems Group, Japan. Total cholesterol and HDL-cholesterol concentrations were determined by the Dimension Clinical Chemistry System manufactured by Dade Behring Inc., Newark, NJ, USA. LDL-cholesterol was estimated with the Friedewald equation (Friedewald W.T., Levy R.I., Fredrickson D.S. 1972).
Clinical events: screening and diagnostics
Clinical interviews were carried out every month for one year after stroke. The following are the clinical events verified: newly diagnosed strokes or TIAs; death for any reason and sudden cardiac death; coronary ischaemic events (myocardial infarction, unstable angina, arrhythmia) that needed hospital admission for cardiovascular reasons, new-onset chronic heart failure and diabetes mellitus. Newly diagnosed strokes were confirmed with CT. Coronary artery disease (CAD), heart failure, and diabetes mellitus were diagnosed according to contemporary clinical guidelines [
18,
21,
22]. All clinical events were presented as cumulative.
Ethical declaration
The study was approved by a local Ethics Committee of the State Medical University, Zaporozhye, Ukraine. All the patients gave an informed consent to participate in the study. The study was carried out in conformity with the Declaration of Helsinki.
Statistical analysis
Statistical analysis was carried out by using SPSS for Windows v. 17.0 (SPSS Inc., Chicago, IL, USA). All the values were presented as the mean value and 95% CI or the median and percentiles. An independent group t-test was used to compare interval parameters that meet the criteria of normality and homogeneity of variance. For interval parameters that do not meet the criteria mentioned, the non-parametric Mann-Whitney test was used to compare groups between them. Comparison of categorical variables between groups was carried out by using the Chi2 test and the Fisher exact test. The VEGF-1 concentration was not normally distributed (the Kolmogorov-Smirnov test) and it was positively skewed. The data were not transformed. The potential factors, which may be associated with Cumulative Clinical Events (CCE), were identified first with the univariate analysis (ANOVA), and then Cox proportional hazards multivariate analyses were used to identify predictors of CCE. Receiver operating characteristic (ROC) curves were configured to establish cutoff points of VEGF-1 levels that predicted optimally the occurrence of cumulative clinical events. The Kaplan-Meier survival curves were estimated for hypertensive patients as a function of incremented VEGF-1 levels. The calculated difference of P <0.05 was considered statistically significant.
Results
The study included 102 patients with mild to moderate arterial hypertension (67 men and 35 women of 58.38 years of age at average [95% CI = 54–72 years]) who were evaluated within a 3-week post ischaemic stroke period.
