Simple Summary
Amphibians mostly live in freshwater. However, crab-eating frogs (Fejervarya cancrivora) can adapt to high saline environments, while the osmoregulatory mechanism was not fully clear. Cyclin-dependent kinase 5 (CDK5), activating the nuclear factor of the activated T cells-5 (NFAT5) pathway, plays an important role in protecting cells from hypertonicity. We investigated the role of CDK5/NFAT5 in crab-eating frogs’ adaptation to saline environments. As a result, we found that the expression of CDK5/NFAT5 and its downregulated genes were higher in crab-eating frogs, which subsequently regulated ion transport to mediate osmotic pressure. The results indicate that CDK5/NFAT5-regulated transporters are involved in osmoregulation in frogs.
Abstract
Crab-eating frogs (Fejervarya cancrivora) can live in brackish water with a salinity of up to 18‰, although most amphibians are not able to tolerate such high saline environments. To investigate its potential osmoregulation, we conducted experiments in F. cancrivora and F. multistriata. The results showed that F. cancrivora made use of ions (such as Na+ and Cl−) to increase intracellular concentrations via the Na+/K+-ATPase (NKA) enzyme. The mRNA expression of aldose reductase (AR) was significantly higher in F. cancrivora (p < 0.05), indicating that more organic osmolytes were produced and transported to maintain cellular homeosis. The mRNA expressions of Aquaporin 1 (AQP1) and AQP3 in kidney were significantly higher in F. cancrivora, while AQP expression in skin was higher in F. multistriata (p < 0.05). The mRNA level in activating the transcription of the nuclear factor of activated T cells-5 (NFAT5) which is one of the target genes of regulating the cellular response to hypertonicity, was higher in F. cancrivora. The protein expression of CDK5, the upstream protein of the NFAT5 pathway, was 2 times higher in F. cancrivora. Therefore, we can conclude that CDK5/NFAT5-regulated transporters might be involved in osmoregulation in F. cancrivora.
1. Introduction
Amphibians, depending on water, throughout their life stages are very sensitive to high saline conditions; therefore, they are at a high risk to severe environmental changes [1]. Some amphibians can adapt to high-saline water, for example, Xenopus laeuis can tolerate up to 600 m-osmoles; Bufo viridis can tolerate 800 m-osmoles; Fejervarya cancrivora can tolerate up to 1000 m-osmoles, with the high concentration comparable to seawater [2], while most frogs, such as rice frogs (Fejervarya multistriata), are not able to tolerate such high-saline environments. Crab-eating frogs (Fejervarya cancrivora), located around the coast of southeastern Asia, mainly inhabit bushes, mangrove swamps and forests [3]. Adult frogs can survive in 18‰ saline water, and their tadpoles can tolerate 35‰ marine water [4,5]. Studies have shown that the concentrations of sodium, chloride and potassium ions in blood plasma increased following a high saline trial in crab-eating frogs [4]. Nevertheless, the osmotic regulations of molecular mechanisms in crab-eating frogs are still largely unknown.
Some strategies of amphibian adaptive responses to hypertonicity have been researched [6], for example, B. viridis and B. calamit showed local adaptation to salinity [7,8]. There has been extensive focus on physiological traits, such as the concentration of permeable ions and ion transporters [2,9,10,11,12]. Na+, Cl− and K+ are the three dominant ions responsible to homeosis in cells, and transporters play a crucial role in transporting these ions effectively. Na+/K+-ATPase (NKA), also known as sodium–potassium pump or sodium pump, is a key enzyme that controls extracellular volume via Na+ reabsorption, located on the membrane [13]. The NKA enzyme utilizes one molecule of ATP to export 3 Na+ and import 2 K+ through membranes in order to maintain intracellular homeostasis [14].
In addition, aldose reductase (AR) [15], the Na+/Cl2− coupled betaine/c-aminobutyric acid transporter (BGT1) [16], the Na+/myo-inositol cotransporter (SMIT) [17], the Na+ and Cl2− dependent taurine transporter (TauT) [18], and serum-and glucocorticoid-inducible kinase (SGK1) [19] also transport organic osmolytes to balance fluid volume. Cells require a large amount of osmolytes to elevate intracellular pressure. It was demonstrated that SGK1 regulates the transportation of Na+ and activates the enzyme activity of NKA in amphibians [20,21,22]. In kidney, aquaporins (AQPs) are responsible for water reabsorption via the renal tubules and collecting ducts from initial urine and therefore regulate urine concentration, which can be divided into 13 types in mammals [23,24,25]. It is reported that in amphibians, AQPs are generally classified into the following six types: 1, 2, 3, 5 and a1, a2. AQPs locate mainly on the urinary bladder and ventral pelvic skin to absorb water. For example, Bufo japonicus inhabits drier land, thus displaying several AQPs in order to transport water [26]. Urea transporter (UT-A) functions in urea accumulation, with four isoforms including UT-A1, UT-A2, UT-A3 and UT-A4. UT-A1 and UT-A2 can activate urea recycling and raise the concentration of urea while heat shock protein 70 (HSP70) can protect cells from high urea [27,28,29,30]. These proteins work together in the system to prevent damage from hypertonicity (see review [31]).
When cells encounter hypertonic conditions, cyclin-dependent kinase 5 (CDK5) can be activated, following which CDK5 regulates the nuclear factor of activated T cells-5 (NFAT5), also named TonEBP, which primarily functions in regulating the cellular response to hypertonicity [32]. Renal medulla metabolizes salt and urea. When renal medulla occurs under hypertonic conditions, it causes double-stranded DNA breaks [33]. CDK5/NFAT5 can up-regulate the expression of osmoprotective genes, including AR, BGT1, SMIT, TauT, SGK1, AQPs, UT-A and HSP70, which could increase the intracellular concentration, and thus protect cells from DNA damage [23]. Therefore, CDK5/NFAT5 pathway plays an essential role in the regulation of maintaining the fluid volume to protect cells from hypertonicity.
