The Use of Chitosan-Coated Nanovesicles in Repairing Alcohol-Induced Damage of Liver Cells in Mice
Abstract
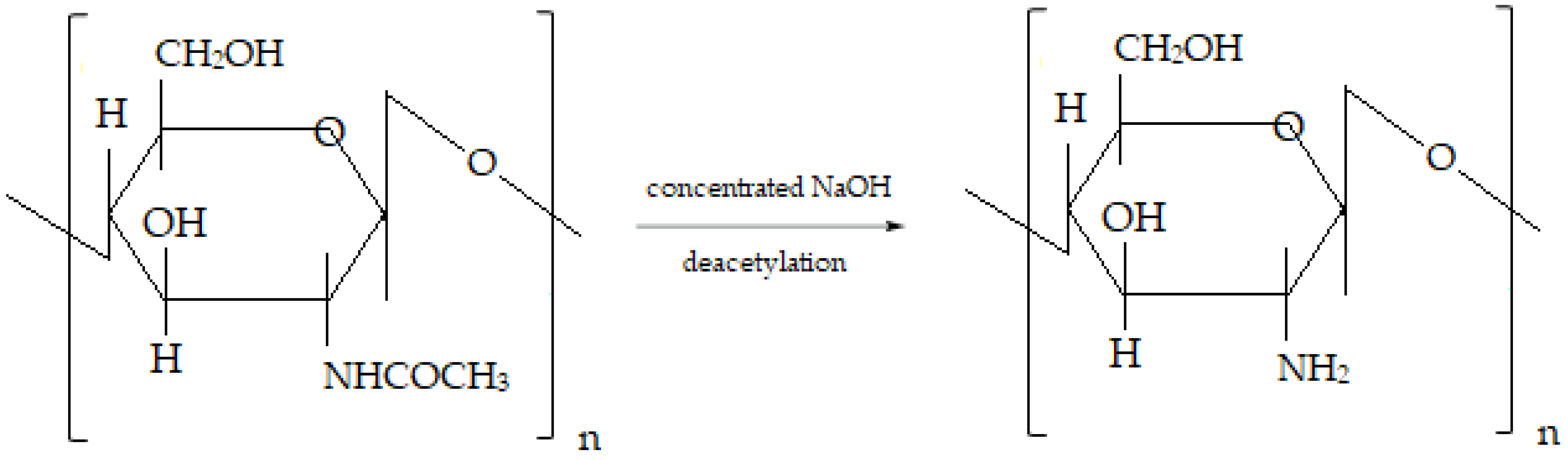
:1. Introduction
2. Materials and Methods
2.1. Substances
2.2. Chitosan Lipid Vesicles Preparation
2.3. Laboratory Animals
2.4. Investigation of Chitosan Vesicles Effects in Mice with Experimental Induced Alcoholic Liver Toxicity
- Group 1 (Control): water ad libitum, without alcohol;
- Group 2 (Control alcohol): 5% alcohol in water ad libitum;
- Group 3 (CHIT): 0.1 mL/10 g body chitosan solution in animals with alcoholic liver toxicity;
- Group 4 (CHIT-ves): 0.1 mL/10 g body solution containing chitosan vesicles in animals with alcoholic liver toxicity;
- Group 4 (AcA): 200 mg/kg body ascorbic acid in animals with alcoholic liver toxicity.
2.5. Statistical Analysis of Data
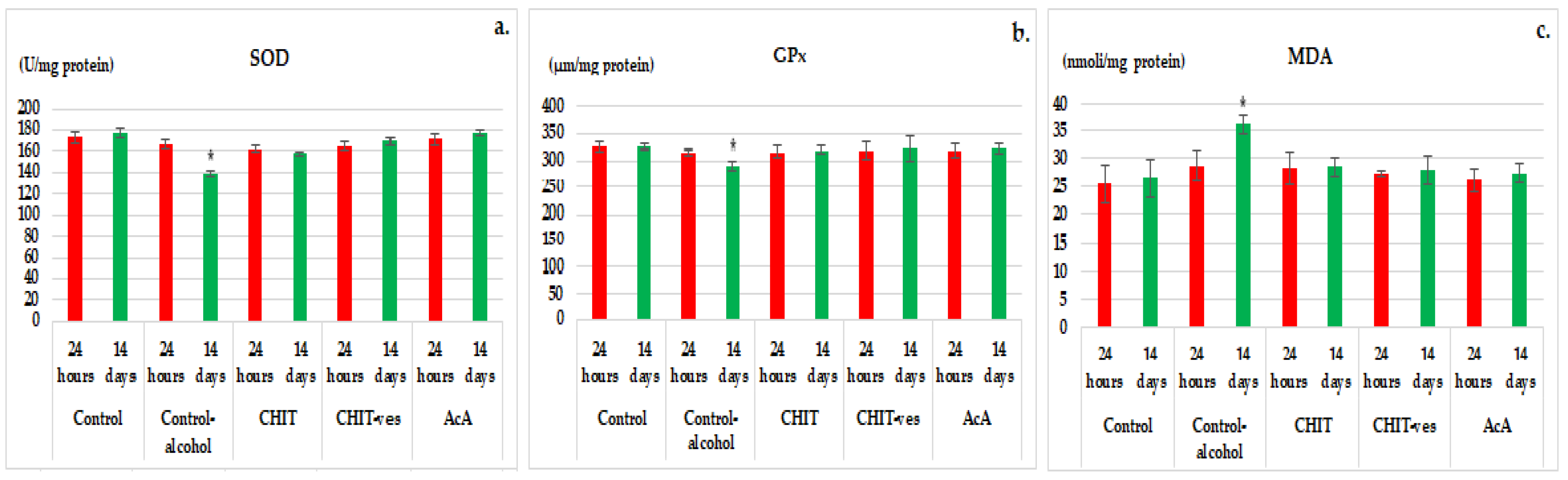
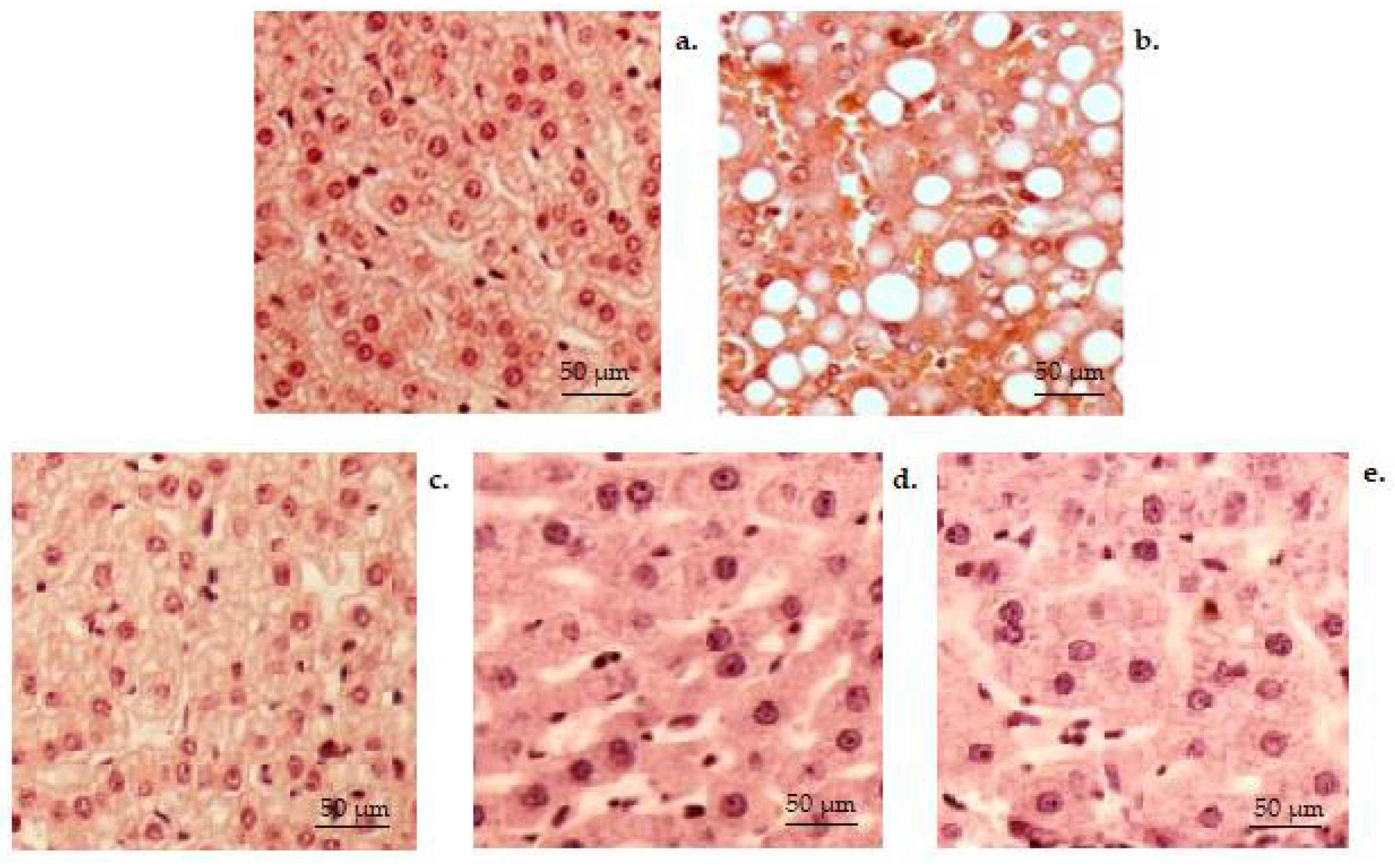
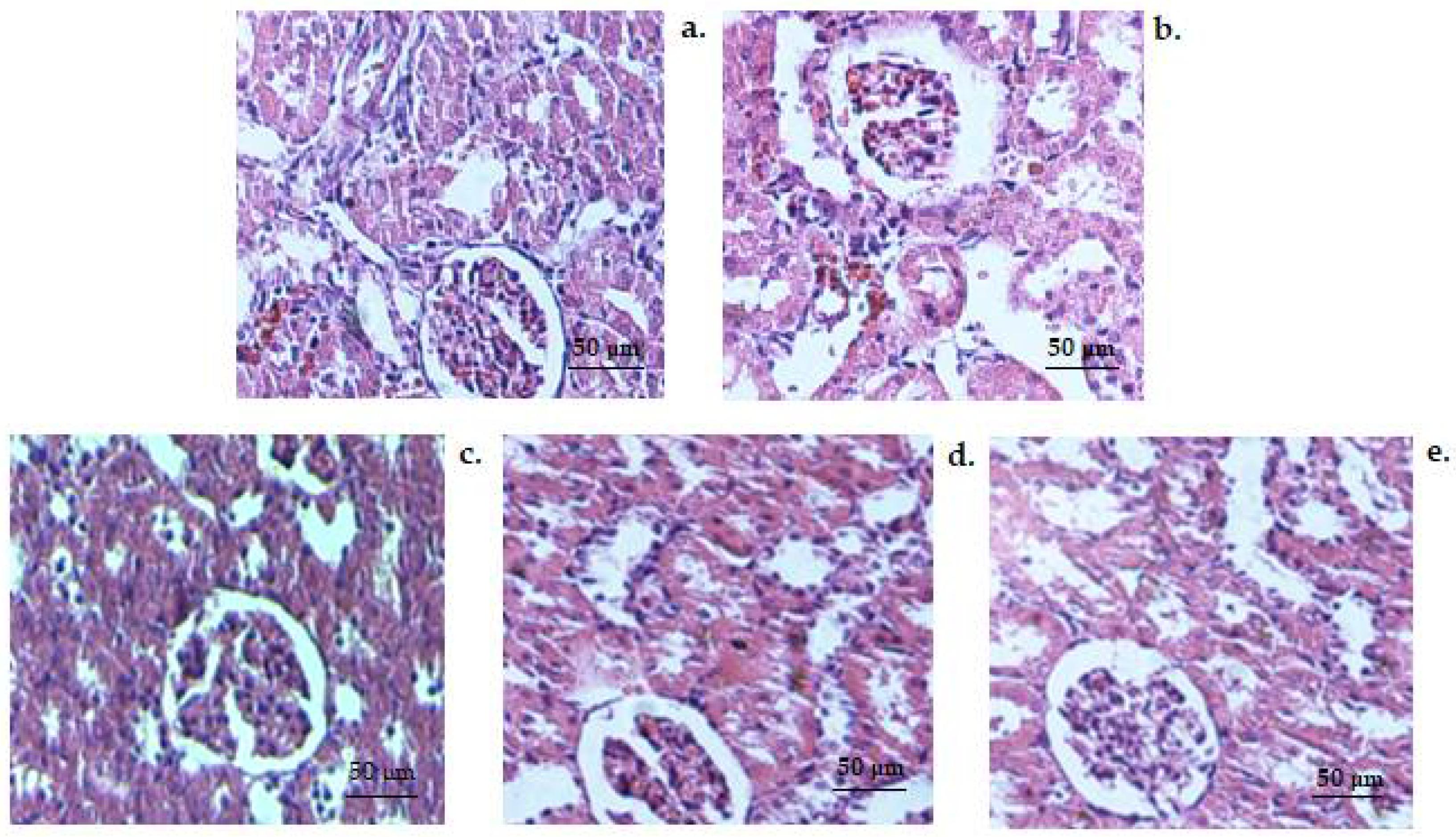
3. Results
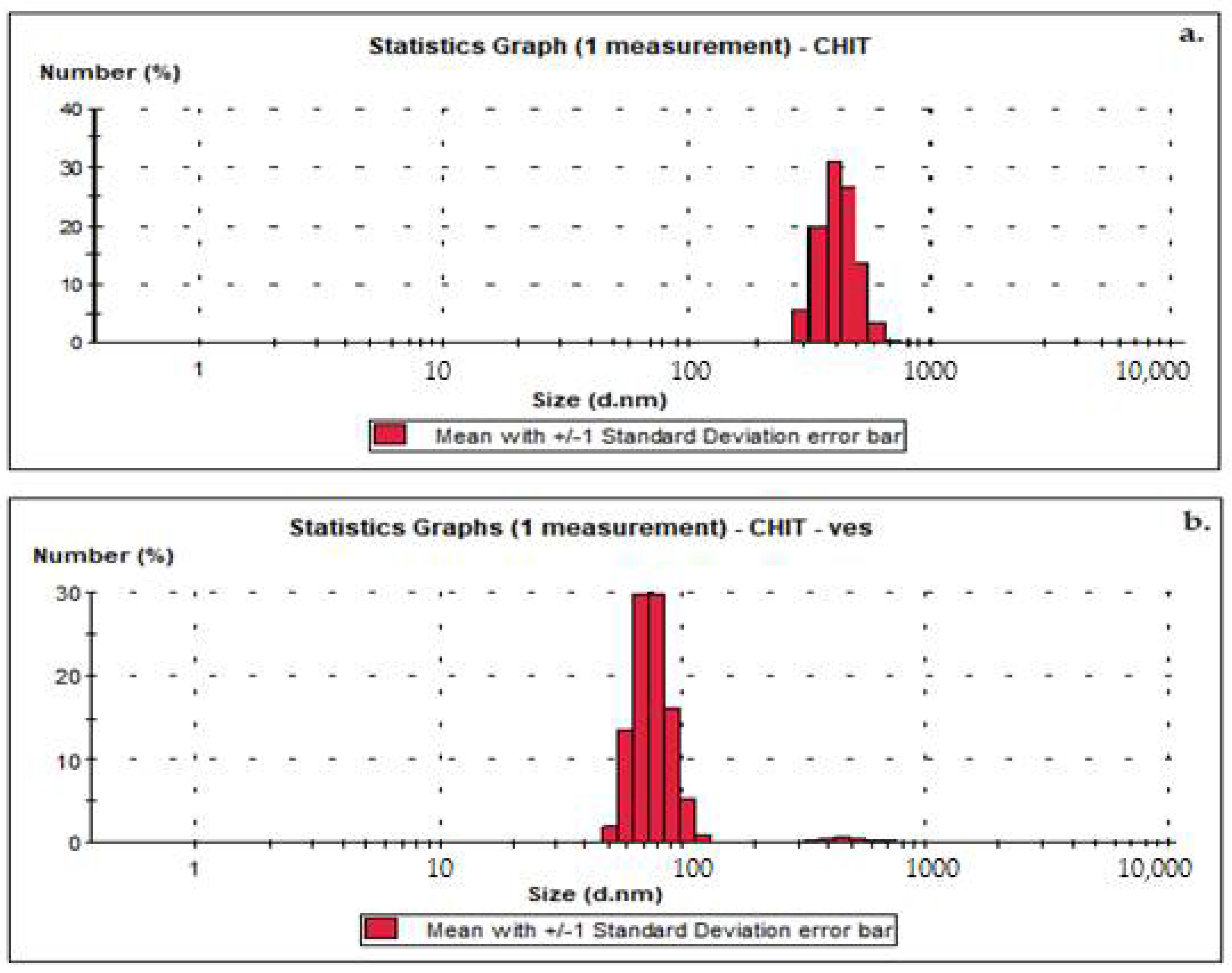
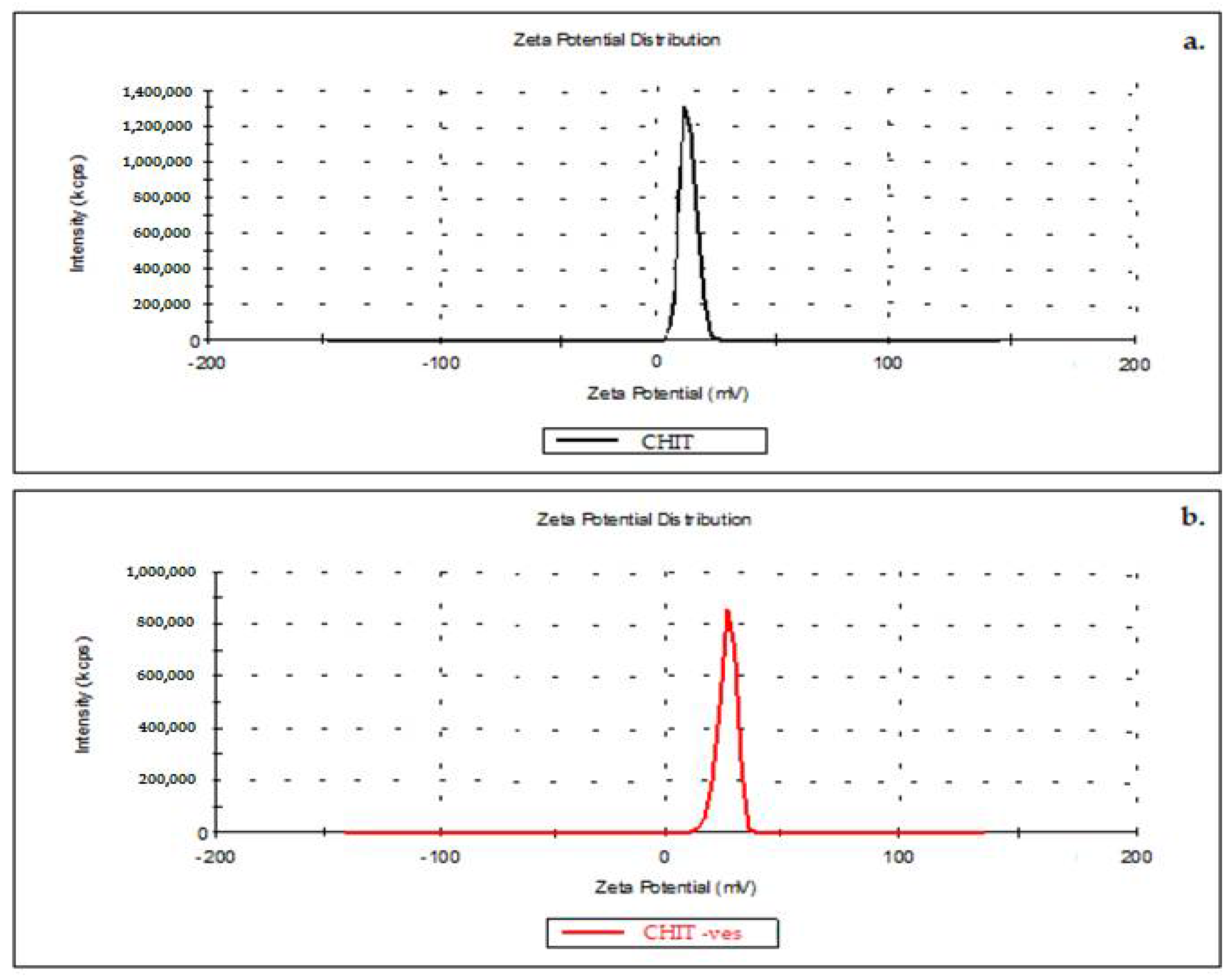
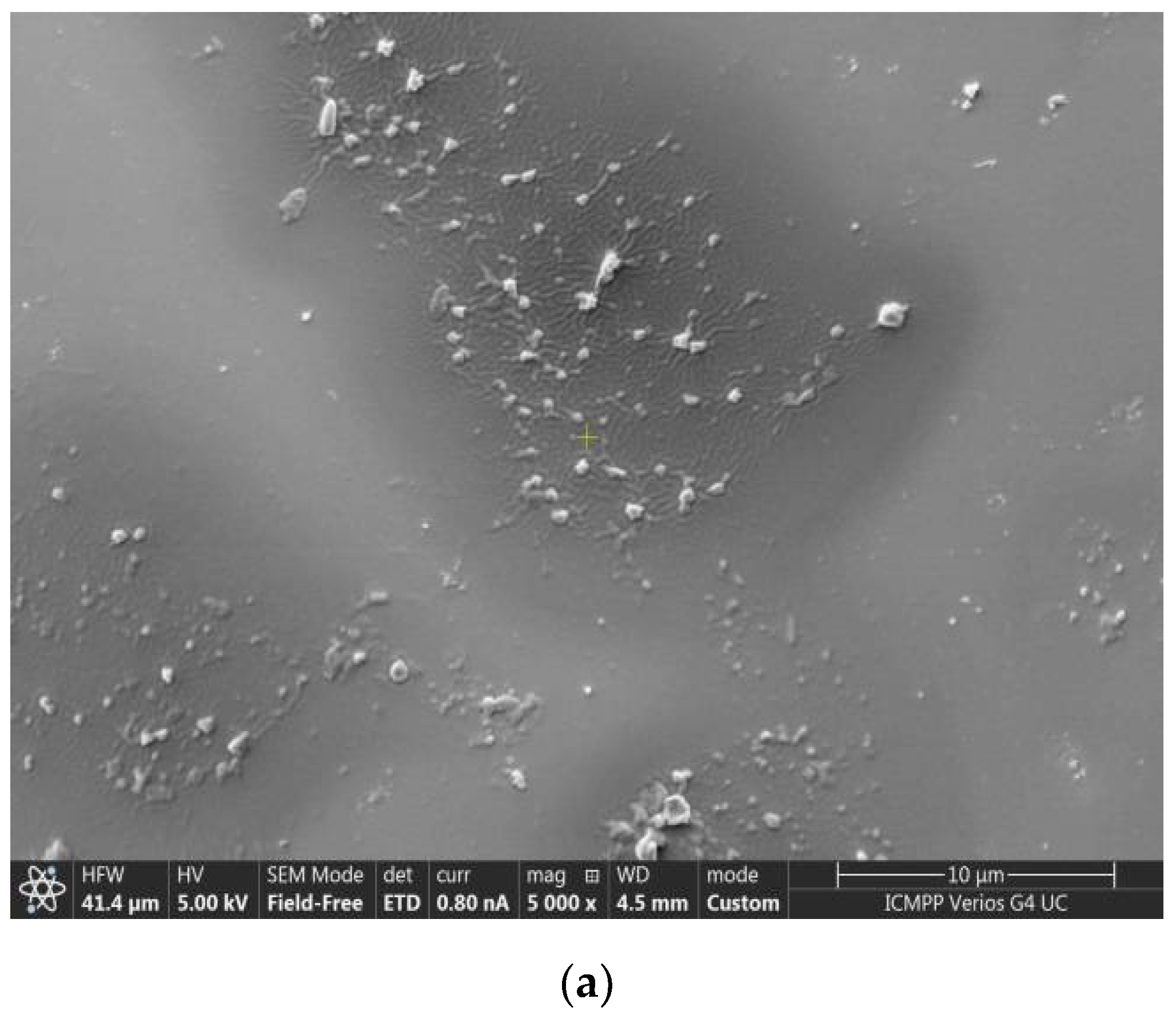
3.1. Size, Zeta Potential and SEM Microscopy of Lipid Vesicles with CHIT
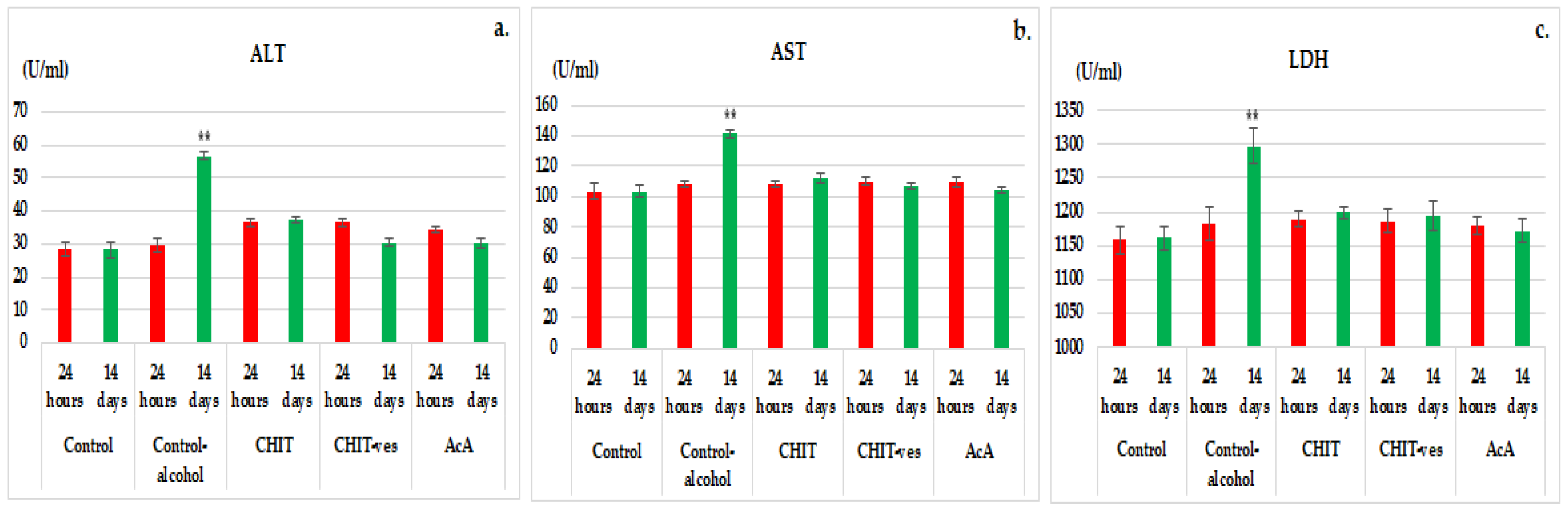
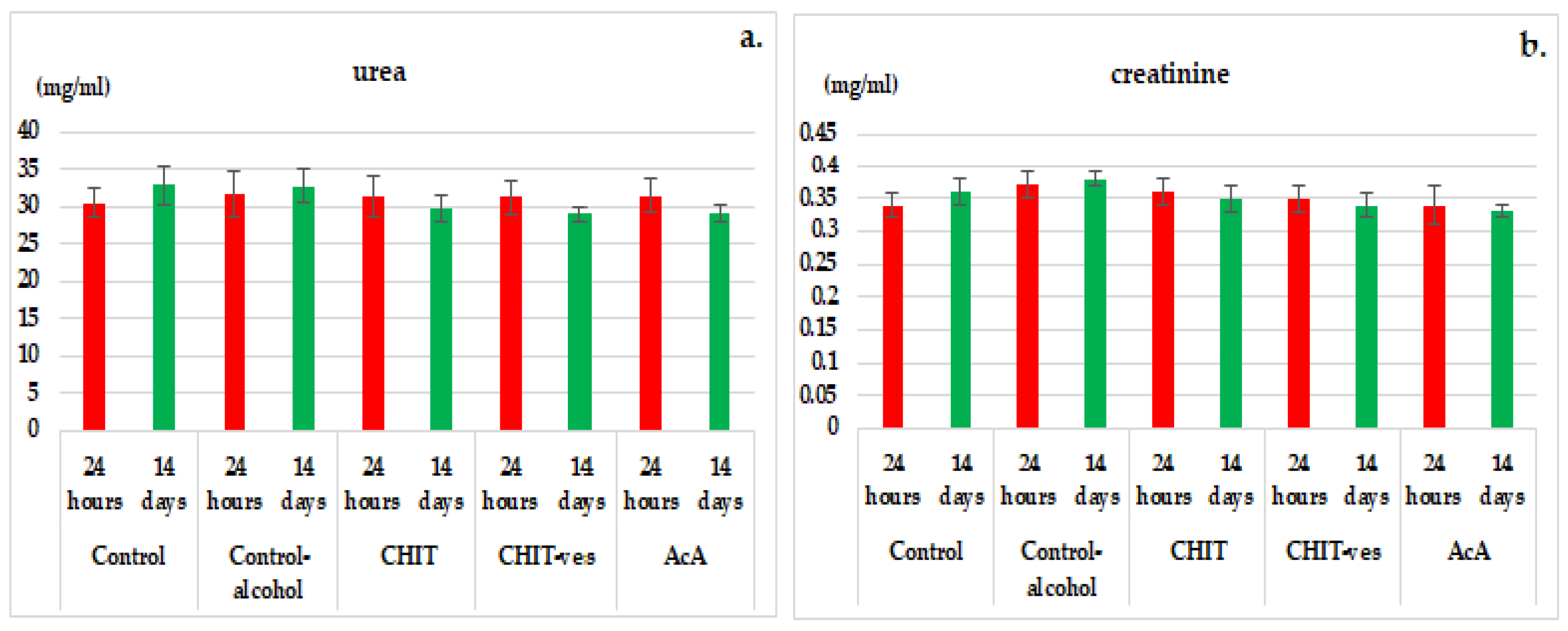
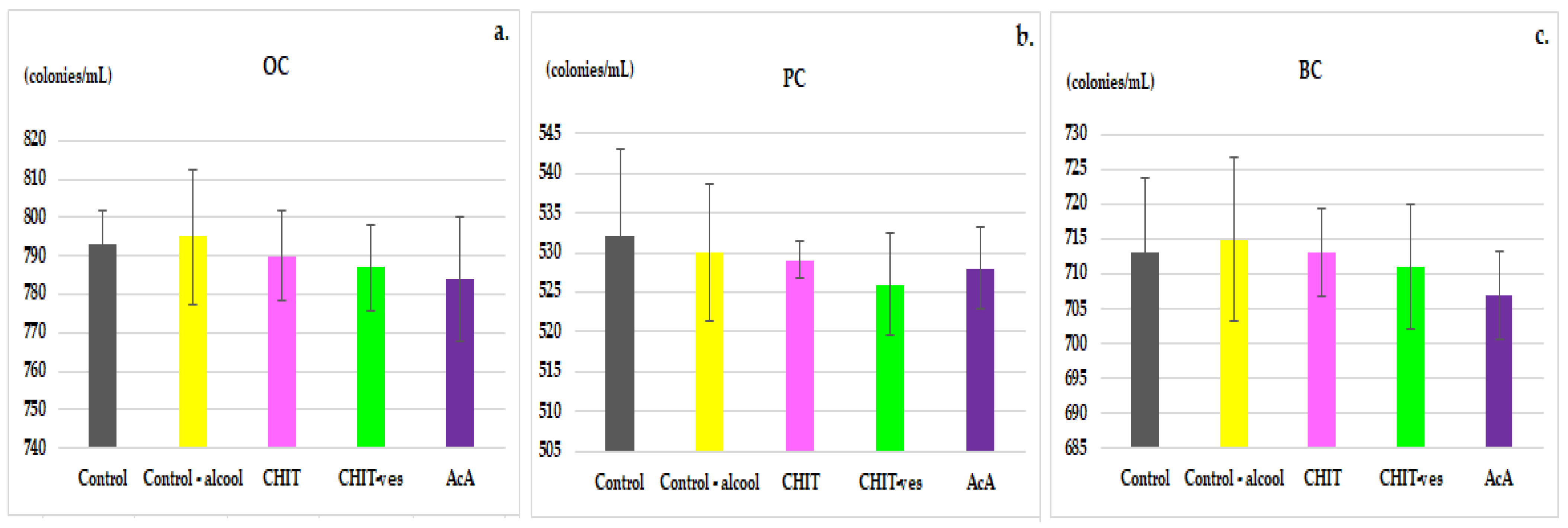
3.2. Hematological Tests
3.3. Histopathology Tests
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| alanine aminotransferase | ALT |
| ascorbic acid (group) | AcA |
| aspartate aminotransferase | AST |
| bactericidal capacity of peritoneal macrophages | BC |
| basophils | B |
| chitosan (group) | CHIT |
| chitosan vesicles (group) | CHIT-ves |
| eosinophils | E |
| glutathione peroxidase | GPx |
| hemoglobin | Hb |
| hematocrit | Ht |
| lactate dehydrogenase | LDH |
| lymphocytes | Ly |
| malondialdehyde | MDA |
| monocytes | M |
| peripheral blood | PC |
| polymorphonuclear neutrophils | MN |
| serum opsonic capacity | OC |
| superoxide dismutase | SOD |
References
- Aravamudhan, A.; Ramos, D.M.; Nada, A.A.; Kumbar, S.G. Natural Polymers: Polysaccharides and Their Derivatives for Biomedical Applications. In Natural and Synthetic Biomedical Polymers; Elsevier Inc.: Amsterdam, The Netherlands, 2014. [Google Scholar] [CrossRef]
- Dash, M.; Chiellini, F.; Ottenbrite, R.M.; Chiellini, E. Chitosan—A versatile semi-synthetic polymer in biomedical applications. Prog. Polym. Sci. 2011, 36, 981–1014. [Google Scholar] [CrossRef]