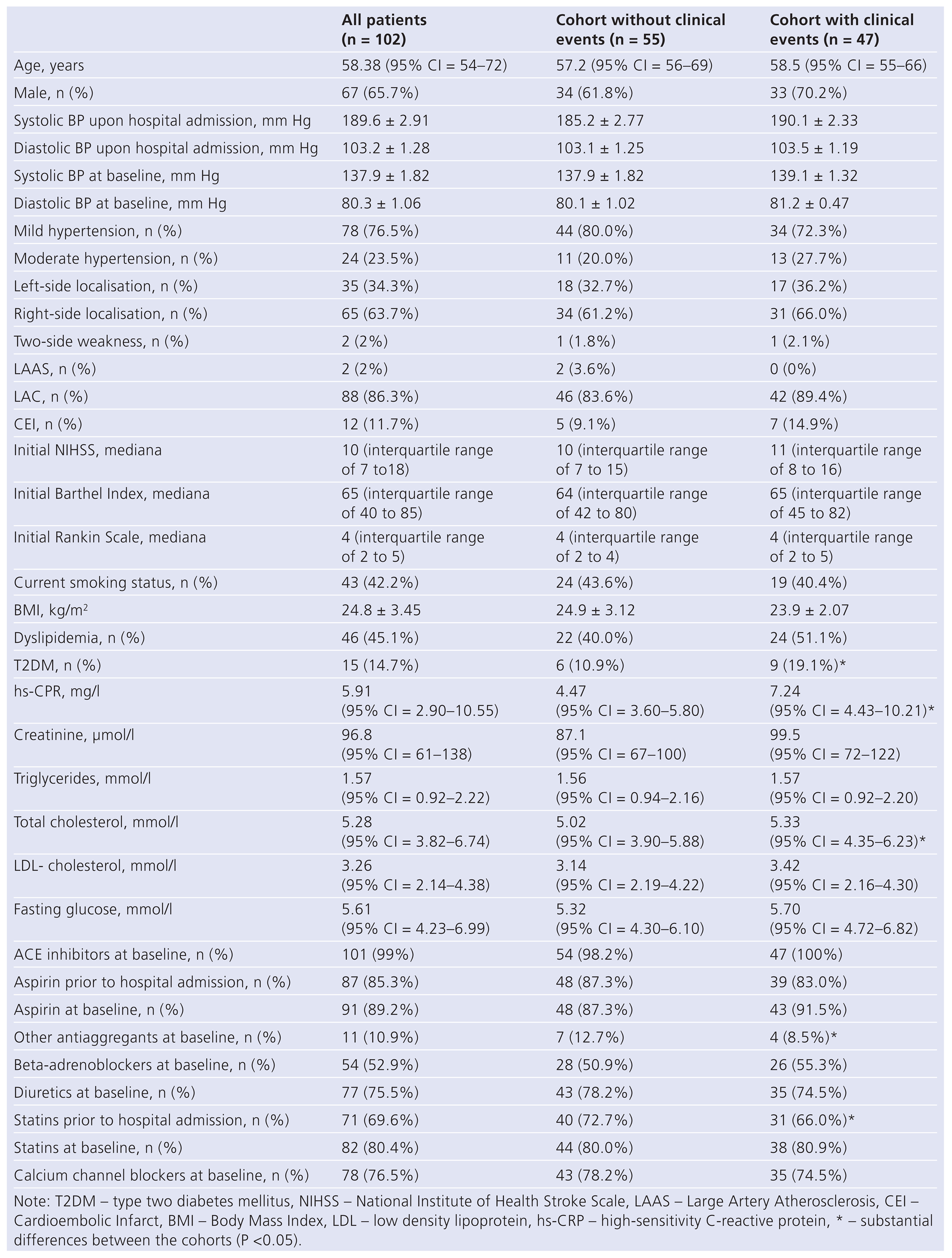
Table 1 shows the baseline characteristics of the study group. All the patients included were hypertensive at the screening stage (78 patients had mild hypertension and 24 patients had moderated hypertension). All the patients were haemodynamically stable prior to the study; they have their target blood pressure controlled (BP was less than 140/90 mm Hg) and remained free of any ischaemic events during the time elapsed between the first qualifying episode and the date when they were enrolled into the study. Moreover, 45.1% of patients had dyslipemia, 42.2% of patients were smokers, and 14.7% of patients had a history of mild diabetes mellitus. LAAS type of ischaemic stroke was found in 2% of patients, LAC and CEI were found in 86.3% and in 11.7% of patients respectively. The authors found right-side brain injuries in 63.7% of patients, left-side brain injuries in 34.3%, and bilateral brain injuries in 2% of patients. NIHSS score upon hospital admission and 21 days after hospital admission was 10 (interquartile range of 7 to 18) and 5 (interquartile range of 3 to 9) respectively. The median Barthel Index was 65 (interquartile range of 40 to 85) upon hospital admission and 75 (interquartile range of 55 to 90) 21 days after hospital admission; and the median Rankin Scale score was 4 (interquartile range of 2 to 5) upon hospital admission and on the 21st day prior to the study.
The median of the hs-CRP concentration was 5.91 mg/l (95% CI = 2.90–10.55 mg/l). The medians of the plasma levels of total cholesterol and low-density cholesterol (LDL-C) were 5.28 mmol/l (95% CI = 3.82–6.74) and 3.26 mmol/l (95% CI = 2.14–4.38) respectively. The target LDL-C levels of less than 1.8 mmol/l and less than 2.5 mmol/l were in 23 (22.5%) and 33 (32.4%) patients at baseline. The type 2 diabetes mellitus (T2DM) incidence was much higher in the cohort of patients with clinical events in comparison with patients without clinical events. The total plasma cholesterol level was substantially higher in the cohort of patients with clinical events in comparison with patients without clinical events.
The treatment strategy was similar in both cohorts, however, anti-aggregants differing from aspirin were used more often in patients with clinical events. Statins were administered more often in the cohort of patients without clinical events prior to hospital admission. The ratios of the patients administered with statins were similar at baseline. Statins were taken by 71 (69.6%) patients (atorvastatin was taken by 56 patients and simvastatin was taken by 15 patients) prior to hospital admission. The average oral daily atorvastatin and simvastatin doses were 30 mg (interquartile range of 20 to 60 mg) and 20 mg (interquartile range of 10 to 40 mg) respectively.
