Abstract
Nonalcoholic fatty liver disease (NAFLD) is the most common chronic liver disease in the world. Sustained hepatic inflammation is a key driver of the transition from simple fatty liver to nonalcoholic steatohepatitis (NASH), the more aggressive form of NAFLD. Hepatic inflammation is orchestrated by chemokines, a family of chemoattractant cytokines that are produced by hepatocytes, Kupffer cells (liver resident macrophages), hepatic stellate cells, endothelial cells, and vascular smooth muscle cells. Over the last three decades, accumulating evidence from both clinical and experimental investigations demonstrated that chemokines and their receptors are increased in the livers of NAFLD patients and that CC chemokine ligand (CCL) 2 and CCL5 in particular play a pivotal role in inducing insulin resistance, steatosis, inflammation, and fibrosis in liver disease. Cenicriviroc (CVC), a dual antagonist of these chemokines’ receptors, CCR2 and CCR5, has been tested in clinical trials in patients with NASH-associated liver fibrosis. Additionally, recent studies revealed that other chemokines, such as CCL3, CCL25, CX3C chemokine ligand 1 (CX3CL1), CXC chemokine ligand 1 (CXCL1), and CXCL16, can also contribute to the pathogenesis of NAFLD. Here, we review recent updates on the roles of chemokines in the development of NAFLD and their blockade as a potential therapeutic approach.
1. Introduction
Chemokines (Greek—kinos, movement) are a large family of chemotactic cytokines that involve immune and inflammatory responses through the chemoattraction and activation of leukocytes [1]. These small proteins (approximately 8–12 kilodaltons) are classified into four different subfamilies (CC, CXC, CX3C and XC) based on the presence of four cysteine residues in the conserved locations of N-terminals that are key to forming their 3-dimensional shape [2]. To date, approximately 50 chemokines expressed in various cell types and tissues have been identified in humans and mice [3]. In the liver, not only Kupffer cells but also hepatocytes, hepatic stellate cells (HSCs), liver sinusoidal endothelial cells, and vascular smooth muscle cells can secrete chemokines upon activation [4].
Chemokine receptors are a group of ~20 typical G protein-coupled seven-transmembrane proteins and are expressed in various leucocytes and immune cells. The directed migration of specific chemokine receptor-expressing cells allows for their recruitment along a chemokine concentration gradient [5]. Upon ligand binding, chemokine receptors mediate cellular calcium influx through phosphatidylinositol 3-kinase and small Rho guanosine triphosphatase activation, thereby increasing the avidity of leukocyte integrins that promote leukocytes’ interactions with intercellular adhesion molecules on sinusoidal endothelial cells [6,7]. Chemokines regulate not only immune cell recruitment during inflammation through inflammatory chemokines (CCL2, CCL3, CCL5, etc.) but also the trafficking of innate immune cells at homeostasis through homeostatic chemokines (CXCL12, etc.); they also modulate the functions of nonimmune cells, such as fibrogenic HSCs [8,9,10].
Regarding the pathogenesis of NAFLD, relevant chemokines and their receptors have been well summarized by excellent reviews [11,12]. Particularly, the pathophysiological roles of CCL2 and CCL5 in the development of NAFLD have been well studied in both NAFLD patients and animal models. In NAFLD patients, elevated serum and hepatic mRNA levels of CCL2 increase the recruitment of CCR2-positive bone marrow-derived monocytes into the liver, resulting in further hepatic inflammation, fibrosis, and steatosis [13,14,15]. Accordingly, the genetic deletion or pharmacological inhibition of CCR2 has been reported to improve NASH and insulin resistance in mice [16,17]. CCL5 production is also increased by excessive lipid accumulation in the liver [18]. CCL5 is required for the progression of liver fibrosis by binding to CCR1 on liver macrophages and CCR5 on hepatic stellate cells [19,20]. Based on these observations, cenicriviroc (CVC), a dual CCR2 and CCR5 antagonist, is expected to improve NASH and has been tested in clinical trials in patients with NASH-associated liver fibrosis [21,22].
In this review, we will highlight recent updates on the roles of chemokines, including CCL3, CCL25, CXCL1, CXCL16, and CX3CL1 (Figure 1), in the development of NAFLD and their blockade as a potential therapeutic approach.
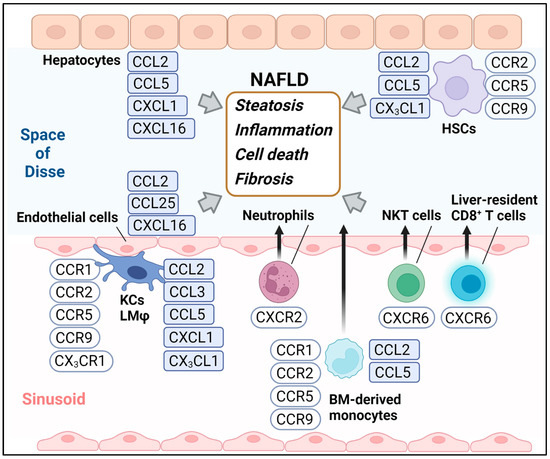
Figure 1.
The chemokines and chemokine receptors highlighted in this review. BM, Bone marrow; KCs, Kupffer cells; LMφ, Liver macrophages; HSCs, Hepatic stellate cells.
2. An Update on the Chemokine System in the Development of NAFLD
2.1. CCL3-CCR1 and CCR5
CCL3 (known as macrophage inflammatory protein-1α) is expressed in macrophages and secreted to recruit macrophages themselves, various leukocyte subtypes, and T cells to inflamed sites [23,24]. Various proinflammatory stimuli, such as viral infections, lipopolysaccharide, tumor necrosis factor-α (TNF-α), interferon-γ, and interleukin-1β (IL-1β), can induce the expression of CCL3 [25,26]. CCL3 signals through its receptors CCR1 and CCR5. T cells, eosinophils, and neutrophils express CCR1 [27]. NK cells and subsets of resting memory T cells, including some but not all Th1 cells, express CCR5 [27]. Monocytes and mature macrophages express both CCR1 and CCR5 [28].
The progression of some inflammatory diseases, including atherosclerosis [29], rheumatoid arthritis [30], and NAFLD [31,32], is associated with the increased expression of CCL3 and its receptors. Circulating CCL3 levels were significantly higher in NASH patients compared with healthy controls [31,32]. Recently, we also reported that the serum and hepatic protein levels of CCL3 were significantly higher in patients with NASH compared with those of healthy controls [33]. Unlike in a previous report [31,32], however, patients with simple fatty liver also showed a trend of increased serum CCL3 levels and a significant increase in hepatic CCL3 protein expression compared with those of healthy controls [33]. Additionally, the circulating levels of CCL3 were high according to the histological severity of ballooning, steatosis, and lobular inflammation [33]. These results suggest that CCL3 might be a causative factor, not just a result of advanced fibrosis, in NAFLD development.