- Adhikari, H.S.; Garai, A.; Khanal, C.; Adhikari, R.; Yadav, P.N. Imidazole-2-carboxaldehyde Chitosan Thiosemicarbazones and Their Copper(II) Complexes: Synthesis, Characterization and Antitumorigenic Activity against Madin-Darby Canine Kidney Cell Line. Asian J. Chem. 2021, 33, 969–976. [Google Scholar] [CrossRef]
- Ladiè, R.; Cosentino, C.; Tagliaro, I.; Antonini, C.; Bianchini, G.; Bertini, S. Supramolecular structuring of hyaluronan-lactose-modified chitosan matrix: Towards high-performance biopolymers with excellent biodegradation. Biomolecules 2021, 11, 389. [Google Scholar] [CrossRef] [PubMed]
- Islam, S.; Bhuiyan, M.A.R.; Islam, M.N. Chitin and Chitosan: Structure, Properties and Applications in Biomedical Engineering. J. Polym. Environ. 2017, 25, 854–866. [Google Scholar] [CrossRef]
- Shaheen, S.; Arafah, M.M.; Alshanwani, A.R.; Fadda, L.M.; Alhusaini, A.M.; Ali, H.M.; Hasan, I.H.; Hagar, H.; Alharbi, F.M.; AlHarthii, A. Chitosan nanoparticles as a promising candidate for liver injury induced by 2-nitropropane: Implications of P53, iNOS, VEGF, PCNA, and CD68 pathways. Sci. Prog. 2021, 104, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Mertins, O.; Dimova, R. Insights on the interactions of chitosan with phospholipid vesicles. Part II: Membrane stiffening and pore formation. Langmuir 2013, 29, 14552–14559. [Google Scholar] [CrossRef]
- Wong, C.Y.; Al-Salami, H.; Dass, C.R. Potential of insulin nanoparticle formulations for oral delivery and diabetes treatment. J. Control. Release 2017, 264, 247–275. [Google Scholar] [CrossRef]
- Li, Y.; He, J.; Lyu, X.; Yuan, Y.; Wang, G.; Zhao, B. Chitosan-based thermosensitive hydrogel for nasal delivery of 446 exenatide: Effect of magnesium chloride. Int. J. Pharm. 2018, 553, 375–385. [Google Scholar] [CrossRef]
- Yan, L.; Crayton, S.H.; Thawani, J.P.; Amirshaghaghi, A.; Tsourkas, A.; Cheng, Z. A pH-responsive drug-delivery platform based on glycol chitosan-coated liposomes. Small 2015, 11, 4870–4874. [Google Scholar] [CrossRef] [Green Version]
- Wang, Q.Z.; Chen, X.G.; Liu, N.; Wang, S.X.; Liu, C.S.; Meng, X.H.; Liu, C.G. Protonation constants of chitosan with different molecular weight and degree of deacetylation. Carbohydr. Polym. 2006, 65, 194–201. [Google Scholar] [CrossRef]
- Sebaaly, C.; Trifan, A.; Sieniawska, E.; Greige-Gerges, H. Chitosan-coating effect on the characteristics of liposomes: A focus on bioactive compounds and essential oils: A review. Processes 2021, 9, 445. [Google Scholar] [CrossRef]