The statins intake was not discontinued after hospital admission, and 82 (80.4%) patients took the average atorvastatin daily oral dose of 40 mg (interquartile range of 20 to 80 mg) at baseline.
Fifty-seven (57) cumulative clinical events occurred in 48 patients (47.1%) within the follow-up, with their distribution being as follows: 4 deaths, 6 cardiac arrhythmias, 17 cardiac ischaemic events, 9 strokes (5 lacunar infarctions and 2 cardioembolic strokes), 10 diabetes mellitus, 4 chronic heart failures and 7 hospital admissions for cardiovascular reasons, new-onset chronic heart failure and diabetes mellitus.
The analysis of study results showed that the VEGF-1 concentration median in patients with recurrent cardiovascular events at baseline was similar to that in patients without newly diagnosed outcomes (Me = 344.87 pg/ml, 95% CI = 245.67–493.46 pg/ml and Me = 352.10 pg/ml, 95% CI = 205.31-573.81 pg/ml respectively, P >0.1). The VEGF-1 concentration medians in patients with and without cardiovascular events were 814.51 pg/ml (95% CI = 428.17–1033.45 pg/ml) and 203.59 pg/ml (95% CI = 200.13–285.81 pg/ml) respectively (P <0.001) after a six-month follow-up. The substantial difference in VEGF-1 concentrations in hypertensive patients were a function of age, gender, types of ischaemic stroke, severity of hypertension, and blood pressure values at baseline, while no association between conventional cardiovascular risk factors, NIHSS, Barthel Index, and Rankin score index was found. The circulating VEGF-1 levels rose substantially (Δ% = 57.7%; P <0.001) in patients with recurrent cardiovascular events during a one-year follow-up. In contrast, the VEGF-1 concentration diminished in patients without cardiovascular events during a sixmonth follow-up (Δ% = –42.2%; P <0.001).
For further analysis, the VEGF-1 concentration was presented as a function of recurrent cardiovascular events in the follow-up. It was found that circulating VEGF-1 levels in patients with one, two, three, and more recurrent cardiovascular events were 373.80 pg/ ml (95% CI = 342.90–479.70 pg/ml), 539.96 pg/ml (95% CI = 444.28–865.56 pg/ml) and 724.66 pg/ml (95% CI = 558.72–890.66 pg/ml) respectively at baseline. Moreover, VEGF-1 levels in those patients at baseline were much higher than in patients without cardiovascular events (Me = 289.28 pg/ml; 95% CI = 279.71–345.88 pg/ ml) (P = 0.001 for all the patients). Within a six-month follow-up, the median of the VEGF-1 concentration in patients with one, two, three, and more recurrent cardiovascular events was 484.51 pg/ml (95% CI = 428.19–588.01 pg/ml), 815.45 pg/ml (95% CI = 583.02–1045.99 pg/ml) and 964.61 pg/ml (95% CI = 806.61–1135.83 pg/ ml) respectively. However, the medians of the VEGF-1 concentration among patients with cardiovascular events were much higher (P = 0.001 for all the patients) than in patients without cardiovascular events (Me = 203.59 pg/ml; 95% CI = 200.14–285.81 pg/ml). Nevertheless, circulating VEGF-1 in the cohort of patients with clinical events both at baseline and in six months was pronounced different in patients who had two and more recurrent cardiovascular events during a oneyear follow-up when compared with patients with one cardiovascular event determined during the same period.
Based on ROC-analysis, the optimal cutoff points of circulating VEGF-1 level in hypertensive patients at baseline (Model 1) and after a six-month follow-up (Model 2) were found to be 403.57 pg/ml and 450.15 pg/ ml respectively. For these cutoff points, sensitivity and specificity were 78.6% and 70.0%, as well as 85.7% and 70.5% in terms of the positive and negative likelihood ratio equal to 1.12 and 0.305, as well as 2.86 and 0.202 respectively. At the same time, areas under ROC curve (AUC) for both models were 0.76 (95% CI = 0.602– 0.917; P = 0.001) and 0.824 (95% CI = 0.707–0.921; P = 0.001). This result showed a higher predictive value of Model 2 compared with Model 1 (
Figure 1).