The aim of this study was to investigate possible molecular response to high saline environments in F. cancrivora, via comparison with F. multistriata. We measured the concentration of ions and NKA enzyme activity to compare two frogs’ responses on the physiological level, followed by the detection of the expression of CDK5/NFAT5 and its downregulated genes.
2. Materials and Methods
2.1. Animal Sampling
F. cancrivora were collected in June 2021 from Dongzhai Port Nature Reserve, China (110°34′31.72 E, 19°57′2.81 N) and F. multistriata were collected in farms near the Dongzhai Port. Six adult crab-eating frogs were caught and placed in boxes with natural salt water in mangrove, and the average salinity of the mangrove water was 14‰ (Supplementary Table S1), while six adult F. multistriata were placed in another box with freshwater, followed by transference to the laboratory immediately to measure their body mass (±0.1 g; BW) and snout-vent length (±1 mm; SVL) (Supplementary Table S2). Frogs were euthanized with ethyl carbamate after measurements. Cardiocentesis for blood collection was performed on frogs with 1 mL clean medical syringes and needles injected in the heart. The blood (200 μL) of each frog was collected in tubes with heparin anticoagulant for biochemical analysis. Then, dissection was performed on frogs and skin, liver and kidney tissues were collected for RNA extraction.
2.2. Biochemical Analysis
Blood samples were immediately centrifugated at 3000 rpm for 5 min. Plasma was pipetted into a fresh 1.5 mL microfuge tube after centrifugation. Concentrations of Na+, Cl−, K+ were detected using the ISE internal standard (HITACHI, Tokyo, Japan) [34]. ISE is a method of using ion-selective electrodes to detect concentrations of ions from samples. The electromotive force produced by ion concentrations and sensitivities from all samples is calculated with Nernst formula:
where E is the electromotive force in the measured samples, E0 is the standard electromotive force in the measurement systems, n is the charge number of ions to be tested, R is the gas constant (8.314 J/K mol), T is the absolute temperature (273 + t °C), F is the Faraday constant (96,487 C/mol), ax is the ionic activity to be tested, C is the concentrations of ions to be tested, and fx is the ionic activity coefficient to be tested.
2.3. NKA Enzyme Measurement
Samples were homogenized 1:10 in normal saline, then centrifuged at 4000 rpm at 4 °C for 10 min after which the supernatant was collected for determining protein concentrations using the BCA method with an assay kit (Nanjing Jiancheng, Ltd., Nanjing, China). The enzyme activity of NKA was detected following the manufacturer’s instructions (Nanjing Jiancheng, Ltd., Nanjing, China). Samples were mixed with the matrix solution, ddH2O as control, followed by reactions at 37 °C for 10 min. Chromogenic reagents were added to the control and NKA groups, then samples were centrifugated at 3500 rpm for 10 min. Supernatant was collected for the phosphate assay. The OD (optical density) value was detected at 636 nm using an enzyme-labeled instrument (BIO-RAD, Hercules, CA, USA) to calculate the enzyme activity of NKA.
2.4. Total RNA Extraction and cDNA Synthesis
Total RNA was extracted from tissues according to the manufacturer’s instructions for the Trizol reagent (Invitrogen, Carlsbad, CA, USA). Briefly, 100 mg tissue samples were added to 1 mL of the Trizol reagent, and were later smashed using a Tissue Grinder (Scientz, Ningbo, China). The supernatants were collected after adding chloroform for the purpose of final precipitation with the same volume of isopropanol. The precipitation was washed twice with 75% ethanol and eventually resuspended in 30 μL DEPC-treated water. The concentration and quality of total RNA was measured by NanoDrop one/onec spectrophotometer (Thermo Fisher, Waltham, MA, USA). The integrity of RNA was detected using 1.2% agarose gel electrophoresis. Qualified RNA then was reverted into cDNA using the cDNA synthesis kit (Invitrogen, Carlsbad, CA, USA).
2.5. Quantitative Real-Time PCR (qRT-PCR)
All primers were designed according to the whole transcriptome sequencing data (Supplementary Tables S3 and S4). The nucleotide sequences of primers are provided in Table 1. β-tubulin, compared with β-actin and GAPDH, was selected as the reference gene for steadiness. A qRT-PCR was performed with the Genius 2 × SYBR Green Fast qPCR MixSKit (ABclonal Technology, Boston, NY, USA) to detect the expression of selected genes. The total volume of the reaction was 20 μL, including 1 μL cDNA sample, 10 μL 2 × Mix, 0.8 μL each of the 10 mM forward and reverse primers and 7.4 μL of dH2O. The 2−ΔΔCt method of processing data was used to obtain the relative expression levels of mRNA. The values of skin in F. multistriata were standardized to 1.

Table 1.
Sequences of primer pairs for qRT-PCR in Fejervarya cancrivora and Fejervarya multistriata.
2.6. Western Blotting
Tissues were cut into pieces and lysed using an RIPA lysis buffer with 3 μL nuclease and 5 μL PMSF separately, then later smashed using a Tissue Grinder (Scientz, Ningbo, China). The supernatants were collected and denatured at 37 °C for 30 min. Subsequently, the concentrations of protein were measured using a BCA assay (Nanjing, Jiancheng). After the application of the BCA assay, all samples were adjusted to the same concentration with a loading buffer and boiled for 6 min, then proteins from F. cancrivora and F. multistriata were mixed, respectively, and stored in a refrigerator. All equivalent amounts of protein samples were separated by 8–10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) followed by transference to polyvinylidene difluoride (PVDF) membranes (Millipore Corp, Atlanta, GA, USA). The membranes were placed in 3% nonfat dried milk at room temperature for 2 h and washed with TBST 3 times and immunoblotted with specific primary antibodies (Supplementary Table S5), followed by incubating at 4 °C overnight. HRP-conjugated secondary antibodies (BOSTER Biological Technology, Wuhan, China) were incubated for another 1 h and washed with TBST 4 times. The signal was detected using Pierce ECL reagent after washing (Thermo Fisher Scientific, Waltham, MAUSA) with LSA 4000 mini ImageQuant instrument (CE Healthcare, Chicago, IL, USA).