We further investigated the role of CCL3 in the pathogenesis of NAFLD in mice fed a high-cholesterol and high-fat (CL) diet, a dietary model of NASH [34]. We found that the circulating levels and hepatic expression of CCL3 were elevated in the CL diet-fed mice and that the hepatic source of CCL3 was particularly M1-like macrophages rather than M2-like macrophages and other cell types [33]. The genetic deletion of CCL3 attenuated the CL diet-induced steatohepatitis and hepatic insulin resistance, at least partly, by decreasing macrophage recruitment and restoring alternative macrophage activation in the liver [33]. Moreover, the specific deletion of CCL3 in bone marrow cells eased CL diet-induced steatohepatitis [33]. These results suggest that CCL3 plays a certain role in the recruitment of bone marrow-derived monocytes into the liver and the M1 polarization of liver macrophages, which contributes to chronic inflammation and hepatic insulin resistance in the development of NAFLD.
2.2. CCL25-CCR9
The chemokine CCL25 is selectively and constitutively expressed in the thymus and small intestine. CCR9, the sole functional receptor of CCL25 [35], is expressed on thymocytes and intestinal lymphocytes [36]. The CCL25-CCR9 axis is crucial for mucosal lymphocyte recruitment to the small intestine followed by accumulating CCR9+CD4+ tissue-infiltrating T cells in both Crohn’s disease and a murine model of inflammatory bowel disease [37,38,39]. With regard to liver immunology, CCR9+ macrophages play a pathogenic role in a murine acute hepatitis model and humans [40]. Peripheral blood samples from patients with acute hepatitis had more TNF-α-producing CCR9+ monocytes than healthy volunteers [40]. Similarly, in concanavalin A-injected mice, bone marrow-derived CCR9+ macrophages accumulate in the liver, which produces high levels of TNF-α and promotes the Th1 differentiation of naive CD4+ T cells, thereby contributing to acute liver inflammation [40]. Additionally, Morikawa et al. provided multiple lines of evidence indicating that the CCL25-CCR9 axis also plays a pivotal role in NASH pathogenesis [41]: (1)Serum CCL25 and hepatic CCR9 and CCL25 levels were higher in patients with NASH compared with healthy volunteers and patients with simple fatty liver. (2) CCL25 was expressed in CD31+/LYVE1+ sinusoidal endothelial cells, whereas CCR9 was expressed in CD68+ macrophages and GFAP+/α-SMA+ HSCs in the livers of patients with NASH, and the numbers of these CCR9+ cells were significantly lower in the control samples. (3) CCR9-deficient mice showed alleviated diet-induced steatohepatitis associated with the decrease in the amount of CD11b+ inflammatory macrophage accumulation in the liver. (4) Consistent with human NASH, CCR9 was also expressed on HSCs in NASH mice and CCR9-deficient HSCs show fewer fibrogenic phenotypes. Finally (5) A CCR9 antagonist, vercirnon (CCX282-B) ameliorated steatohepatitis and the development of diethylnitrosamine-induced hepatocellular carcinoma in a high-fat diet-fed mice. These results indicate a therapeutic potential of CCR9 blockade in NAFLD.
2.3. CXCL1-CXCR2
CXCL1 is one of the major chemoattractants for neutrophils [42]. After binding to its receptor CXCR2, CXCL1 activates PI3K/Akt, MAP kinases, or phospholipase-β signaling pathways, increasing the recruitment of neutrophils into inflamed sites [43]. CXCL1 is also involved in the processes of wound healing, angiogenesis, tumorigenesis, and cell motility [44]. CXCL1 is highly expressed in the liver of NASH patients but not in the simple fatty livers in obese individuals or in high-fat diet (HFD)-fed mice [45,46]. In the choline-deficient amino acid-defined (CDAA) diet-induced mouse NASH model, the hepatic mRNA levels of CXCL1 are increased in a toll-like receptor 4 (TLR4)-MyD88-dependent manner, resulting in increased neutrophil infiltration associated with hepatic inflammation and fibrosis [47]. Additionally, adenoviral overexpression of CXCL1 in the liver is sufficient to activate progression from steatosis to steatohepatitis in HFD-fed mice by inducing hepatic neutrophile infiltration, oxidative stress, and hepatocyte apoptosis [48]. These studies indicate the importance of CXCL1-/CXCR2-mediated neutrophile recruitment during NAFLD development.
2.4. CXCL16-CXCR6
In conjunction with CD4, CXCR6 can serve as a co-receptor for the entry of human and most simian immunodeficiency viruses (human immunodeficiency virus type I and simian immunodeficiency virus) [49]. Similar to CCR5 and CXCR3, the expression pattern of CXCR6 is restricted to memory/effector T cells such as natural killer T (NKT) cells [50,51] and CD8+ T cells [52]. In the liver, CXCR6+ NKT cells patrol liver sinusoids and provide the intravascular immune surveillance of pathogens [53]. CXCL16, a membrane-bound ligand for CXCR6, is expressed on the hepatocytes and biliary epithelial cells in the portal tracts and on sinusoidal cells in both normal and chronically inflamed liver tissue such as hepatitis C [54]. CXCL16 promotes the adhesion of CXCR6+ cells to cholangiocytes and hepatocytes by triggering the conformational activation of β1 integrins and the binding to vascular cell adhesion molecule-1 (VCAM-1), thereby promoting liver inflammation [54]. Regarding NAFLD, Jing et al. demonstrated that serum levels of CXCL16 were elevated in NAFLD patients and that CXCL16 was strongly expressed around the steatotic hepatocytes in liver biopsy specimens [55]. Additionally, in the co-culture of murine hepatocytes and HSCs, lentiviral overexpression of CXCL16 increased lipid accumulation and mitochondrial stress in hepatocytes and induced the activation and proliferation of HSCs [55], suggesting that the CXCL16-CXCR6 axis mediates the crosstalk between hepatocytes and HSCs in NAFLD development.
In the liver, CXCR6 is also expressed in CD8+ T cells. The auto-aggression of CD8+ T cells may be involved in the development of hepatocellular carcinoma from NASH. CXCR6+ CD8+ T cells accumulate in the livers of a preclinical mouse model of NASH (mice fed a choline-deficient and HFD) and of patients with NASH [52]. The T cells are susceptible to metabolic stimuli such as acetate and extracellular ATP, showing auto-aggressive killing of cells in an MHC-class-I-independent fashion [52].