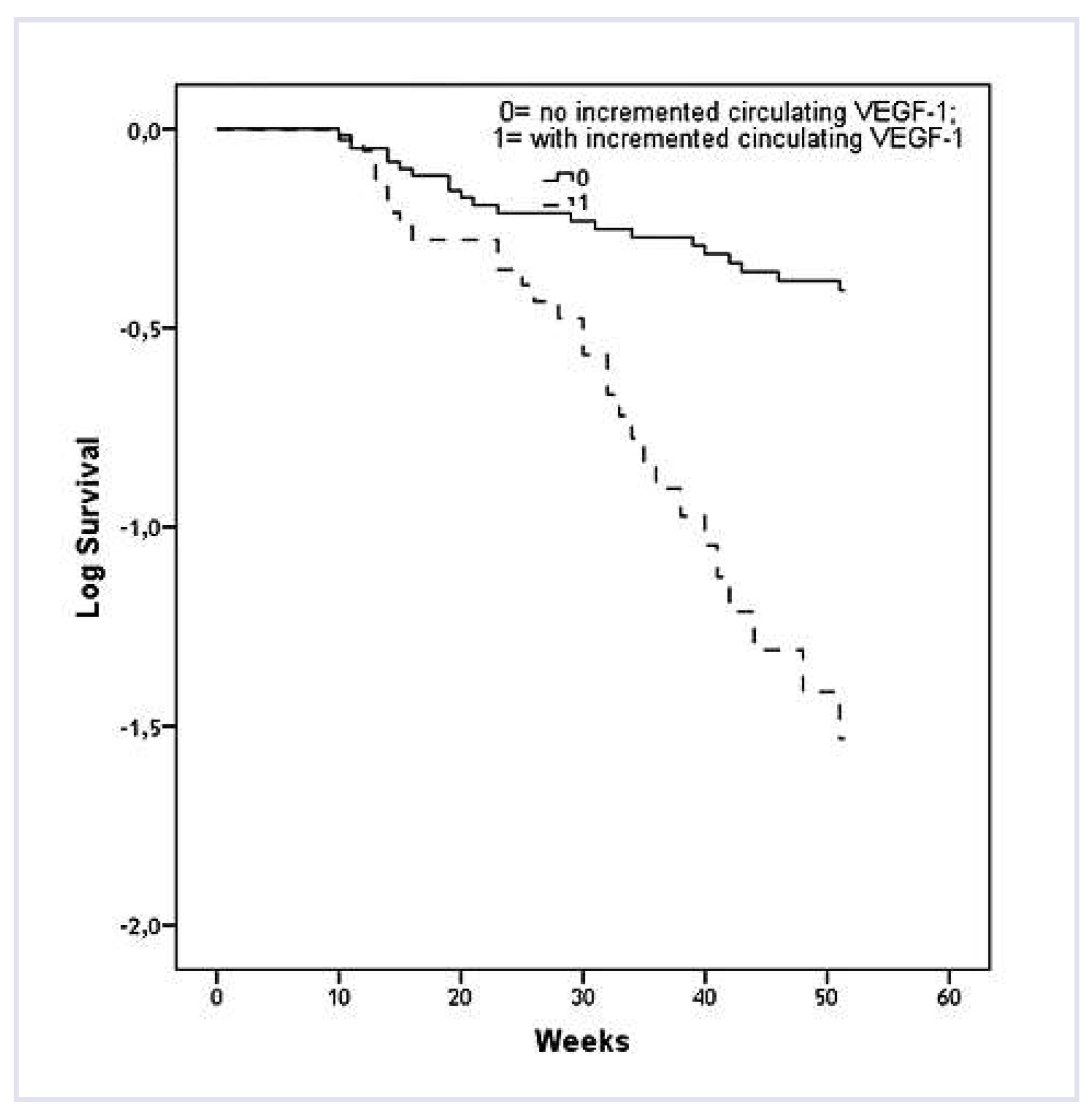
The Cox regression analysis showed that the odds ratio (OR) of cumulative cardiovascular events was 4.11 (95% CI = 2.66–7.28; P = 0.001) in hypertensive patients whose VEGF-1 level was more than 403.57 pg/ ml at baseline versus that in patients with VEGF-1 level of less than 403.57 pg/ml. The circulating VEGF-1 concentration rose to more than 450.15 pg/ml for six months in comparison with a low VEGF-1 concentration associated with the OR increased to 5.46 (95% CI = 3.12–7.90; P = 0.001). In this regard, the authors verified the hypothesis that the repeatedly measured VEGF-1 concentration increased by 2 standard deviations in each hypertensive patient during a six-month follow-up may have even a greater predictive value than a single-measured peak increase in circulating VEGF-1 concentration. The analysis of the study results showed that the recurrent cardiovascular events rate was much higher in patients with an increased VEGF-1 level than in those without an increased circulating VEGF-1 level during follow-up (
Figure 2). The Kaplan-Meier curves divergence was observed in 10 weeks, and it was continued up to the end of the followup. In addition, determined by serial measurements, the adjusted odds ratio (OR) for the occurrence of cumulative cardiovascular events in hypertensive patients with increased circulating VEGF-1 level was 6.10 (95% CI = 4.70–8.30, P = 0.001) versus in those without such changes in the VEGF-1 level. Thus, these data allowed the authors to establish the fact that an increased circulating VEGF-1 level within six months after stroke is associated closely with a high risk of recurrent cardiovascular events in the cohort of patients with controlled arterial hypertension.