2.7. Statistical Analysis
All data were represented as Mean ± S.E. analyzed using the SPSS 16.0 software (IBM SPSS, Chicago, IL, USA). An independent-sample T test was used to test the significance of the ion concentration and activity of NKA between the two species. One-sample Kolmogorov–Smirnov and Homogeneity of variance tests were carried out to evaluate the normal distribution and statistical homogeneity of data. The significance of the relative expressions of selected genes and proteins between frogs was determined using an independent-sample T test or Mann–Whitney U test. Statistical significance was set at p < 0.05. In order to quantify the results of the Western blot analysis, a gray level test was used to calculate the protein expression level of CDK5. ImageJ was utilized to calculate the gray level of proteins. All figures are presented via Graphpad prism 7.0 (GraphPad Software, San Diego, CA, USA).
3. Results
3.1. Ion Concentration in Plasma
The mean concentration of K+ in F. cancrivora was 5.03 mmol/L and F. multistriata was 4.54 mmol/L; however, there was no significant difference between these two species (p > 0.05). Nonetheless, Na+ and Cl−, the two main ions in seawater, were significantly different in the two species. The mean concentration of Na+ in F. cancrivora was 171.73 mmol/L, higher than 109.34 mmol/L in F. multistriata (p < 0.01), as the mean concentrations of Cl− in two species were 149.4 mmol/L and 82.4 mmol/L, respectively (p < 0.05) (Figure 1, Supplementary Table S6).
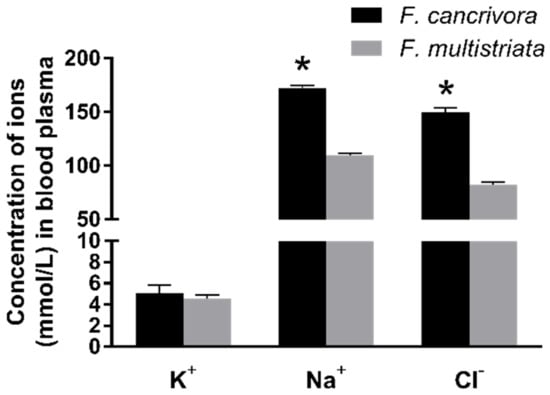
Figure 1.
Concentrations of Na+, Cl−, K+ in F. cancrivora and F. multistriata in blood plasma. * Statistical significance when p < 0.05 in independent-sample t-test.
3.2. NKA Enzyme Activity
The activity of NKA was detected in F. cancrivora as 149.36 ± 23.11 U/mgprot, while in F. multistriata the activity was 83.67 ± 16.7 U/mgprot. The activity of F. cancrivora was 1.8 times higher, but with no significant difference (p > 0.05) (Figure 2).
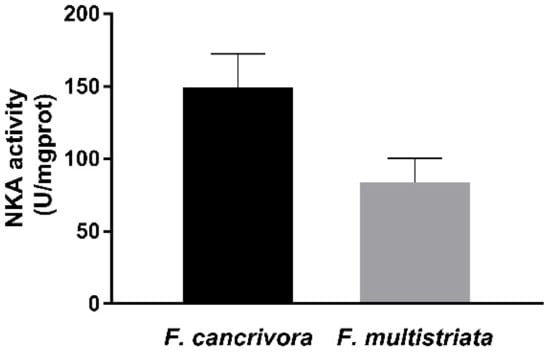
Figure 2.
Activity of NKA enzyme in F. cancrivora and F. multistriata in liver. Independent-sample T test was used to calculate significance.
3.3. The mRNA Expression of Osmoregulative Genes
The expressions of osmoregulative genes overall were highest in kidney and the expression level in skin and liver was almost identical in the two species.
In kidney, AR was significantly expressed to a higher degree in F. cancrivora (p < 0.05), while SGK1, SMIT, TauT were slightly higher (p > 0.05). However, the expression of BGT1 was a little higher in F. multistriata (p > 0.05). It showed no significant difference between these genes in skin and liver (p > 0.05).
In kidney, the mRNA expressions of AQP1, AQP3 were significantly higher in F. cancrivora (p < 0.05), increasing by 2.3–2.5 fold, but no significant difference was detected in AQP4 (p > 0.05). In skin, the expressions of AQPs were higher in F. multistriata with a significant expression of AQP4 (p < 0.05). In liver, there was no significant expression variance in the AQPs between two species (p > 0.05).
No significant difference in the expression of UT-A appeared in all tissues (p > 0.05), but in kidney, F. cancrivora increased by 1.6 fold. In skin and liver, UT-A also presented a slightly higher expression in F. cancrivora. As for HSP70, it was significantly expressed at a higher degree in skin and liver tissues in F. cancrivora (p < 0.05). (Figure 3).
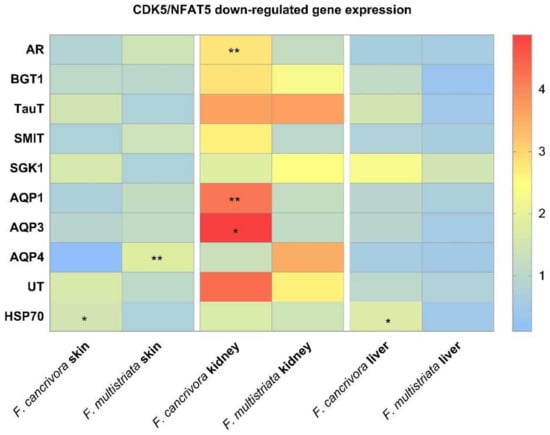
Figure 3.