- Murugesan, S.; Scheibel, T. Chitosan-based nanocomposites for medical applications. J. Polym. Sci. 2021, 59, 1610–1642. [Google Scholar] [CrossRef]
- Behr, M.; Ganesan, K. Improving Polysaccharide-Based Chitin/Chitosan-Aerogel Materials by Learning from Genetics and Molecular Biology. Materials 2022, 15, 1041. [Google Scholar] [CrossRef] [PubMed]
- Jhundoo, H.D.; Siefen, T.; Liang, A.; Schmidt, C.; Lokhnauth, J.; Béduneau, A.; Pellequer, Y.; Larsen, C.; Lamprecht, A. Anti-inflammatory activity of chitosan and 5-amino salicylic acid combinations in experimental colitis. Pharmaceutics 2020, 12, 1038. [Google Scholar] [CrossRef]
- Niculescu, A.G.; Grumezescu, A.M. Applications of Chitosan-Alginate-Based Nanoparticles-An Up-to-Date Review. Nanomaterials 2022, 12, 186. [Google Scholar] [CrossRef]
- Shakil, M.S.; Mahmud, K.M.; Sayem, M.; Niloy, M.S.; Halder, S.K.; Hossen, M.S.; Uddin, M.F.; Hasan, M.A. Using Chitosan or Chitosan Derivatives in Cancer Therapy. Polysaccharides 2021, 2, 48. [Google Scholar] [CrossRef]
- Elkeiy, M.M.; Khamis, A.A.; El-Gamal, M.M.; Abo Gazia, M.M.; Zalat, Z.A.; El-Magd, M.A. Chitosan nanoparticles from Artemia salina inhibit progression of hepatocellular carcinoma in vitro and in vivo. Environ. Sci. Pollut. Res. 2020, 27, 19016–19028. [Google Scholar] [CrossRef]
- Adhikari, H.S.; Garai, A.; Thapa, M.; Adhikari, R.; Yadav, P.N. Chitosan functionalized thiophene-2-thiosemicarbazones, and their copper(II) complexes: Synthesis, characterization, and anticancer activity. J. Macromol. Sci. Part A 2022, 59, 211–227. [Google Scholar] [CrossRef]
- Fong, D.; Hoemann, C.D. Chitosan immunomodulatory properties: Perspectives on the impact of structural properties and dosage. Future Sci. OA 2018, 4, FSO225. [Google Scholar] [CrossRef] [Green Version]
- Abd-Allah, H.; Nasr, M.; Ahmed-Farid, O.A.H.; Ibrahim, B.M.M.; Bakeer, R.M.; Ahmed, R.F. Nicotinamide and ascorbic acid nanoparticles against the hepatic insult induced in rats by high fat high fructose diet: A comparative study. Life Sci. 2020, 263, 118540. [Google Scholar] [CrossRef]
- Zheng, J.; Yuan, X.; Cheng, G.; Jiao, S.; Feng, C.; Zhao, X.; Yin, H.; Du, Y.; Liu, H. Chitosan oligosaccharides improve the disturbance in glucose metabolism and reverse the dysbiosis of gut microbiota in diabetic mice. Carbohyd. Polym. 2018, 190, 77–86. [Google Scholar] [CrossRef] [PubMed]