2.5. CX3CL1-CX3CR1
CX3CL1, also known as fractalkine, a membrane-anchored chemokine, is expressed on epithelial cells, dendritic cells, and neurons and could be induced by inflammatory cytokines, such as TNF-α and IFN-γ [56,57,58,59,60,61]. CX3CL1 drives integrin-dependent adhesion and promotes the retention of specific CX3CR1-expressing leukocytes. The receptor is mainly expressed on circulating monocytes, tissue-resident macrophages, dendritic cells, and T cells [56,62,63]. The N-terminal domain of CX3CL1, containing a CX3C motif, can be cleaved by ADAM Metallopeptidase Domain 10 (ADAM10) [64] and ADAM17 [65], yielding a soluble form that also ligates CX3CR1 and exerts potent chemotactic activity [66].
Similar to the other chemokines, both animal and clinical studies have demonstrated that CX3CL1-CX3CR1 signaling is enhanced in various inflammatory diseases, such as rheumatoid arthritis [67], atherosclerosis [68,69], and chronic hepatitis C [70]. However, the pathophysiological role of CX3CL1-CX3CR1 signaling in NAFLD development remains controversial. In the mouse liver, CX3CL1 is expressed in Kupffer cells/liver macrophages and HSCs [71], while CX3CR1 is mainly expressed in Kupffer cells [71,72]. Sutti et al. reported that CX3CR1-positive monocyte-derived dendritic cells (moDCs) contribute to hepatocyte injury by producing TNF-α in a murine model of steatohepatitis induced by a methionine/choline-deficient (MCD) diet [73] or carbon tetrachloride (CCl4) [74]. Following CCl4 exposure, whole-body CX3CR1-deficient mice (CX3CR1gfp/gfp) showed less moDC recruitment into the liver associated with incomplete maturation of monocytes into moDCs [74]. Additionally, in C57BL/6 wild-type mice, treatment with the CX3CR1 antagonist CX3-AT eased CCl4-induced hepatic injury and inflammation along with the decreased moDC accumulation in the liver [75]. These results suggest that CX3CR1 mediates hepatic inflammation by driving moDC recruitment and development. In contrast, Aoyama et al. reported that whole-body CX3CR1 knockout (CX3CR1−/−) mice exposed to CCl4 exhibited increased inflammatory cell recruitment into the liver and pro-inflammatory cytokine/chemokine production, including TNF-α, IL-1β, CCL2, and CCL5, but decreased expression of anti-inflammatory IL-10 and arginase-1 in Kupffer cells, resulting in enhanced HSC activation and subsequent liver fibrosis [71]. Additionally, our group reported that CX3CR1−/− mice were more prone to HFD-induced obesity, insulin resistance, and hepatic steatosis and inflammation compared with wild-type control mice [76]. We also found that CX3CL1 expression was lower in the epididymal white adipose tissue (eWAT) of HFD-induced obese C57BL/6J mice, and the long-term (4 weeks) in vivo expression of CX3CL1 by pLIVE® vector (plasma CX3CL1 concentration; 220–250 ng/mL vs. 150–170 ng/mL by empty vector) alleviated insulin resistance and inflammation in the liver and eWAT of obese mice [76]. Collectively, this discrepancy in previous studies may be due to the different roles of the CX3CL1-CX3CR1 signaling in different cell types/tissues.
3. Chemokine-Chemokine Receptor Axis as a Therapeutic Target of NAFLD (Small Molecules and Food Factors)
3.1. Cenicriviroc (CVC)
Since CCR2 and CCR5 play an important role in the infiltration of myeloid cells and the activation of HSCs, CVC, a once-daily, orally available CCR2/CCR5 dual antagonist, has been expected to improve NASH by suppressing both inflammation and fibrosis, as shown in animal models of steatohepatitis [77,78]. In the Phase 2b CENTAUR study (NCT02217475) of adults with NASH and liver fibrosis (NAFLD activity score ≥ 4 and NASH Clinical Research Network stage 1–3 fibrosis), CVC treatment showed a favorable safety and tolerability profile and improved liver fibrosis without worsening steatohepatitis compared with the placebo [22]. However, and unfortunately, the Phase 3 AURORA study (NCT03028740), which enrolled 1778 participants, of whom 1293 participated in Part 1 of the study [21], was terminated early due to lack of efficacy based on the results of the planned interim analysis of the Part 1 data. The disappointing results of the Phase III trial likely reflect the complexity of the pathogenesis of NAFLD, which involves diverse immune and metabolic pathways. Currently, for CVC, a Phase IIb study has been planned testing the combination therapy with a farnesoid X receptor agonist candidate involving bile acid, cholesterol, and lipid and glucose metabolism [79,80], for treating NASH.
3.2. Dietary Carotenoids and Sulforaphane
Many epidemiological studies have demonstrated that the development of NAFLD is closely linked to lifestyle factors (e.g., nutrition, physical activity) [81]. A nutritional intervention with fruits and vegetables could be effective in preventing NAFLD since dietary factors, including antioxidant carotenoids, are useful for decreasing the risk of inflammation-related diseases, including cancer, cardiovascular diseases, and obesity [82,83,84]. β-Cryptoxanthin and lycopene, carotenoids that specifically exist in Citrus unshiu (Satsuma mandarin orange) and Solanum lycopersicum (tomato), respectively, are relatively abundant in human blood [85,86,87] and have been reported to provide beneficial effects in a murine model of NAFLD. The supplementation of these carotenoids attenuated hepatic lipid accumulation and fibrosis in CL diet- or HFD-fed mice along with the decreased accumulation of T cells in the liver and enhanced anti-inflammatory M2-dominant liver macrophages [88,89,90]. The mechanism of this action was mediated, at least partly, through the downregulation of chemokines, including CCL2, CCL3, CCL5, and CXCL10 [88,89].
Sulforaphane, an isothiocyanate derived from cruciferous vegetables, such as broccoli, is a potent inducer of nuclear factor (erythroid-derived 2)–like 2 (Nrf2), a master transcription factor that regulates oxidative stress responses [90,91]. In addition to its antioxidative effects, sulforaphane has anti-inflammatory properties, suppressing pro-inflammatory IL-8 and CCL2 synthesis by inhibiting NF-κB, STAT6, and MAP kinase pathways [92,93]. We also reported that broccoli extract supplementation mitigated HFD-induced insulin resistance, hepatic steatosis, and the upregulation of CCL2-CCR2 axis [94]. Improved NAFLD by the broccoli extract supplementation was associated with decreased hepatic macrophage accumulation and the M2-dominant polarization of hepatic and adipose macrophages [94]. Additionally, the randomized, placebo-controlled, double-blind trial conducted by Kikuchi et al. demonstrated that supplementation with a dietary dose of broccoli extract for 2 months significantly decreased plasma liver enzymes, ALT, and AST in male participants, suggesting improved fatty liver by sulforaphane [95]. Further nutritional intervention studies, including large, long-term randomized clinical trials with histological assessment of NAFLD, are warranted.