The mRNA expression of transporters: F. cancrivora skin standardized to 1, * Statistical significance when p < 0.05, ** when p < 0.01.
3.4. The Expression of NFAT5 and CDK5
The mRNA expression of NFAT5 was highest in kidney, and NFAT5 was also expressed in skin and liver tissues. F. cancrivora demonstrated a slightly higher expression level in skin, kidney and liver, but with no significance (p > 0.05). We calculated the quantity of the protein expression of CDK5 using gray level (Supplementary Table S7, Figure S1) and found that F. cancrivora was 2 times higher (Figure 4).
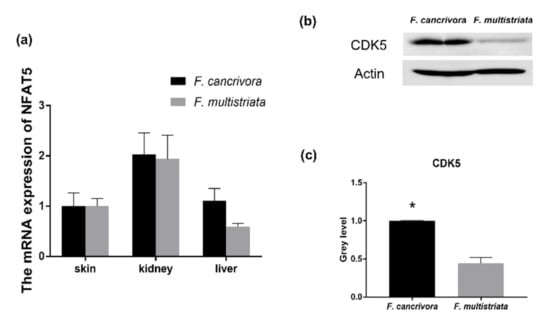
Figure 4.
The mRNA expression of NFAT5 and the protein expression of CDK5 in kidney. (a) The mRNA expression of NFAT5: F. cancrivora skin standardized to 1; (b) the representative bands of CDK5 protein expression; (c) gray analysis of CDK5: F. cancrivora standardized to 1. * Statistical significance.
4. Discussion
Ions play an important role in regulating cell conditions when organisms are transferred from seawater to freshwater to adapt to environments which has been reported in fishes [35], crustacea [36], and frogs [37]. For example, Liza haematocheila juveniles tend to raise concentrations of K+, Na+ and Cl− in serum with elevating salinity [38], as do Acipenser schrenckiiin, leading to increased concentrations of Na+ and Cl− in serum while no significant difference of K+ is observable [39]. In the present study, concentrations of Na+ and Cl− in the plasma of F. cancrivora were much higher than F. multistriata. Frogs with permeable skin could allow ions to alter higher external salinity to promote lower internal concentrations. Hence, the progressive increment of these ions in F. cancrivora might be due to permeation, which was also reported in Rana pipiens after they were exposed to seawater [2]. Nevertheless, the concentration of K+ was 3.6~5.6 mmol/L in two species and presented no significant difference, consistent with Acipenser schrenckiiin’s state, which can be attributed to the preservation of the integrity of cell membranes [39,40].
Na+/K+-ATPase (NKA) is a crucial enzyme in ion transport, where Na+ could permeate from intracellular to extracellular fluids while K+ does the opposite in order to reach osmotic equilibrium [13]. It was reported that Bufo balearicus, which also lives in brackish water, presented much higher activity of the NKA enzyme than Bufo bufo. Furthermore, the activity increased in response to seawater exposure [12]. Juvenile bull sharks (Carcharhinus leucas) showed an elevated enzyme activity when transferred from freshwater to seawater [41]. In this study, the activity of the NKA enzyme in F. cancrivora was higher according to the ion concentration results, implying that NKA is an important ion transporter for F. cancrivora to ensure sufficient ions are provided quickly to maintain cellular homeostasis, and adapt to the brackish environment, similarly to teleost fish [42,43].
To maintain cellular homeostasis, organic osmolytes are also important to increase fluid volume. AR is the key enzyme of the glucose metabolism sorbitol pathway, which can transform glucose into sorbitol, an organic osmolyte [44,45], thereby increasing concentrations in cells. According to our study, the mRNA expression of AR was expressed at a significantly higher rate in F. cancrivora. In rats, hypertonicity induces the mRNA expression of AR [46] as AR is an osmoprotective gene. Inositol and taurine are synthesized in renal cells and can be transported by SMIT and TauT [47,48]. Under hypertonic conditions, inositol and taurine are transported into cells rather than synthesized in vivo; therefore, hypertonicity activates the mRNA expression of inositol and taurine transporters [18,49,50,51]. In this study, SMIT and TauT were expressed at a slightly higher rate in F. cancrivora. We assumed that frogs have a different osmoregulation mechanism in that they mainly utilize AR to produce osmolytes to maintain higher concentration in cells rather than transport osmolytes; therefore, SMIT and TauT could be “backup genes” in F. cancrivora. Besides, SMIT and TauT can also transport Na+ and Cl− into cells. It is possible that these transporters could co-transport ions with NKA. Betaine is synthesized from choline both in liver and kidney [47]. BGT1 transports betaine into cells and hypertonicity stimulates its expression in rats [50]. However, our results showed that BGT1 was expressed at a higher rate in F. multistriata in kidney. BGT1 is not only regulated by transcription but is also related to plasma membrane insertion [52]. Therefore, the result could be altered depending on the effects of multiple factors under this specific circumstance. Moreover, SGK1 mediates the aldosterone regulation of Na+ transport [20,21], resulting in the activation of NKA in amphibian renal epithelial cells [22]. Based on our results, the expression of SGK1 was higher in F. cancrivora, which is in accordance with the results of the enzyme activity of NKA. Therefore, Na+, Cl−, and osmolytes can be transported into cells via these transporters and NKA, causing an increase in the intracellular concentration (Figure 5).
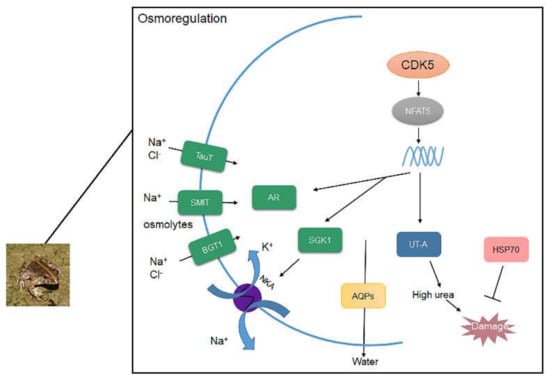
Figure 5.