- Buzlama, A.; Doba, S.; Daghir, S.; Leonidovna, K.E.; Balloul, G. Study of Antiulcer activity of a hydrogel based on chitosan. Res. J. Pharm. Technol. 2021, 14, 4101–4106. [Google Scholar] [CrossRef]
- Muthu, M.; Gopal, J.; Chun, S.; Devadoss, A.J.P.; Hasan, N.; Sivanesan, I. Crustacean waste-derived chitosan: Antioxidant properties and future perspective. Antioxidants 2021, 10, 228. [Google Scholar] [CrossRef] [PubMed]
- Naveed, M.; Phil, L.; Sohail, M.; Hasnat, M.; Baig, M.M.F.A.; Ihsan, A.U.; Shumzaid, M.; Kakar, M.U.; Khan, T.M.; Akabar, M.D.; et al. Chitosan oligosaccharide (COS): An overview. Int. J. Biol. Macromol. 2019, 129, 827–843. [Google Scholar] [CrossRef]
- Boroumand, H.; Badie, F.; Mazaheri, S.; Seyedi, Z.S.; Nahand, J.S.; Nejati, M.; Baghi, H.B.; Abbasi-Kolli, M.; Badehnoosh, B.; Ghandali, M.; et al. Chitosan-Based Nanoparticles Against Viral Infections. Front. Cell Infect. Microbiol. 2021, 11, 643953. [Google Scholar] [CrossRef]
- Ahmed, T.; Aljaeid, B. Preparation, characterization, and potential application of chitosan, chitosan derivatives, and chitosan metal nanoparticles in pharmaceutical drug delivery. Drug Des. Devel. Ther. 2016, 10, 483–507. [Google Scholar] [CrossRef] [Green Version]
- Herdiana, Y.; Wathoni, N.; Shamsuddin, S.; Joni, I.M.; Muchtaridi, M. Chitosan-Based Nanoparticles of Targeted Drug Delivery System in Breast Cancer Treatment. Polymers 2021, 13, 1717. [Google Scholar] [CrossRef]
- Yi, H.; Wu, L.-Q.; Bentley, W.E.; Ghodssi, R.; Rubloff, G.W.; Culver, J.N.; Payne, G.F. Biofabrication with chitosan. Biomacromolecules 2005, 6, 2881–2894. [Google Scholar] [CrossRef]
- Abdelhakim, M.A.; Radwan, M.S.; Rady, A.H. Chitosan nanoparticles as hepato-protective agent against alcohol and fatty diet stress in rats. Biochem. Int. 2017, 4, 5–10. [Google Scholar]
- Tao, W.; Sun, W.; Liu, L.; Wang, G.; Xiao, Z.; Pei, X.; Wang, M. Chitosan Oligosaccharide Attenuates Nonalcoholic Fatty Liver Disease Induced by High Fat Diet through Reducing Lipid Accumulation, Inflammation and Oxidative Stress in C57BL/6 Mice. Mar. Drugs 2019, 17, 645. [Google Scholar] [CrossRef] [Green Version]
- Satitsri, S.; Muanprasat, C. Chitin and Chitosan Derivatives as Biomaterial Resources for Biological and Biomedical Applications. Molecules 2020, 25, 5961. [Google Scholar] [CrossRef] [PubMed]
- Alcohol in the European Union—Consumption, Harm and Policy Approaches. Available online: https://www.euro.who.int/__data/assets/pdf_file/0003/160680/e96457.pdf (accessed on 27 March 2022).