4. Conclusions
Accumulating evidence from in vitro and in vivo studies reveals that some chemokine-chemokine receptor axes play a central role in liver inflammation during the development of NAFLD. However, as the results from the AURORA study demonstrate, therapeutic applications for targeting chemokines and chemokine receptors to resolve steatohepatitis and fibrosis are still challenging. Further basic and clinical research is essential for better understanding the molecular mechanisms by which the chemokine system mediates hepatic and adipose inflammation as well as their interaction in the progression of NAFLD. To improve the response rates among patients with NAFLD, combination approaches including lifestyle interventions that are personally tailored to the patient’s disease drivers, such as obesity and type 2 diabetes, are required.
Author Contributions
Writing—original draft preparation, N.N. and G.C.; writing—review and editing, L.X. and H.A.; supervision, H.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Acknowledgments
The authors thank Tsuguhito OTA (Kanazawa University) for his meaningful advice and kind support for the research. The authors also thank Yuko TSURUMI (Kanazawa University) for her proofreading of this manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Baggiolini, M. Chemokines and leukocyte traffic. Nature 1998, 392, 565–568. [Google Scholar] [CrossRef] [PubMed]
- Rollins, B.J. Chemokines. Blood 1997, 90, 909–928. [Google Scholar] [CrossRef] [PubMed]
- Hughes, C.E.; Nibbs, R.J.B. A guide to chemokines and their receptors. Febs J. 2018, 285, 2944–2971. [Google Scholar] [CrossRef] [PubMed]
- Karlmark, K.R.; Wasmuth, H.E.; Trautwein, C.; Tacke, F. Chemokine-directed immune cell infiltration in acute and chronic liver disease. Expert Rev. Gastroenterol. Hepatol. 2008, 2, 233–242. [Google Scholar] [CrossRef] [PubMed]
- Proudfoot, A.E.I.; Handel, T.M.; Johnson, Z.; Lau, E.K.; Wang, P.; Clark-Lewis, I.; Borlat, F.; Wells, T.N.C.; Kosco-Vilbois, M.H. Glycosaminoglycan binding and oligomerization are essential for the in vivo activity of certain chemokines. Proc. Natl. Acad. Sci. USA 2003, 100, 1885–1890. [Google Scholar] [CrossRef]
- McIntyre, T.M.; Prescott, S.M.; Weyrich, A.S.; Zimmerman, G.A. Cell-cell interactions: Leukocyte-endothelial interactions. Curr. Opin. Hematol. 2003, 10, 150–158. [Google Scholar] [CrossRef]
- Mellado, M.; Rodríguez-Frade, J.M.; Mañes, S.; Martínez, A.C. Chemokine signaling and functional responses: The role of receptor dimerization and TK pathway activation. Annu. Rev. Immunol. 2001, 19, 397–421. [Google Scholar] [CrossRef]
- Sokol, C.L.; Luster, A.D. The Chemokine System in Innate Immunity. Cold Spring Harb. Perspect. Biol. 2015, 7, a016303. [Google Scholar] [CrossRef]
- Marra, F. Chemokines in liver inflammation and fibrosis. Front. Biosci. 2002, 7, d1899–d1914. [Google Scholar] [CrossRef]
- Chen, K.; Bao, Z.; Tang, P.; Gong, W.; Yoshimura, T.; Wang, J.M. Chemokines in homeostasis and diseases. Cell Mol. Immunol. 2018, 15, 324–334. [Google Scholar] [CrossRef]
- Roh, Y.-S.; Seki, E. Chemokines and Chemokine Receptors in the Development of NAFLD. Adv. Exp. Med. Biol. 2018, 1061, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Marra, F.; Tacke, F. Roles for chemokines in liver disease. Gastroenterology 2014, 147, 577–594.e1. [Google Scholar] [CrossRef] [PubMed]
- Haukeland, J.W.; Damås, J.K.; Konopski, Z.; Løberg, E.M.; Haaland, T.; Goverud, I.; Torjesen, P.A.; Birkeland, K.; Bjøro, K.; Aukrust, P. Systemic inflammation in nonalcoholic fatty liver disease is characterized by elevated levels of CCL2. J. Hepatol. 2006, 44, 1167–1174. [Google Scholar] [CrossRef] [PubMed]
- Greco, D.; Kotronen, A.; Westerbacka, J.; Puig, O.; Arkkila, P.; Kiviluoto, T.; Laitinen, S.; Kolak, M.; Fisher, R.M.; Hamsten, A.; et al. Gene expression in human NAFLD. Am. J. Physiol. Gastrointest. Liver Physiol. 2008, 294, G1281–G1287. [Google Scholar] [CrossRef]
- Obstfeld, A.E.; Sugaru, E.; Thearle, M.; Francisco, A.-M.; Gayet, C.; Ginsberg, H.N.; Ables, E.V.; Ferrante, A.W., Jr. C-C chemokine receptor 2 (CCR2) regulates the hepatic recruitment of myeloid cells that promote obesity-induced hepatic steatosis. Diabetes 2010, 59, 916–925. [Google Scholar] [CrossRef]
- Miura, K.; Yang, L.; Van Rooijen, N.; Ohnishi, H.; Seki, E. Hepatic recruitment of macrophages promotes nonalcoholic steatohepatitis through CCR2. Am. J. Physiol. Gastrointest. Liver Physiol. 2012, 302, G1310–G1321. [Google Scholar] [CrossRef]
- Tamura, Y.; Sugimoto, M.; Murayama, T.; Minami, M.; Nishikaze, Y.; Ariyasu, H.; Akamizu, T.; Kita, T.; Yokode, M.; Arai, H. C-C chemokine receptor 2 inhibitor improves diet-induced development of insulin resistance and hepatic steatosis in mice. J. Atheroscler. Thromb. 2010, 17, 219–228. [Google Scholar] [CrossRef]
- Kirovski, G.; Gäbele, E.; Dorn, C.; Moleda, L.; Niessen, C.; Weiss, T.S.; Wobser, H.; Schacherer, D.; Buechler, C.; Wasmuth, H.E.; et al. Hepatic steatosis causes induction of the chemokine RANTES in the absence of significant hepatic inflammation. Int. J. Clin. Exp. Pathol. 2010, 3, 675–680. [Google Scholar]