The conclusion of CDK5/NFAT5 mediating downstream genes to response to high saline environments.
It is widely reported that NFAT5 can activate transporters and AR to regulate organic osmolytes permeating through cell membranes [53,54]. NFAT5 was originally identified as a key transcription factor in the kidney medulla under hypertonic conditions [23,32,55]. Cyclin-dependent kinase 5 (CDK5) is one of the activators of NFAT5. In this study, the protein expression of CDK5 was 2 times higher in F. cancrivora; however, the transcriptional expression of NFAT5 was not significantly different, as it was slightly higher in F. cancrivora in three different tissues. This suggests that up-stream protein CDK5 might activate NFAT5, and that NFAT5 relies on its transcriptional activation activity to regulate its target genes and stimulate the production of transporters. Therefore, under high saline environments, in order to adapt to environmental conditions and regulate hypertonic cellular conditions, CDK5/NFAT might be triggered in F. cancrivora (Figure 5).
CDK5/NFAT5 also stimulates aquaporins (AQPs) and the urea transporter (UT-A) to produce the amount of urea in kidney and HSP70, which is often overexpressed to protect cells from high urea [31]. AQPs is a complex family with 13 different types. AQP1 is highly expressed in kidney to control water reabsorption. AQP3 is more efficient in transporting glycerol. AQP4 amplifies the function of urea concentration through AQP3 [25]. In kidney, the expression of AQPs was significantly higher in F. cancrivora, while in skin, F. multistriata had a much higher trend. It is believed that expressing more AQP1 is beneficial for excluding water from kidneys and AQP3 could transport small molecules such as urea into cells in F. cancrivora, thereby increasing the concentration of urea to maintain a hyperosmotic cell pressure. Therefore, a high expression of AQPs is necessary in kidney, and a lower expression of AQPs in skin prevents environmental water from entering the body. Takifugu obscurus also presented the same results when subjected to salinity change [56] (Figure 5).
High urea is crucial to frogs to elevate concentrations in the body [2,57]. UT-A can carry a large number of small urea molecules quickly through membranes, thereby maintaining an extremely high osmotic pressure and concentrated urine [58]. However, high urea can be damaging to health; to protect cells, organisms should develop an appropriate response mechanism. Moreover, hypertonicity can cause protein oxidation in cells [23]. Liver, as the main metabolic organ, plays a key role in producing protective proteins such as HSP70. For instance, HSP70 is overexpressed to inhibit apoptosis, induced by various stressors [59], or to protect cells from high urea. It was reported in rats that HSP70 was expressed under high salinity for cell survival [60]. In this study, UT-A and HSP70 were also highly expressed in F. cancrivora, indicating that urea plays a crucial role in osmoregulation, and HSP70 can protect against high urea for F. cancrivora (Figure 5).
Various environmental factors affect physiological indicators; however, we found that the salinity of the two species’ habitats differs greatly. The average salinity of mangrove water was 14‰ based on our detection and that of fresh water was less than 0.05‰. Therefore, salinity could be a very important factor that affects physiological mechanisms. It is well established that the environment that individuals inhabit can strongly affect physiology as an adult. Therefore, any differences observed may be simply from occupying different environments, not due to species-specific differences in physiology. For example, F. cancrivora, Bufo viridis and Xenopua laeuis had increased ion concentrations when transported into high saline environments. Hence, environments affect physiological mechanisms in species. However, these frogs still displayed much higher ion concentrations in freshwater compared with other species in the same taxa [2]. This indicates that the species is capable of adapting to high salinities, and the levels of plasma sodium can be very high, implying considerable tolerance to high plasma sodium in these species, which can also be seen in our results. This could provide evidence of adaptive responses to high saline environment in F. cancrivora. When comparing the osmoregulatory systems of two closely related kajika frogs, Buergeria japonica (brackish water living) and B. buergeri (freshwater living), the plasma Na+ concentration was also significantly higher in B. japonica than in B. buergeri, suggesting differences in the mechanisms for salinity adaptation [61], which is similar to our results. Therefore, the differences among our results in the two species could be attributed to salinity adaptation.
5. Conclusions
In conclusion, F. cancrivora relies on Na+ and Cl− transport through NKA and perhaps SMIT, TauT and BGT1 co-transport ions with NKA. AR and AQPs in kidney were highly expressed in F. cancrivora, which indicated that more organic osmolytes were produced and more water was filtered to maintain cellular homeosis. CDK5, as the upstream protein, was highly expressed in F. cancrivora and activated the transcription of NFAT5, which primarily plays a role in regulating cellular responses to hypertonicity. Hence, CDK5 activates NFAT5, and NFAT5 therefore regulates these target transporters to protect cells from hypertonicity. F. cancrivora could rely on these transporters to survive in high saline environments (Figure 5).
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/biology11060858/s1, Figure S1: Original images of Full Western Blot, Table S1: The salinity of mangrove water; Table S2: Morphometrical parameters of Fejervarya cancrivora and Fejervarya multistriata; Table S3: KEGG pathway of unigenes Fejervarya cancrivora. Table S4: GO enrichment of unigene in Fejervarya cancrivora; Table S5: Primary antibodies of Western blotting; Table S6: Concentration of ions in blood plasma in Fejervarya cancrivora and Fejervarya multistriata; Table S7: Gray level of CDK5 and β-actin in Fejervarya cancrivora and Fejervarya multistriata.