- Benić, M.S.; Nežić, L.; Vujić-Aleksić, V.; Mititelu-Tartau, L. Novel therapies for the treatment of drug induced liver injury: A systematic review. Front. Pharmacol. 2021, 12, 785790. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.; Zhang, L.; Hu, Z.; Kong, S.; Zhang, Z.; Li, G. Optimized preparation of gastric acid-response sulfhydryl functionalized chitosan/alginate/tilapia peptide hydrogel and its protective effects on alcohol-induced liver and brain injury. RSC Adv. 2021, 11, 34544–34557. [Google Scholar] [CrossRef]
- Lamas-Paz, A.; Hao, F.; Nelson, L.J.; Vázquez, M.T.; Canals, S.; Del Moral, M.G.; Martínez-Naves, E.; Nevzorova, Y.A.; Cubero, F.J. Alcoholic liver disease: Utility of animal models. World J. Gastroenterol. 2018, 24, 5063–5075. [Google Scholar] [CrossRef] [PubMed]
- Ghosh Dastidar, S.; Warner, J.B.; Warner, D.R.; McClain, C.J.; Kirpich, I.A. Rodent models of alcoholic liver disease: Role of binge ethanol administration. Biomolecules 2018, 8, 3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brandon-Warner, E.; Schrum, L.W.; Schmidt, C.M.; McKillop, I.H. Rodent models of alcoholic liver disease: Of mice and men. Alcohol 2012, 46, 715–725. [Google Scholar] [CrossRef] [Green Version]
- Ueno, A.; Lazaro, R.; Wang, P.Y.; Higashiyama, R.; Machida, K.; Tsukamoto, H. Mouse intragastric infusion (iG) model. Nat. Protoc. 2012, 7, 771–781. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsukamoto, H.; Mkrtchyan, H.; Dynnyk, A. Intragastric ethanol infusion model in rodents. Methods Mol. Biol. 2008, 447, 33–48. [Google Scholar] [CrossRef]
- Okamura, Y.; Omori, A.; Asada, N.; Ono, A. Effects of vitamin C and E on toxic action of alcohol on partial hepatectomy-induced liver regeneration in rats. J. Clin. Biochem. Nutr. 2018, 63, 50–57. [Google Scholar] [CrossRef] [Green Version]
- Oyinbo, C.A.; Dare, W.N.; Okogun, G.R.A.; Anyanwu, L.C.; Ibeabuchi, N.M.; Noronha, C.C.; Okanlawon, O.A. The hepatoprotective effect of vitamin C and E on hepatotoxicity induced by ethanol in Sprague Dawley rats. Pak. J. Nutr. 2012, 5, 507–511. [Google Scholar]
- Abdulrazzaq, A.M.; Badr, M.; Gammoh, O.; Abu Khalil, A.A.; Ghanim, B.Y.; Alhussainy, T.M.; Qinna, N.A. Hepatoprotective actions of ascorbic acid, alpha lipoic acid and silymarin or their combination against acetaminophen-induced hepatotoxicity in rats. Medicina 2019, 55, 181. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Su, M.; Liang, X.; Xu, X.; Wu, X.; Yang, B. Hepatoprotective benefits of vitamin C against perfluorooctane sulfonate-induced liver damage in mice through suppressing inflammatory reaction and ER stress. Environ. Toxicol. Pharmacol. 2019, 65, 60–65. [Google Scholar] [CrossRef] [PubMed]