- Seki, E.; De Minicis, S.; Gwak, G.-Y.; Kluwe, J.; Inokuchi, S.; Bursill, C.A.; Llovet, J.M.; Brenner, D.A.; Schwabe, R.F. CCR1 and CCR5 promote hepatic fibrosis in mice. J. Clin. Investig. 2009, 119, 1858–1870. [Google Scholar] [CrossRef]
- Berres, M.-L.; Koenen, R.; Rueland, A.; Zaldivar, M.M.; Heinrichs, D.; Sahin, H.; Schmitz, P.; Streetz, K.L.; Berg, T.; Gassler, N.; et al. Antagonism of the chemokine Ccl5 ameliorates experimental liver fibrosis in mice. J. Clin. Investig. 2010, 120, 4129–4140. [Google Scholar] [CrossRef]
- Anstee, Q.M.; Neuschwander-Tetri, B.A.; Wong, V.W.-S.; Abdelmalek, M.F.; Younossi, Z.M.; Yuan, J.; Pecoraro, M.L.; Seyedkazemi, S.; Fischer, L.; Bedossa, P.; et al. Cenicriviroc for the treatment of liver fibrosis in adults with nonalcoholic steatohepatitis: AURORA Phase 3 study design. Contemp. Clin. Trials 2020, 89, 105922. [Google Scholar] [CrossRef] [PubMed]
- Ratziu, V.; Sanyal, A.; Harrison, S.A.; Wong, V.W.S.; Francque, S.; Goodman, Z.; Aithal, G.P.; Kowdley, K.V.; Seyedkazemi, S.; Fischer, L.; et al. Cenicriviroc Treatment for Adults With Nonalcoholic Steatohepatitis and Fibrosis: Final Analysis of the Phase 2b CENTAUR Study. Hepatology 2020, 72, 892–905. [Google Scholar] [CrossRef] [PubMed]
- Olson, T.S.; Ley, K. Chemokines and chemokine receptors in leukocyte trafficking. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2002, 283, R7–R28. [Google Scholar] [CrossRef]
- Menten, P.; Wuyts, A.; Van Damme, J. Macrophage inflammatory protein-1. Cytokine Growth Factor Rev. 2002, 13, 455–481. [Google Scholar] [CrossRef]
- Lukacs, N.W.; Strieter, R.M.; Elner, V.M.; Evanoff, H.L.; Burdick, M.; Kunkel, S.L. Intercellular adhesion molecule-1 mediates the expression of monocyte-derived MIP-1 alpha during monocyte-endothelial cell interactions. Blood 1994, 83, 1174–1178. [Google Scholar] [CrossRef] [PubMed]
- Trifilo, M.J.; Bergmann, C.C.; Kuziel, W.A.; Lane, T.E. CC chemokine ligand 3 (CCL3) regulates CD8(+)-T-cell effector function and migration following viral infection. J. Virol. 2003, 77, 4004–4014. [Google Scholar] [CrossRef] [PubMed]
- Rabin, R.L. CC, C, and CX3C Chemokines. In Encyclopedia of Hormones; Henry, H.L., Norman, A.W., Eds.; Academic Press: New York, NY, USA, 2003; pp. 255–263. [Google Scholar] [CrossRef]
- Kaufmann, A.; Salentin, R.; Gemsa, D.; Sprenger, H. Increase of CCR1 and CCR5 expression and enhanced functional response to MIP-1 alpha during differentiation of human monocytes to macrophages. J. Leukoc. Biol. 2001, 69, 248–252. [Google Scholar]
- Kennedy, A.; Gruen, M.L.; Gutierrez, D.A.; Surmi, B.K.; Orr, J.S.; Webb, C.D.; Hasty, A.H. Impact of macrophage inflammatory protein-1α deficiency on atherosclerotic lesion formation, hepatic steatosis, and adipose tissue expansion. PLoS ONE 2012, 7, e31508. [Google Scholar] [CrossRef]
- Zhang, G.; Liu, H.-B.; Zhou, L.; Cui, X.-Q.; Fan, X.-H. CCL3 participates in the development of rheumatoid arthritis by activating AKT. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 6625–6632. [Google Scholar] [CrossRef]
- Du Plessis, J.; Korf, H.; Van Pelt, J.; Windmolders, P.; Vander Elst, I.; Verrijken, A.; Hubens, G.; Van Gaal, L.; Cassiman, D.; Nevens, F.; et al. Pro-Inflammatory Cytokines but Not Endotoxin-Related Parameters Associate with Disease Severity in Patients with NAFLD. PLoS ONE 2016, 11, e0166048. [Google Scholar] [CrossRef]
- Pan, X.; Kaminga, A.C.; Liu, A.; Wen, S.W.; Chen, J.; Luo, J. Chemokines in Non-alcoholic Fatty Liver Disease: A Systematic Review and Network Meta-Analysis. Front. Immunol. 2020, 11, 1802. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Chen, Y.; Nagashimada, M.; Ni, Y.; Zhuge, F.; Chen, G.; Li, H.; Pan, T.; Yamashita, T.; Mukaida, N.; et al. CC chemokine ligand 3 deficiency ameliorates diet-induced steatohepatitis by regulating liver macrophage recruitment and M1/M2 status in mice. Metabolism 2021, 125, 154914. [Google Scholar] [CrossRef] [PubMed]
- Nishikawa, T.; Nagata, N.; Shimakami, T.; Shirakura, T.; Matsui, C.; Ni, Y.; Zhuge, F.; Xu, L.; Chen, G.; Nagashimada, M.; et al. Xanthine oxidase inhibition attenuates insulin resistance and diet-induced steatohepatitis in mice. Sci. Rep. 2020, 10, 815. [Google Scholar] [CrossRef] [PubMed]
- Uehara, S.; Grinberg, A.; Farber, J.M.; Love, P.E. A role for CCR9 in T lymphocyte development and migration. J. Immunol. 2002, 168, 2811–2819. [Google Scholar] [CrossRef]
- Svensson, M.; Agace, W.W. Role of CCL25/CCR9 in immune homeostasis and disease. Expert Rev. Clin. Immunol. 2006, 2, 759–773. [Google Scholar] [CrossRef]
- Papadakis, K.A.; Prehn, J.; Moreno, S.T.; Cheng, L.; Kouroumalis, E.A.; Deem, R.; Breaverman, T.; Ponath, P.D.; Andrew, D.P.; Green, P.H.; et al. CCR9-positive lymphocytes and thymus-expressed chemokine distinguish small bowel from colonic Crohn’s disease. Gastroenterology 2001, 121, 246–254. [Google Scholar] [CrossRef]
- Rivera–Nieves, J.; Ho, J.; Bamias, G.; Ivashkina, N.; Ley, K.; Oppermann, M.; Cominelli, F. Antibody blockade of CCL25/CCR9 ameliorates early but not late chronic murine ileitis. Gastroenterology 2006, 131, 1518–1529. [Google Scholar] [CrossRef]