Discussion
Atherosclerosis is the main pathophysiological mechanism in patients with noncardioembolic stroke, including large- and small-vessel disease. Obtained by the authors, the results support the hypothesis that the circulating VEGF-1 level is an independent predictor of cardiovascular outcomes during a one-year period, including atherothrombotic events, in hypertensive pa-tients after a serious ischaemic brain event. However, this conclusion was valid for patients at a very high cardiovascular risk with substantially increased circulating VEGF-1 levels during a six-month follow-up. Interestingly, an increase in the circulating VEGF-1 level appears to be associated with the accumulation of cardiovascular events before achieving the optimal predictive cutoff point of circulating VEGF-1 during a sixthmonth follow-up. Many recent clinical trials failed to indicate a predictive value of VEGF-1 peak concentrations among symptomatic atherosclerotic patients atherosclerotic carotid plaque patients after stroke [
13], while theoretical backgrounds of such hypothesis are very attractive [
23,
24]. In particular, the VEGF-1 secretion due to focal brain ischaemia was found to be able to create a neuroprotection, to improve neoangiogenesis and neurogenesis [
25,
26]. On the other hand, VEGF-1 is able to induce post-ischaemic neurovascular remodeling and apoptosis [
27]. Probably, these mechanisms underlie the derangement of progressive threedimensional perivascular citoarchitectonics, expanding the penumbra zone and worsening cerebral ischaemia [
28]. Since the angiopoetic VEGF-1 effect is systemic, it might be assumed that neovascularisation in the vulnerable atheroma site should promote progressive worsening of the mechanical capacity of the atheroma cap, the formation of the phenomenon of “fatigue” cap, the appearance of endothelial dysfunction and deregulation of vascular tone, which ultimately leads to a corresponding atherothrombotic events in any vascular territories [
29]. Thus, the authors suppose that immediate VEGF-1 effects are probably adaptive in nature in hypertensive patients after ischaemic stroke, while delayed VEGF-1 effects may be associated with recurrent clinical events, in particular, mediated by atherothrombosis [
23,
30,
31]. This hypothesis was confirmed by the results of this study. The authors consider increased VEGF-1 concentrations to be not only associated with a higher incidence of recurrent cardiovascular events, but may be a reflection of the phenomenon of progressive vascular remodeling in a long-term period. It should be noted that all the patients included in the trial had their blood pressure controlled, and the majority of them continued receiving ACE inhibitors, calcium channel blockers, statins, and antiplatelet therapy after stroke. However, despite the use of statins, the target LDL-C levels were not achieved in the majority of patients. Taking into consideration the fact that statins have antiproliferative and antiinflammatory effects, the study findings may be interpreted as an indirect argument in favour of expanding the use of statins in hypertensive patients immediately after stroke. This assumption needs to be confirmed in studies of greater statistical power.
In conclusion, the authors found that an increase in circulating VEGF-1 level in hypertensive patients after ischaemic stroke within a one-year period had bigger predictive value for cardiovascular recurrent events than a single VEGF-1 concentration measurement made within the follow-up.
Limitations of the study
This study had some limitations. We considered that a greater cohort would be desirable to improve the power of the study as low rates of recurrent strokes and deaths were detected. The authors also relied on clinical data to rule out infection and other inflammatory diseases prior to sampling, but they cannot rule out that some patients had unrecognised conditions responsible for the elevated VEGF-1 levels observed. However, additional verification of atherosclerosis as well as intracranial artery occlusive disease may be required. The authors supposed that these limitations might not have a significant effect on the interpretation of the study data.