- Adikwu, E.; Deo, O. Hepatoprotective effect of vitamin C (ascorbic acid). Pharmacol. Pharm. 2013, 4, 84–92. [Google Scholar] [CrossRef] [Green Version]
- Uboh, F.E.; Ebong, P.E.; Akpan, H.D.; Usoh, I.F. Hepatoprotective effect of vitamins C and E against gasoline vapor-induced liver injury in male rats. Turk. J. Biol. 2012, 36, 217–223. [Google Scholar] [CrossRef]
- Zhong, X.; Zeng, M.; Bian, H.; Zhong, C.; Xiao, F. An evaluation of the protective role of vitamin C in reactive oxygen species-induced hepatotoxicity due to hexavalent chromium in vitro and in vivo. J. Occup. Med. Toxicol. 2017, 12, 15. [Google Scholar] [CrossRef] [Green Version]
- KhaldounOularbi, H.; Richeval, C.; Lebaili, N.; Zerrouki-Daoudi, N.; Baha, M.; Djennas, N.; Allorge, D. Ameliorative effect of vitamin C against hepatotoxicity induced by emamectin benzoate in rats. Hum. Exp. Toxicol. 2017, 36, 709–717. [Google Scholar] [CrossRef]
- Aroor, A.R.; Jackson, D.E.; Shukla, S.D. Elevated activation of ERK1 and ERK2 accompany enhanced liver injury following alcohol binge in chronically ethanol-fed rats. Alcohol Clin. Exp. Res. 2011, 35, 2128–2138. [Google Scholar] [CrossRef] [Green Version]
- Lambert, J.C.; Zhou, Z.; Wang, L.; Song, Z.; McClain, C.J.; Kang, Y.J. Prevention of alterations in intestinal permeability is involved in zinc inhibition of acute ethanol-induced liver damage in mice. J. Pharmacol. Exp. Ther. 2003, 305, 880–886. [Google Scholar] [CrossRef]
- Lima, M.C.; Marks, G.; Silva, I.S.; Silva, B.A.; Cônsolo, L.Z.; Nogueira, G.B. Evaluation of oxidative stress in mice subjected to aerobic exercise. Acta Cir. Bras. 2012, 27, 544–551. [Google Scholar] [CrossRef]
- Ikechukwu, U.R.; Amarachi, A.; Nancy, U.O.; Kalu, A.N.; Ikechukwu, E.S.; Chukwuemaka, N.-A.P.; Rita, N.O. Evaluation of Antioxidant Activity of Aqueous Extracts of Palm Fruits (Elaeisguineensis). Asian J. Biochem. 2017, 12, 49–58. [Google Scholar] [CrossRef]
- European Union. DIRECTIVE 2010/63/EU of the European Parliament and of the Council of 22 September 2010 on the Protection of Animals Used for Scientific Purposes; European Union: Brussels, Belgium, 2010; Available online: https://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2010:276:0033:0079:en:PDF (accessed on 7 January 2022).