- Wermers, J.D.; McNamee, E.N.; Wurbel, M.; Jedlicka, P.; Rivera–Nieves, J. The chemokine receptor CCR9 is required for the T-cell-mediated regulation of chronic ileitis in mice. Gastroenterology 2011, 140, 1526–1535.e3. [Google Scholar] [CrossRef]
- Nakamoto, N.; Ebinuma, H.; Kanai, T.; Chu, P.-S.; Ono, Y.; Mikami, Y.; Ojiro, K.; Lipp, M.; Love, P.E.; Saito, H.; et al. CCR9+ macrophages are required for acute liver inflammation in mouse models of hepatitis. Gastroenterology 2012, 142, 366–376. [Google Scholar] [CrossRef]
- Morikawa, R.; Nakamoto, N.; Amiya, T.; Chu, P.-S.; Koda, Y.; Teratani, T.; Suzuki, T.; Kurebayashi, Y.; Ueno, A.; Taniki, N.; et al. Role of CC chemokine receptor 9 in the progression of murine and human non-alcoholic steatohepatitis. J. Hepatol. 2021, 74, 511–521. [Google Scholar] [CrossRef]
- Kobayashi, Y. The role of chemokines in neutrophil biology. Front. Biosci. 2008, 13, 2400–2407. [Google Scholar] [CrossRef] [PubMed]
- Silva, R.L.; Lopes, A.H.; Guimarães, R.M.; Cunha, T.M. CXCL1/CXCR2 signaling in pathological pain: Role in peripheral and central sensitization. Neurobiol. Dis. 2017, 105, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Amiri, K.I.; Richmond, A. Fine Tuning the Transcriptional Regulation of the CXCL1 Chemokine. Prog. Nucleic Acid Res. Mol. Biol. 2003, 74, 1–36. [Google Scholar] [PubMed]
- Bertola, A.; Bonnafous, S.; Anty, R.; Patouraux, S.; Saint-Paul, M.-C.; Iannelli, A.; Gugenheim, J.; Barr, J.; Mato, J.; Le Marchand-Brustel, Y.; et al. Hepatic expression patterns of inflammatory and immune response genes associated with obesity and NASH in morbidly obese patients. PLoS ONE 2010, 5, e13577. [Google Scholar] [CrossRef]
- Chang, B.; Xu, M.-J.; Zhou, Z.; Cai, Y.; Li, M.; Wang, W.; Feng, D.; Bertola, A.; Wang, H.; Kunos, G.; et al. Short- or long-term high-fat diet feeding plus acute ethanol binge synergistically induce acute liver injury in mice: An important role for CXCL1. Hepatology 2015, 62, 1070–1085. [Google Scholar] [CrossRef]
- Yang, L.; Miura, K.; Zhang, B.; Matsushita, H.; Yang, Y.M.; Liang, S.; Song, J.; Roh, Y.S.; Seki, E. TRIF Differentially Regulates Hepatic Steatosis and Inflammation/Fibrosis in Mice. Cell Mol. Gastroenterol. Hepatol. 2017, 3, 469–483. [Google Scholar] [CrossRef]
- Hwang, S.; He, Y.; Xiang, X.; Seo, W.; Kim, S.; Ma, J.; Ren, T.; Park, S.H.; Zhou, Z.; Feng, D.; et al. Interleukin-22 Ameliorates Neutrophil-Driven Nonalcoholic Steatohepatitis Through Multiple Targets. Hepatology 2020, 72, 412–429. [Google Scholar] [CrossRef]
- Deng, H.K.; Unutmaz, D.; Kewal-Ramani, V.N. Littman DR. Expression cloning of new receptors used by simian and human immunodeficiency viruses. Nature 1997, 388, 296–300. [Google Scholar] [CrossRef]
- Matloubian, M.; David, A.; Engel, S.; Ryan, J.E.; Cyster, J.G. A transmembrane CXC chemokine is a ligand for HIV-coreceptor Bonzo. Nat. Immunol. 2000, 1, 298–304. [Google Scholar] [CrossRef]
- Wilbanks, A.; Zondlo, S.C.; Murphy, K.; Mak, S.; Soler, D.; Langdon, P.; Andrew, D.P.; Wu, L.; Briskin, M. Expression cloning of the STRL33/BONZO/TYMSTRligand reveals elements of CC, CXC, and CX3C chemokines. J. Immunol. 2001, 166, 5145–5154. [Google Scholar] [CrossRef]
- Dudek, M.; Pfister, D.; Donakonda, S.; Filpe, P.; Schneider, A.; Laschinger, M.; Hartmann, D.; Hüser, N.; Meiser, P.; Bayerl, F.; et al. Auto-aggressice CXCR6+ CD8 T cells cause liver immune pathology in NASH. Nature 2021, 592, 444–449. [Google Scholar] [CrossRef] [PubMed]
- Geissmann, F.; Cameron, T.O.; Sidobre, S.; Manlongat, N.; Kronenberg, M.; Briskin, M.J.; Dustin, M.L.; Littman, D.R. Intravascular immune surveillance by CXCR6+ NKT cells patrolling liver sinusoids. PLoS Biol. 2005, 3, e113. [Google Scholar] [CrossRef] [PubMed]
- Heydtmann, M.; Lalor, P.; Eksteen, J.A.; Hübscher, S.G.; Briskin, M.; Adams, D. CXC chemokine ligand 16 promotes integrin-mediated adhesion of liver-infiltrating lymphocytes to cholangiocytes and hepatocytes within the inflamed human liver. J. Immunol. 2005, 174, 1055–1062. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Yang, M.; Li, X.; Wang, Y.; Zhou, G.; Zhao, J. CXC Motif Ligand 16 Promotes Nonalcoholic Fatty Liver Disease Progression via Hepatocyte-Stellate Cell Crosstalk. J. Clin. Endocrinol. Metab. 2018, 103, 3974–3985. [Google Scholar] [CrossRef] [PubMed]
- Imai, T.; Hieshima, K.; Haskell, C.; Baba, M.; Nagira, M.; Nishimura, M.; Kakizaki, M.; Takagi, S.; Nomiyama, H.; Schall, T.J.; et al. Identification and Molecular Characterization of Fractalkine Receptor CX3CR1, which Mediates Both Leukocyte Migration and Adhesion. Cell 1997, 91, 521–530. [Google Scholar] [CrossRef]
- Auffray, C.; Fogg, D.; Garfa, M.; Elain, G.; Join-Lambert, O.; Kayal, S.; Sarnacki, S.; Cumano, A.; Lauvau, G.; Geissmann, F. Monitoring of blood vessels and tissues by a population of monocytes with patrolling behavior. Science 2007, 317, 666–670. [Google Scholar] [CrossRef]