Author Contributions
Conceptualization, J.L. and L.D.; methodology, J.L., X.W., T.L., Y.L., L.D.; software, J.L.; validation, J.L., X.W., T.L.; formal analysis, J.L.; writing—original draft preparation, J.L.; writing—review and editing, M.H., L.D.; visualization, J.L., L.D.; supervision, M.H., L.D.; project administration, L.W.; funding acquisition, L.W. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Hainan Natural Science Foundation, 320MS039.
Institutional Review Board Statement
This research was conducted under Animal Research Ethics Committee of Hainan Provincial Education Center for Ecology and Environment, Hainan Normal University. The number is HNECEE-2020-003.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data relevant to this study are provided in the manuscript and Supplementary Materials.
Acknowledgments
We thank Zhijun Lin, Rongping Pu for field help, and Jing Lin, Na Li, Xiaoqi Ai for experimental help. We also want to thank Majorbio Company and their staff for patient help and instructions.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Hoffmann, M.; Hilton-Taylor, C.; Angulo, A.; Böhm, M.; Brooks, T.M.; Butchart, S.H.; Carpenter, K.E.; Chanson, J.; Collen, B.; Cox, N.A.; et al. The impact of conservation on the status of the world’s vertebrates. Science 2010, 330, 1503–1509. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Balinsky, J.B. Adaptation of nitrogen metabolism to hyperosmotic environment in amphibia. J. Exp. Zool. Part A 1980, 215, 335–350. [Google Scholar] [CrossRef]
- Kurniawan, N.; Djong, T.H.; Islam, M.M.; Nishizawa, T.; Belabut, D.M.; Sen, Y.H.; Wanichanon, R.; Yasir, I.; Sumida, M. Taxonomic status of three types of Fejervarya cancrivora from Indonesia and other Asian countries based on morphological observations and crossing experiments. Zool. Sci. 2011, 28, 12–24. [Google Scholar] [CrossRef] [PubMed]
- Gordon, S.; Schmidtnielsen, K.; Kelly, M. Osmotic regulation in the crab-eating frog (Rana cancrivora). J. Exp. Biol. 1961, 38, 659–678. [Google Scholar] [CrossRef]
- Gordon, M.S.; Tucker, V.A. Osmotic regulation in tadpoles of crab-eating frog (Rana cancrivora). J. Exp. Biol. 1965, 42, 437–445. [Google Scholar] [CrossRef]
- Albecker, M.A.; McCoy, M.W. Adaptive responses to salinity stress across multiple life stages in anuran amphibians. Front. Zool. 2017, 14, 40. [Google Scholar] [CrossRef] [Green Version]
- Gomez-Mestre, I.; Tejedo, M. Local adaptation of an Anuran amphibian to osmotically stressful environments. Evolution 2003, 57, 1889–1899. [Google Scholar] [CrossRef] [Green Version]
- Gomez-Mestre, I.; Tejedo, M. Contrasting patterns of quantitative and neutral genetic variation in locally adapted populations of the natterjack toad. Evolution 2004, 58, 2343–2352. [Google Scholar] [CrossRef] [Green Version]
- Gordon, M.S.; Tucker, V.A. Further observations on the physiology of salinity adaptation in the crab-eating frog (Rana cancrivora). J. Exp. Biol. 1968, 48, 185–193. [Google Scholar] [CrossRef]
- Katz, U.; Degani, G.; Gabbay, S. Acclimation of the euryhaline toad Bufo viridis to hyperosmotic solution (Nacl, Urea and Mannitol). J. Exp. Biol. 1984, 108, 403–409. [Google Scholar] [CrossRef]
- Liggins, G.W.; Grigg, G.C. Osmoregulation of the cane toad, Bufo marinus, in salt water. Comp. Biochem. Physiol. A Comp. Physiol. 1985, 82, 613–619. [Google Scholar] [CrossRef] [Green Version]
- Bernabò, I.; Bonacci, A.; Coscarelli, F.; Tripepi, M.; Brunelli, E. Effects of salinity stress on Bufo balearicus and Bufo bufo tadpoles: Tolerance, morphological gill alterations and Na(+)/K(+)-ATPase localization. Aquat. Toxicol. 2013, 132–133, 119–133. [Google Scholar] [CrossRef] [PubMed]
- Geering, K. Na, K-ATPase. Curr. Opin. Nephrol. Hypertens. 1997, 6, 434–439. [Google Scholar] [CrossRef] [PubMed]
- Scheiner-Bobis, G. The sodium pump-its molecular properties and mechanics of ion transport. Eur. J. Biochem. 2002, 269, 2424–2433. [Google Scholar] [CrossRef]
- Ko, B.C.; Ruepp, B.; Bohren, K.M.; Gabbay, K.H.; Chung, S.S. Identification and characterization of multiple osmotic response sequences in the human aldose reductase gene. J. Biol. Chem. 1997, 272, 16431–16437. [Google Scholar] [CrossRef] [Green Version]
- Miyakawa, H.; Rim, J.S.; Handler, J.S.; Kwon, H.M. Identification of the second tonicity-responsive enhancer for the betaine transporter (BGT1) Gene. Biochim. Biophys. Acta 1999, 1446, 359–364. [Google Scholar] [CrossRef]
- Rim, J.S.; Atta, M.G.; Dahl, S.C.; Berry, G.T.; Handler, J.S.; Kwon, H.M. Transcription of the sodium/myo-inositol cotransporter gene is regulated by multiple tonicity-responsive enhancers spread over 50 kilobase pairs in the 5’-flanking region. J. Biol. Chem. 1998, 273, 20615–20621. [Google Scholar] [CrossRef] [Green Version]