- Goodman, J.; Chandna, A.; Roe, K. Trends in animal use at US research facilities. J. Med. Ethics 2015, 41, 567–569. [Google Scholar] [CrossRef] [PubMed]
- Lunardi, C.N.; Gomes, A.J.; Rocha, F.S.; De Tommaso, J.; Patience, G.S. Experimental methods in chemical engineering: Zeta potential. Can. J. Chem. Eng. 2021, 99, 627–639. [Google Scholar] [CrossRef]
- Larsson, M.; Hill, A.; Duffy, J. Suspension stability; why particle size, zeta potential and rheology are important. Annu. Trans. Nord. Rheol. Soc. 2012, 12, 209–214. [Google Scholar]
- Midekessa, G.; Godakumara, K.; Ord, J.; Viil, J.; Lättekivi, F.; Dissanayake, K.; Kopanchuk, S.; Rinken, A.; Andronowska, A.; Bhattacharjee, S.; et al. Zeta potential of extracellular vesicles: Toward understanding the attributes that determine colloidal stability. ACS Omega 2020, 5, 16701–16710. [Google Scholar] [CrossRef]
- Nguyen, T.X.; Huang, L.; Liu, L.; Abdalla, A.M.E.; Gauthier, M.; Yang, G. Chitosan-coated nano-liposomes for the oral delivery of berberine hydrochloride. Acta Pharm. Sin. B 2014, 2, 7149–7159. [Google Scholar] [CrossRef]
- Tan, C.; Feng, B.; Zhang, X.; Xia, W.; Xia, S. Biopolymer-coated liposomes by electrostatic adsorption of chitosan (chitosomes) as novel delivery systems for carotenoids. Food Hydrocoll. 2016, 52, 774–784. [Google Scholar] [CrossRef]
- Ahlawat, J.; Henriquez, G.; Narayan, M. Enhancing the delivery of chemotherapeutics: Role of biodegradable polymeric nanoparticles. Molecules 2018, 23, 2157. [Google Scholar] [CrossRef] [Green Version]
- Subhapradha, N.; Shanmugam, V.; Shanmugam, A. Chitosan nanoparticles from marine squid protect liver cells against N-diethylnitrosoamine-induced hepatocellular carcinoma. Carbohydr. Polym. 2017, 171, 18–26. [Google Scholar] [CrossRef]
- Bonferoni, M.C.; Gavini, E.; Rassu, G.; Maestri, M.; Giunchedi, P. Chitosan Nanoparticles for Therapy and Theranostics of Hepatocellular Carcinoma (HCC) and Liver-Targeting. Nanomaterials 2020, 10, 870. [Google Scholar] [CrossRef]
- Bugnicourt, L.; Peers, S.; Dalverny, C.; Ladavière, C. Tunable morphology of lipid/chitosan particle assemblies. J. Colloid Interface Sci. 2019, 534, 105–109. [Google Scholar] [CrossRef]
- Huang, G.; Liu, Y.; Chen, L. Chitosan and its derivatives as vehicles for drug delivery. Drug Deliv. 2017, 24, 108–113. [Google Scholar] [CrossRef] [PubMed]
- Ozcelik, E.; Uslu, S.; Erkasap, N.; Karimi, H. Protective effect of chitosan treatment against acetaminophen-induced hepatotoxicity. KJMS 2014, 30, 286–290. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Login, C.; Nagy Andras, L.; Muresan, A.; Moldovan, R.; Decea, N.; Daicoviciu, D.; Clichici, S. Antioxidant and hepatoprotective effect of chitosan versus vitamin E in experimental carbon tetrachloride-induced liver injuries. Studia Univ. Babeș-Bolyai. Chem. 2015, 60, 389–397. [Google Scholar]
- Wang, Z.; Wang, M.; Yu, D.; Zhao, Y.; Xu, H.; Zhong, S.; Sun, W.-Y.; He, Y.-F.; Niu, J.-Q.; Gao, P.-J.; et al. Therapeutic effect of chitosan on CCl4-induced hepatic fibrosis in rats. Mol. Med. Rep. 2018, 18, 3211–3218. [Google Scholar] [CrossRef] [Green Version]
- Shaban, N.Z.; Yehia, S.A.; Awad, D.; Shaban, S.Y.; Saleh, S.R. A Titanium (IV)-Dithiophenolate Complex and Its Chitosan Nanocomposite: Their Roles Towards Rat Liver Injuries In Vivo and against Human Liver Cancer Cell Lines. Int. J. Mol. Sci. 2021, 22, 11219. [Google Scholar] [CrossRef]
- Yahya, S.; Shalaby, R.K.; Mannaa, F.A.; Abdel-Wahhab, K.G.; Mohamed, N.R.; Shabana, M.S.; Elwakeel, S.H.B. Hepatoprotective effects of chitosan on thioacetamide induced liver toxicity in male albino rats. Biointerface Res. Appl. Chem. 2021, 11, 14490–14505. [Google Scholar] [CrossRef]
- Abou Zaid, O.; Elsonbaty, S.; Moawad, F.; Abdelghaffar, M. Antioxidants and hepatoprotective effects of chitosan nanoparticles against hepatotoxicity induced in rats. Benha Vet. Med. J. 2019, 36, 252–261. [Google Scholar] [CrossRef]