- Harrison, J.K.; Jiang, Y.; Chen, S.; Xia, Y.; Maciejewski, D.; McNamara, R.K.; Streit, W.J.; Salafranca, M.N.; Adhikari, S.; Thompson, D.A.; et al. Role for neuronally derived fractalkine in mediating interactions between neurons and CX3CR1-expressing microglia. Proc. Natl. Acad. Sci. USA 1998, 95, 10896–10901. [Google Scholar] [CrossRef]
- Kanazawa, N.; Nakamura, T.; Tashiro, K.; Muramatsu, M.; Morita, K.; Yoneda, K.; Inaba, K.; Imamura, S.; Honjo, T. Fractalkine and macrophage-derived chemokine: T cell-attracting chemokines expressed in T cell area dendritic cells. Eur. J. Immunol. 1999, 29, 1925–1932. [Google Scholar] [CrossRef]
- Fraticelli, P.; Sironi, M.; Bianchi, G.; D’Ambrosio, D.; Albanesi, C.; Stoppacciaro, A.; Chieppa, M.; Allavena, P.; Ruco, L.; Girolomoni, G.; et al. Fractalkine (CX3CL1) as an amplification circuit of polarized Th1 responses. J. Clin. Investig. 2001, 107, 1173–1181. [Google Scholar] [CrossRef]
- Matsumiya, T.; Ota, K.; Imaizumi, T.; Yoshida, H.; Kimura, H.; Satoh, K. Characterization of Synergistic Induction of CX3CL1/Fractalkine by TNF-α and IFN-γ in Vascular Endothelial Cells: An Essential Role for TNF-α in Post-Transcriptional Regulation of CX3CL1. J. Immunol. 2010, 184, 4205. [Google Scholar] [CrossRef]
- Imaizumi, T.; Yoshida, H.; Satoh, K. Regulation of CX3CL1/fractalkine expression in endothelial cells. J. Atheroscler. Thromb. 2004, 11, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Bazan, J.F.; Bacon, K.B.; Hardiman, G.; Wang, W.; Soo, K.; Rossi, D.; Greaves, D.R.; Zlotnik, A.; Schall, T.J. A new class of membrane-bound chemokine with a CX3C motif. Nature 1997, 385, 640–644. [Google Scholar] [CrossRef] [PubMed]
- Hundhausen, C.; Misztela, D.; Berkhout, T.A.; Broadway, N.; Saftig, P.; Reiss, K.; Hartmann, D.; Fahrenholz, F.; Postina, R.; Matthews, V.; et al. The disintegrin-like metalloproteinase ADAM10 is involved in constitutive cleavage of CX3CL1 (fractalkine) and regulates CX3CL1-mediated cell-cell adhesion. Blood 2003, 102, 1186–1195. [Google Scholar] [CrossRef] [PubMed]
- Garton, K.J.; Gough, P.J.; Blobel, C.P.; Murphy, G.; Greaves, D.; Dempsey, P.J.; Raines, E.W. Tumor Necrosis Factor-α-converting Enzyme (ADAM17) Mediates the Cleavage and Shedding of Fractalkine (CX3CL1)*. J. Biol. Chem. 2001, 276, 37993–38001. [Google Scholar] [CrossRef] [PubMed]
- Chapman, G.A.; Moores, K.; Harrison, D.; Campbell, C.A.; Stewart, B.R.; Strijbos, P.J.L.M. Fractalkine cleavage from neuronal membranes represents an acute event in the inflammatory response to excitotoxic brain damage. J. Neurosci. Off. J. Soc. Neurosci. 2000, 20, RC87. [Google Scholar] [CrossRef]
- Kasama, T.; Isozaki, T.; Takahashi, R.; Odai, T.; Wakabayashi, K.; Kanemitsu, H.; Nohtomi, K.; Takeuchi, H.T.; Matsukura, S.; Tezuka, M. Synergistic induction of CX3CL1 by TNF alpha and IFN gamma in osteoblasts from rheumatoid arthritis: Involvement of NF-kappa B and STAT-1 signaling pathways. J. Inflamm. Res. 2008, 1, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Lesnik, P.; Haskell, C.A.; Charo, I.F. Decreased atherosclerosis in CX3CR1–/– mice reveals a role for fractalkine in atherogenesis. J. Clin. Investig. 2003, 111, 333–340. [Google Scholar] [CrossRef]
- Combadiere, C.; Potteaux, S.; Gao, J.-L.; Esposito, B.; Casanova, S.; Lee, E.J.; Debré, P.; Tedgui, A.; Murphy, P.M.; Mallat, Z. Decreased atherosclerotic lesion formation in CX3CR1/apolipoprotein E double knockout mice. Circulation 2003, 107, 1009–1016. [Google Scholar] [CrossRef]
- Wasmuth, H.E.; Zaldivar, M.M.; Berres, M.-L.; Werth, A.; Scholten, D.; Hillebrandt, S.; Tacke, F.; Schmitz, P.; Dahl, E.; Wiederholt, T.; et al. The fractalkine receptor CX3CR1 is involved in liver fibrosis due to chronic hepatitis C infection. J. Hepatol. 2008, 48, 208–215. [Google Scholar] [CrossRef]
- Aoyama, T.; Inokuchi, S.; Brenner, D.A.; Seki, E. CX3CL1-CX3CR1 interaction prevents carbon tetrachloride-induced liver inflammation and fibrosis in mice. Hepatology 2010, 52, 1390–1400. [Google Scholar] [CrossRef]
- Karlmark, K.R.; Zimmermann, H.W.; Roderburg, C.; Gassler, N.; Wasmuth, H.E.; Luedde, T.; Trautwein, C.; Tacke, F. The fractalkine receptor CX₃CR1 protects against liver fibrosis by controlling differentiation and survival of infiltrating hepatic monocytes. Hepatology 2010, 52, 1769–1782. [Google Scholar] [CrossRef] [PubMed]
- Sutti, S.; Locatelli, I.; Bruzzi’, S.; Jindal, A.; Vacchiano, M.; Bozzola, C.; Albano, E. CX3CR1-expressing inflammatory dendritic cells contribute to the progression of steatohepatitis. Clin. Sci. 2015, 129, 797–808. [Google Scholar] [CrossRef] [PubMed]
- Sutti, S.; Heymann, F.; Bruzzì, S.; Peusquens, J.; Trautwein, C.; Albano, E.; Tacke, F. CX3CR1 modulates the anti-inflammatory activity of hepatic dendritic cells in response to acute liver injury. Clin. Sci. 2017, 131, 2289–2301. [Google Scholar] [CrossRef]
- Sutti, S.; Bruzzì, S.; Heymann, F.; Liepelt, A.; Krenkel, O.; Toscani, A.; Ramavath, N.N.; Cotella, D.; Albano, E.; Tacke, F. CX3CR1 Mediates the Development of Monocyte-Derived Dendritic Cells during Hepatic Inflammation. Cells 2019, 8, 1099. [Google Scholar] [CrossRef] [PubMed]