- Ito, T.; Fujio, Y.; Hirata, M.; Takatani, T.; Matsuda, T.; Muraoka, S.; Takahashi, K.; Azuma, J. Expression of taurine transporter is regulated through the TonE (tonicity-responsive element)/TonEBP (tone-binding protein) pathway and contributes to cytoprotection in Hepg2 cells. Biochem. J. 2004, 382, 177–182. [Google Scholar] [CrossRef] [Green Version]
- Chen, S.; Grigsby, C.L.; Law, C.S.; Ni, X.; Nekrep, N.; Olsen, K.; Humphreys, M.H.; Gardner, D.G. Tonicity-dependent induction of sgk1 expression has a potential role in dehydration-induced natriuresis in rodents. J. Clin. Investig. 2009, 119, 1647–1658. [Google Scholar] [CrossRef] [Green Version]
- Pearce, D. The role of SGK1 in hormone-regulated sodium transport. Trends Endocrinol. Metab. 2001, 12, 341–347. [Google Scholar] [CrossRef]
- Stockand, J.D. New ideas about aldosterone signaling in epithelia. Am. J. Physiol. Renal. Physiol. 2002, 282, 559–576. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alvarez de la Rosa, D.; Gimenez, I.; Forbush, B.; Canessa, C.M. SGK1 Activates Na+-K+-ATPase in amphibian renal epithelial cells. Am. J. Physiol. Cell Physiol. 2006, 290, 492–498. [Google Scholar] [CrossRef]
- Burg, M.B.; Ferraris, J.D.; Dmitrieva, N.I. Cellular response to hyperosmotic stresses. Physiol. Rev. 2007, 87, 1441–1474. [Google Scholar] [CrossRef] [PubMed]
- Bourque, C.W. Central mechanisms of osmosensation and systemic osmoregulation. Nat. Rev. Neurosci. 2008, 9, 519–531. [Google Scholar] [CrossRef] [PubMed]
- Su, W.; Cao, R.; Zhang, X.Y.; Guan, Y. Aquaporins in the kidney: Physiology and pathophysiology. Am. J. Physiol. Renal. Physiol. 2020, 318, 193–203. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, M.; Hasegawa, T.; Ogushi, Y.; Tanaka, S. Amphibian aquaporins and adaptation to terrestrial environments: A review. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2007, 148, 72–81. [Google Scholar] [CrossRef]
- Nakayama, Y.; Peng, T.; Sands, J.M.; Bagnasco, S.M. The TonE/TonEBP pathway mediates tonicity-responsive regulation of UT-A urea transporter expression. J. Biol. Chem. 2000, 275, 38275–38280. [Google Scholar] [CrossRef] [Green Version]
- Woo, S.K.; Kwon, H.M. Adaptation of kidney medulla to hypertonicity: Role of the transcription factor TonEBP. Int. Rev. Cytol. 2002, 215, 189–202. [Google Scholar]
- Woo, S.K.; Lee, S.D.; Kwon, H.M. TonEBP transcriptional activator in the cellular response to increased osmolality. Pflugers Arch. 2002, 444, 579–585. [Google Scholar]
- Ito, T.; Fujio, Y.; Takahashi, K.; Azuma, J. Degradation of NFAT5, a transcriptional regulator of osmotic stress-related genes, is a critical event for doxorubicin-induced cytotoxicity in cardiac myocytes. J. Biol. Chem. 2007, 282, 1152–1160. [Google Scholar] [CrossRef] [Green Version]
- Jeon, U.S.; Kim, J.A.; Sheen, M.R.; Kwon, H.M. How tonicity regulates genes: Story of TonEBP transcriptional activator. Acta Physiol. 2006, 187, 241–247. [Google Scholar] [CrossRef] [PubMed]
- Miyakawa, H.; Woo, S.K.; Dahl, S.C.; Handler, J.S.; Kwon, H.M. Tonicity-responsive enhancer binding protein, a Rel-like protein that stimulates transcription in response to hypertonicity. Proc. Natl. Acad. Sci. USA. 1999, 96, 2538–2542. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kültz, D.; Chakravarty, D. Hyperosmolality in the form of elevated NaCl but not urea causes DNA damage in murine kidney cells. Proc. Natl. Acad. Sci. USA. 2001, 98, 1999–2004. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- James, T.J. Ion-selective electrodes. Postgrad. Med. J. 1999, 75, 254. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hwang, P.P.; Lee, T.H.; Lin, L.Y. Ion regulation in fish gills: Recent progress in the cellular and molecular mechanisms. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2011, 301, 28–47. [Google Scholar] [CrossRef] [PubMed]
- McNamara, J.C.; Faria, S.C. Evolution of osmoregulatory patterns and gill ion transport mechanisms in the decapod crustacea: A review. J. Comp. Physiol. B 2012, 182, 997–1014. [Google Scholar] [CrossRef]
- Uchiyama, M.; Konno, N. Hormonal regulation of ion and water transport in anuran amphibians. Gen. Comp. Endocrinol. 2006, 147, 54–61. [Google Scholar] [CrossRef]
- Shui, C.; Zhang, H.M.; Shi, Y.H.; Xie, Y.D.; Liu, Y.S.; Lu, G.H.; Xu, J.B. Effects of salinity on growth, osmophysiology and body composition of juvenile Soiuy liza haematocheila. J. Dalian Ocean. Univ. 2015, 30, 634–640. (In Chinese) [Google Scholar]
- Zhao, F.; Zhuang, P.; Zhang, L.Z.; Huang, X.R.; Tian, H.J.; Zhang, T.; Feng, G.P. The influence of salinity acclimation on activity of Na+/K+-ATPase in branchial epithelium, concentration of ions and osmolarity in serum of Acipenser schrenckii. J. Fish. China 2006, 4, 444–449. (In Chinese) [Google Scholar]