- Dawoud, S.F.; Al-Akra, T.M.; Zedan, A.M. Hepatoprotective effects of chitosan and chitosan nanoparticles against biochemical, genetic, and histological disorders induced by the toxicity of emamectin benzoate. Rep. Biochem. Mol. Biol. 2021, 10, 506–514. [Google Scholar] [CrossRef]
- Santhosh, S.; Sini, T.K.; Anandan, R.; Mathew, P.T. Hepatoprotective activity of chitosan against isoniazid and rifampicin-induced toxicity in experimental rats. Eur. J. Pharmacol. 2007, 572, 69–73. [Google Scholar] [CrossRef]
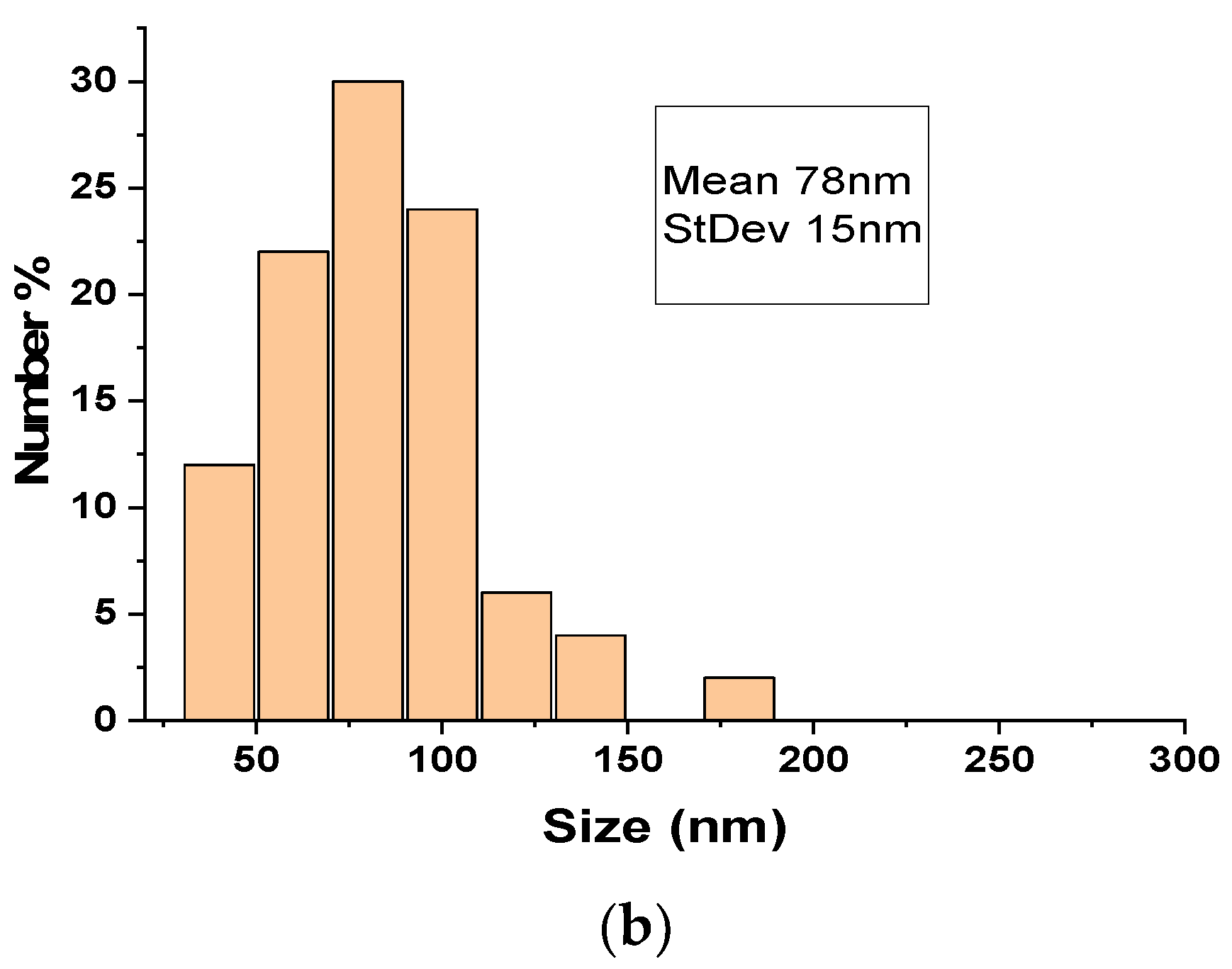
| Investigation Steps | Day of Experiment | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | |
| Control (water ad libitum) | ||||||||||||||
| 5% alcohol in water ad libitum | ||||||||||||||
| Control alcohol | ||||||||||||||
| CHIT | ||||||||||||||
| CHIT-ves | ||||||||||||||
| AcA | ||||||||||||||
| 5 g/kg orally of 10% alcohol solution | ||||||||||||||
| Blood collection | ||||||||||||||
| Liver fragment collection | ||||||||||||||
| Groups | Time Points of Determination | RBCs (mil/μL) | Hb (g/mL) | Ht (%) |
|---|---|---|---|---|
| Control | 24 h | 8.47 ± 1.60 | 13.79 ± 2.29 | 38.35 ± 6.09 |
| 14 days | 8.55 ± 0.98 | 14.07 ± 2.41 | 38.22 ± 5.67 | |
| Control alcohol | 24 h | 8.41 ± 1.33 | 14.37 ± 2.17 | 38.12 ± 5.39 |
| 14 days | 7.79 ± 1.06 | 13.72 ± 2.11 | 39.19 ± 6.33 | |
| CHIT | 24 h | 8.28 ± 2.09 | 13.44 ± 2.43 | 37.64 ± 5.81 |
| 14 days | 8.45 ± 1.43 | 13.58 ± 2.67 | 37.42 ± 5.43 | |
| CHIT-ves | 24 h | 8.34 ± 1.15 | 13.97 ± 2.81 | 38.24 ± 5.29 |
| 14 days | 7.97 ± 1.37 | 13.19 ± 1.33 | 37.79 ± 5.55 | |
| AcA | 24 h | 8.12 ± 1.67 | 14.48 ± 3.21 | 38.72 ± 6.13 |
| 14 days | 8.24 ± 1.55 | 14.13 ± 2.45 | 38.47 ± 5.37 |
| Groups | Time points of Determination | Leukocyte Formula Elements (%) | ||||
|---|---|---|---|---|---|---|
| PMN | Ly | E | M | B | ||
| Control | 24 h | 14.26 ± 6.59 | 83.22 ± 9.03 | 0.45 ± 0.17 | 1.78 ± 1.23 | 0.29 ± 0.15 |
| 14 days | 14.55 ± 7.48 | 82.61 ± 9.11 | 0.51 ± 0.27 | 2.05 ± 1.81 | 0.28 ± 0.19 | |
| Controlalcohol | 24 h | 13.67 ± 7.11 | 83.77 ± 9.23 | 0.49 ± 0.11 | 1.78 ± 1.23 | 0.29 ± 0.13 |
| 14 days | 13.43 ± 6.72 | 83.74 ± 9.13 | 0.48 ± 0.15 | 2.05 ± 1.81 | 0.30 ± 0.37 | |
| CHIT | 24 h | 13.36 ± 7.41 | 83.88 ± 8.07 | 0.47 ± 0.13 | 2.09 ± 0.67 | 0.22 ± 0.13 |
| 14 days | 13.52 ± 7.55 | 84.17 ± 8.12 | 0.50 ± 0.11 | 1.56 ± 0.43 | 0.25 ± 0.11 | |
| CHIT-ves | 24 h | 14.15 ± 7.26 | 83.81 ± 8.07 | 0.50 ± 0.23 | 1.23 ± 1.17 | 0.31 ± 0.25 |
| 14 days | 13.09 ± 8.48 | 85.84 ± 8.12 | 0.49 ± 0.17 | 1.03 ± 0.56 | 0.33 ± 0.57 | |
| AcA | 24 h | 14.84 ± 8.33 | 82.29 ± 9.21 | 0.48 ± 0.13 | 2.07 ± 1.03 | 0.32 ± 0.19 |
| 14 days | 14.52 ± 8.07 | 82.57 ± 8.33 | 0.46 ± 0.21 | 2.15 ± 1.26 | 0.30 ± 0.11 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hilițanu, L.N.; Mititelu-Tarțău, L.; Bogdan, M.; Buca, B.R.; Păuna, A.-M.R.; Pavel, L.L.; Pelin, A.-M.; Meca, A.-D.; Popa, G.E. The Use of Chitosan-Coated Nanovesicles in Repairing Alcohol-Induced Damage of Liver Cells in Mice. Medicina 2022, 58, 762. https://doi.org/10.3390/medicina58060762
Hilițanu LN, Mititelu-Tarțău L, Bogdan M, Buca BR, Păuna A-MR, Pavel LL, Pelin A-M, Meca A-D, Popa GE. The Use of Chitosan-Coated Nanovesicles in Repairing Alcohol-Induced Damage of Liver Cells in Mice. Medicina. 2022; 58(6):762. https://doi.org/10.3390/medicina58060762
Chicago/Turabian StyleHilițanu, Loredana Nicoleta, Liliana Mititelu-Tarțău, Maria Bogdan, Beatrice Rozalina Buca, Ana-Maria Raluca Păuna, Liliana Lăcrămioara Pavel, Ana-Maria Pelin, Andreea-Daniela Meca, and Grațiela Eliza Popa. 2022. "The Use of Chitosan-Coated Nanovesicles in Repairing Alcohol-Induced Damage of Liver Cells in Mice" Medicina 58, no. 6: 762. https://doi.org/10.3390/medicina58060762
APA StyleHilițanu, L. N., Mititelu-Tarțău, L., Bogdan, M., Buca, B. R., Păuna, A.-M. R., Pavel, L. L., Pelin, A.-M., Meca, A.-D., & Popa, G. E. (2022). The Use of Chitosan-Coated Nanovesicles in Repairing Alcohol-Induced Damage of Liver Cells in Mice. Medicina, 58(6), 762. https://doi.org/10.3390/medicina58060762