- Nagashimada, M.; Sawamoto, K.; Ni, Y.; Kitade, H.; Nagata, N.; Xu, L.; Kobori, M.; Mukaida, N.; Yamashita, T.; Kaneko, S.; et al. CX3CL1-CX3CR1 Signaling Deficiency Exacerbates Obesity-induced Inflammation and Insulin Resistance in Male Mice. Endocrinology 2021, 162, bqab064. [Google Scholar] [CrossRef] [PubMed]
- Kruger, A.J.; Fuchs, B.C.; Masia, R.; Holmes, J.A.; Salloum, S.; Sojoodi, M.; Ferreira, D.S.; Rutledge, S.M.; Caravan, P.; Alatrakchi, N.; et al. Prolonged cenicriviroc therapy reduces hepatic fibrosis despite steatohepatitis in a diet-induced mouse model of nonalcoholic steatohepatitis. Hepatol. Commun. 2018, 2, 529–545. [Google Scholar] [CrossRef] [PubMed]
- Krenkel, O.; Puengel, T.; Govaere, O.; Abdallah, A.T.; Mossanen, J.C.; Kohlhepp, M.; Liepelt, A.; Lefebvre, E.; Luedde, T.; Hellerbrand, C.; et al. Therapeutic inhibition of inflammatory monocyte recruitment reduces steatohepatitis and liver fibrosis. Hepatology 2018, 67, 1270–1283. [Google Scholar] [CrossRef] [PubMed]
- Tully, D.C.; Rucker, P.V.; Chianelli, D.; Williams, J.; Vidal, A.; Alper, P.B.; Mutnick, D.; Bursulaya, B.; Schmeits, J.; Wu, X.; et al. Discovery of Tropifexor (LJN452), a Highly Potent Non-bile Acid FXR Agonist for the Treatment of Cholestatic Liver Diseases and Nonalcoholic Steatohepatitis (NASH). J. Med. Chem. 2017, 60, 9960–9973. [Google Scholar] [CrossRef]
- Kremoser, C. FXR agonists for NASH: How are they different and what difference do they make? J. Hepatol. 2021, 75, 12–15. [Google Scholar] [CrossRef]
- Powell, E.; Wong, V.W.-S.; Rinella, M. Non-alcoholic fatty liver disease. Lancet 2021, 397, 2212–2224. [Google Scholar] [CrossRef]
- Krinsky, N.I.; Johnson, E.J. Carotenoid actions and their relation to health and disease. Mol. Aspects Med. 2005, 26, 459–516. [Google Scholar] [CrossRef] [PubMed]
- Senkus, K.E.; Tan, L.; Crowe-White, K.M. Lycopene and Metabolic Syndrome: A Systematic Review of the Literature. Adv. Nutr. 2019, 10, 19–29. [Google Scholar] [CrossRef] [PubMed]
- He, F.J.; Nowson, C.A.; MacGregor, G.A. Fruit and vegetable consumption and stroke: Meta-analysis of cohort studies. Lancet 2006, 367, 320–326. [Google Scholar] [CrossRef]
- Sugiura, M.; Nakamura, M.; Ikoma, Y.; Yano, M.; Ogawa, K.; Matsumoto, H.; Kato, M.; Ohshima, M.; Nagao, A. The homeostasis model assessment-insulin resistance index is inversely associated with serum carotenoids in non-diabetic subjects. J. Epidemiol. 2006, 16, 71–78. [Google Scholar] [CrossRef]
- Ota, T. Molecular Mechanisms of Nonalcoholic Fatty Liver Disease (NAFLD)/Nonalcoholic Steatohepatitis (NASH). Adv. Exp. Med. Biol. 2021, 1261, 223–229. [Google Scholar] [CrossRef]
- Burrows, T.L.; Williams, R.; Rollo, M.; Wood, L.; Garg, M.L.; Jensen, M.; Collins, C.E. Plasma carotenoid levels as biomarkers of dietary carotenoid consumption: A systematic review of the validation studies. J. Nutr. Intermed. Metab. 2015, 2, 15–64. [Google Scholar] [CrossRef]
- Ni, Y.; Nagashimada, M.; Zhan, L.; Nagata, N.; Kobori, M.; Sugiura, M.; Ogawa, K.; Kaneko, S.; Ota, T. Prevention and reversal of lipotoxicity-induced hepatic insulin resistance and steatohepatitis in mice by an antioxidant carotenoid, β-cryptoxanthin. Endocrinology 2015, 156, 987–999. [Google Scholar] [CrossRef]
- Chen, G.; Ni, Y.; Nagata, N.; Zhuge, F.; Xu, L.; Nagashimada, M.; Yamamoto, S.; Ushida, Y.; Fuke, N.; Suganuma, H.; et al. Lycopene Alleviates Obesity-Induced Inflammation and Insulin Resistance by Regulating M1/M2 Status of Macrophages. Mol. Nutr. Food Res. 2019, 63, e1900602. [Google Scholar] [CrossRef]
- Motohashi, H.; Yamamoto, M. Nrf2-Keap1 defines a physiologically important stress response mechanism. Trends Mol. Med. 2004, 10, 549–557. [Google Scholar] [CrossRef]
- Fahey, J.W.; Zhang, Y.; Talalay, P. Broccoli sprouts: An exceptionally rich source of inducers of enzymes that protect against chemical carcinogens. Proc. Natl. Acad. Sci. USA 1997, 94, 10367–10372. [Google Scholar] [CrossRef]
- Starrett, W.; Blake, D.J. Sulforaphane inhibits de novo synthesis of IL-8 and MCP-1 in human epithelial cells generated by cigarette smoke extract. J. Immunotoxicol. 2011, 8, 150–158. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Liu, P.; Zhao, X.; Yang, C.; Li, B.; Liu, Y.; Liu, Y. Sulforaphane inhibits cytokine-stimulated chemokine and adhesion molecule expressions in human corneal fibroblasts: Involvement of the MAPK, STAT, and NF-κB signaling pathways. Exp. Eye Res. 2022, 216, 108946. [Google Scholar] [CrossRef] [PubMed]
- Nagata, N.; Xu, L.; Kohno, S.; Ushida, Y.; Aoki, Y.; Umeda, R.; Fuke, N.; Zhuge, F.; Ni, Y.; Nagashimada, M.; et al. Glucoraphanin Ameliorates Obesity and Insulin Resistance Through Adipose Tissue Browning and Reduction of Metabolic Endotoxemia in Mice. Diabetes 2017, 66, 1222–1236. [Google Scholar] [CrossRef] [PubMed]
- Kikuchi, M.; Ushida, Y.; Shiozawa, H.; Umeda, R.; Tsuruya, K.; Aoki, Y.; Suganuma, H.; Nishizaki, Y. Sulforaphane-rich broccoli sprout extract improves hepatic abnormalities in male subjects. World J. Gastroenterol. 2015, 21, 12457–12467. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).