- Mcenroe, M.; Cech, J.J. Osmoregulation in juvenile and adult white sturgeon, Acipenser transmontanus. Environ. Biol. Fish. 1985, 14, 23–30. [Google Scholar] [CrossRef]
- Pillans, R.D.; Good, J.P.; Anderson, W.G.; Hazon, N.; Franklin, C.E. Freshwater to seawater acclimation of juvenile bull sharks (Carcharhinus leucas): Plasma osmolytes and Na+/K+-ATPase Activity in gill, rectal gland, kidney and intestine. J. Comp. Physiol. B 2005, 175, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Wheatly, M.G.; Gannon, A.T. Ion regulation in crayfish: Freshwater adaptations and the problem of molting. Am. Zool. 1995, 35, 49–59. [Google Scholar] [CrossRef]
- Evans, D.H. Teleost fish osmoregulation: What have we learned since August Krogh, Homer Smith, and Ancel Keys. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2008, 295, 704–713. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moriyama, T.; Garcia-Perez, A.; Burg, M.B. Osmotic regulation of aldose reductase protein synthesis in renal medullary cells. J. Biol. Chem. 1989, 264, 16810–16814. [Google Scholar] [CrossRef]
- Smardo, F.L., Jr.; Burg, M.B.; Garcia-Perez, A. Kidney aldose reductase gene transcription is osmotically regulated. Am. J. Physiol. 1992, 262, 776–782. [Google Scholar] [CrossRef]
- Grunewald, R.W.; Wagner, M.; Schubert, I.; Franz, H.E.; Müller, G.A.; Steffgen, J. Rat renal expression of mRNA coding for aldose reductase and sorbitol dehydrogenase and its osmotic regulation in inner medullary collecting duct cells. Cell Physiol. Biochem. 1998, 8, 293–303. [Google Scholar] [CrossRef]
- Garcia-Perez, A.; Burg, M.B. Renal medullary organic osmolytes. Physiol. Rev. 1991, 71, 1081–1115. [Google Scholar] [CrossRef]
- Nakanishi, T.; Uyama, O.; Sugita, M. Osmotically regulated taurine content in rat renal inner medulla. Am. J. Physiol. 1991, 261, 957–962. [Google Scholar] [CrossRef]
- Uchida, S.; Nakanishi, T.; Kwon, H.M.; Preston, A.S.; Handler, J.S. Taurine behaves as an osmolyte in Madin-Darby canine kidney cells. protection by polarized, regulated transport of taurine. J. Clin. Investig. 1991, 88, 656–662. [Google Scholar] [CrossRef] [Green Version]
- Uchida, S.; Yamauchi, A.; Preston, A.S.; Kwon, H.M.; Handler, J.S. Medium tonicity regulates expression of the Na(+)- and Cl(−)-dependent betaine transporter in Madin-Darby canine kidney cells by increasing transcription of the transporter gene. J. Clin. Investig. 1993, 91, 1604–1607. [Google Scholar] [CrossRef] [Green Version]
- Yamauchi, A.; Uchida, S.; Preston, A.S.; Kwon, H.M.; Handler, J.S. Hypertonicity stimulates transcription of gene for Na(+)-myo-inositol cotransporter in MDCK Cells. Am. J. Physiol. 1993, 264, 20–23. [Google Scholar] [CrossRef] [PubMed]
- Kempson, S.A.; Montrose, M.H. Osmotic regulation of renal betaine transport: Transcription and beyond. Pflugers Arch. 2004, 449, 227–234. [Google Scholar] [CrossRef] [PubMed]
- Cowley, B.D., Jr.; Ferraris, J.D.; Carper, D.; Burg, M.B. In vivo osmoregulation of aldose reductase mRNA, protein, and sorbitol in renal medulla. Am. J. Physiol. 1990, 258, 154–161. [Google Scholar] [CrossRef]
- Martial, S.; Price, S.R.; Sands, J.M. Regulation of aldose reductase, sorbitol dehydrogenase, and taurine cotransporter mRNA in rat medulla. J. Am. Soc. Nephrol. 1995, 5, 1971–1978. [Google Scholar] [CrossRef] [PubMed]
- Ko, B.C.; Turck, C.W.; Lee, K.W.; Yang, Y.; Chung, S.S. Purification, identification, and characterization of an osmotic response element binding protein. Biochem. Biophys. Res. Commun. 2000, 270, 52–61. [Google Scholar] [CrossRef] [PubMed]
- Jeong, S.Y.; Kim, J.H.; Lee, W.O.; Dahms, H.U.; Han, K.N. Salinity changes in the anadromous river pufferfish, Takifugu obscurus, mediate gene regulation. Fish Physiol. Biochem. 2014, 40, 205–219. [Google Scholar] [CrossRef] [PubMed]
- Shoemaker, V.H.; Mcclanahan, L.L. Evaporative water loss, nitrogen excretion and osmoregulation in phyllomedusine frogs. J. Comp. Phys. 1975, 100, 331–345. [Google Scholar] [CrossRef]
- Sands, J.M. Molecular approaches to urea transporte.ers. J. Am. Soc. Nephrol. 2002, 13, 2795–2806. [Google Scholar] [CrossRef] [Green Version]
- Kumar, S.; Stokes, J., 3rd; Singh, U.P.; Scissum Gunn, K.; Acharya, A.; Manne, U.; Mishra, M. Targeting Hsp70: A possible therapy for cancer. Cancer Lett. 2016, 374, 156–166. [Google Scholar] [CrossRef] [Green Version]
- Shim, E.H.; Kim, J.I.; Bang, E.S.; Heo, J.S.; Lee, J.S.; Kim, E.Y.; Lee, J.E.; Park, W.Y.; Kim, S.H.; Kim, H.S.; et al. Targeted disruption of Hsp70.1 sensitizes to osmotic stress. EMBO Rep. 2002, 3, 857–861. [Google Scholar] [CrossRef] [Green Version]
- Haramura, T.; Ikegami, T.; Wong, M.K.S.; Takei, Y. Preparatory mechanisms for salinity tolerance in two congeneric Anuran species inhabiting distinct osmotic habitats. Zool. Sci. 2019, 36, 215–222. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).