Does a Moderately Warming Climate Compensate for the Negative Effects of UV-B Radiation on Amphibians at High Altitudes? A Test of Rana kukunoris Living on the Qinghai–Tibetan Plateau
Abstract
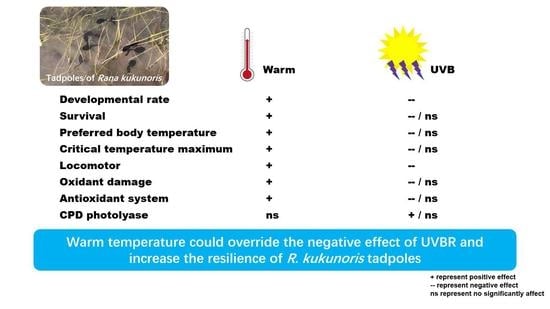
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Experimental Design
2.3. Animal Collection and Maintenance
2.4. Measurement of Thermal Biology
2.5. Measurement of Burst Swimming Performance
2.6. Measurement of Oxidative Damage and Antioxidant Capacity
2.7. Quantitative Real-Time PCR
2.8. Skin Section and Body Coloration Determination
2.9. Statistical Analyses
3. Results
3.1. Development and Survival
3.2. Thermal Biological Characteristics
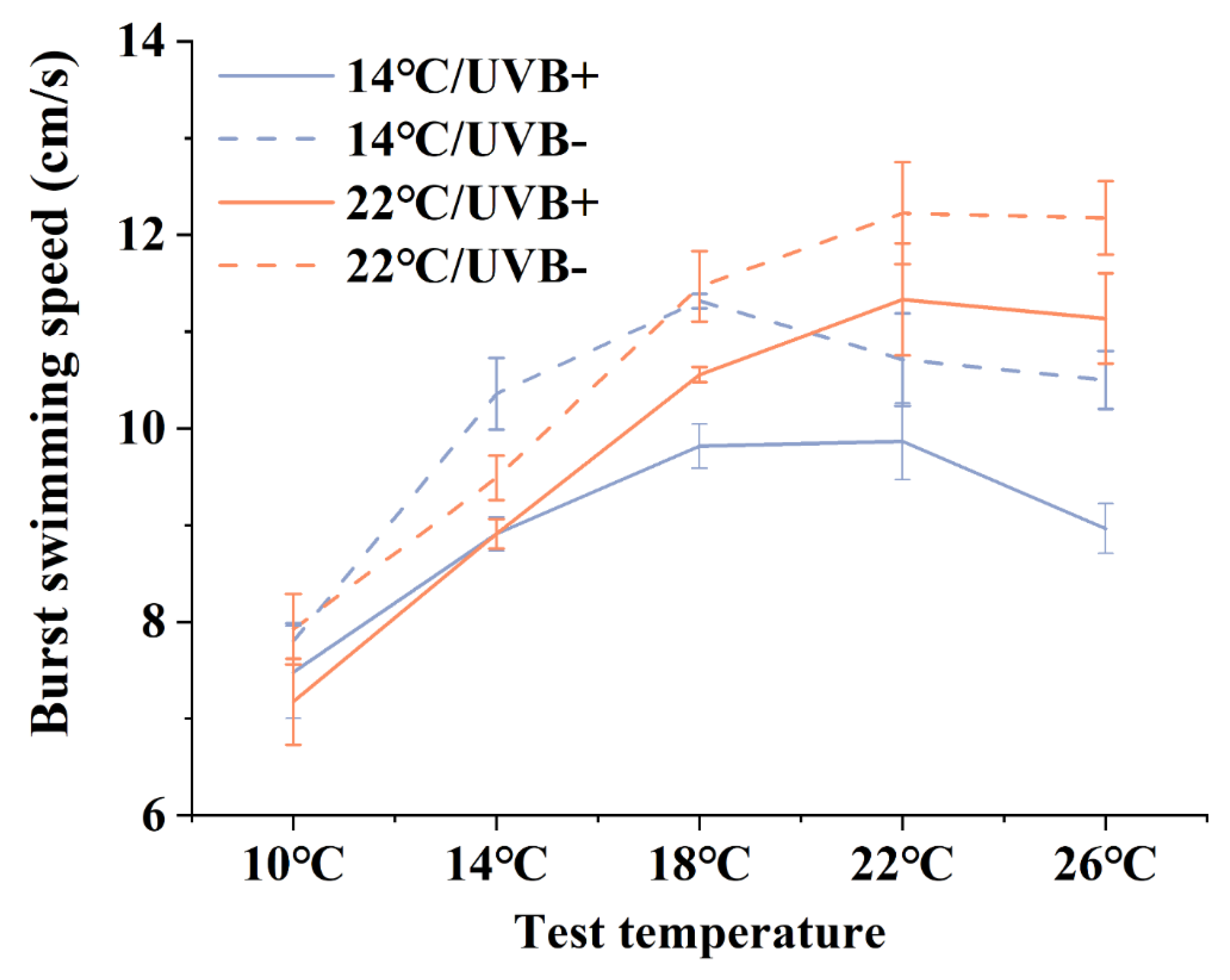
3.3. Burst Swimming Speed
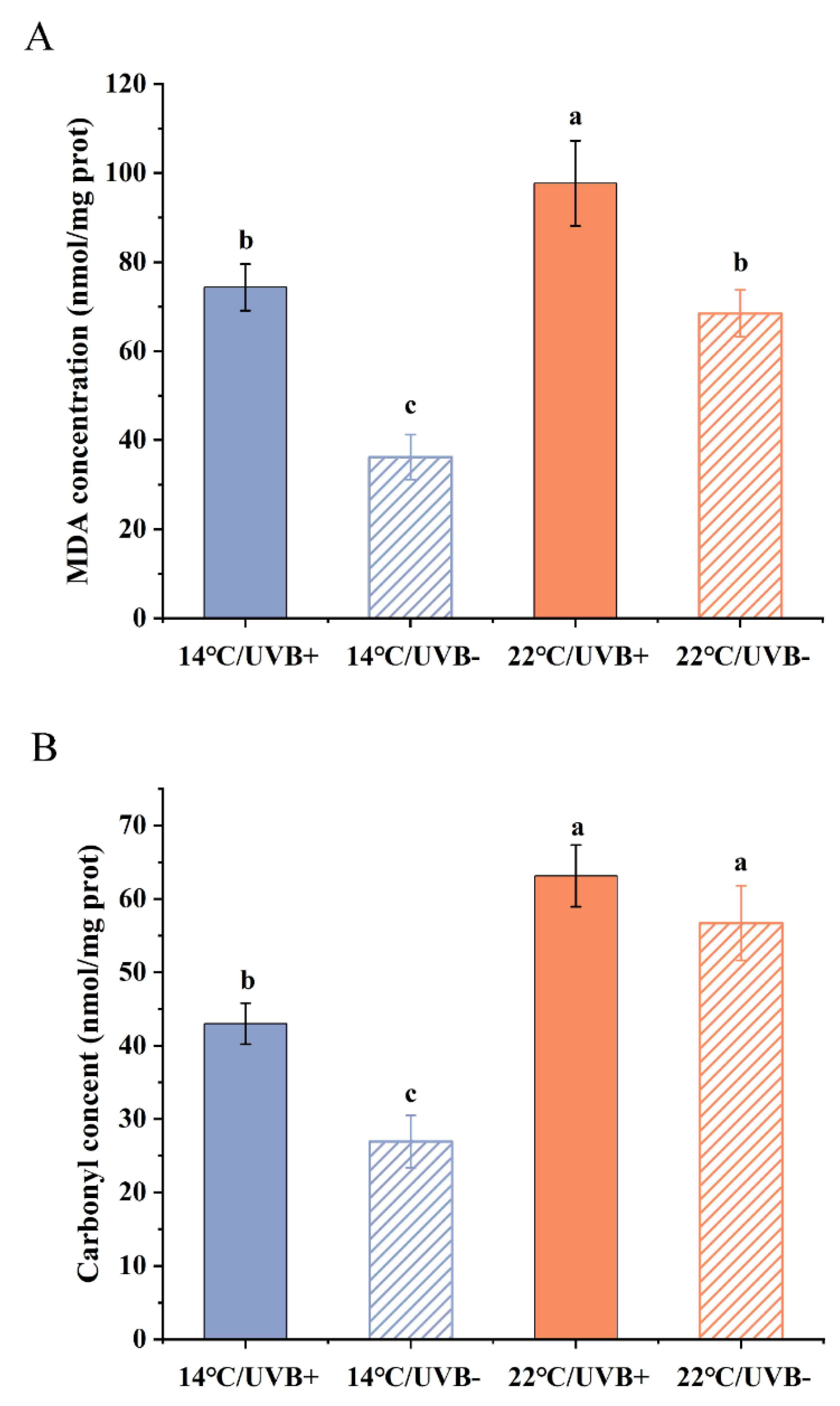
3.4. MDA and Protein Carbonyl Determination
3.5. Antioxidation System Assay
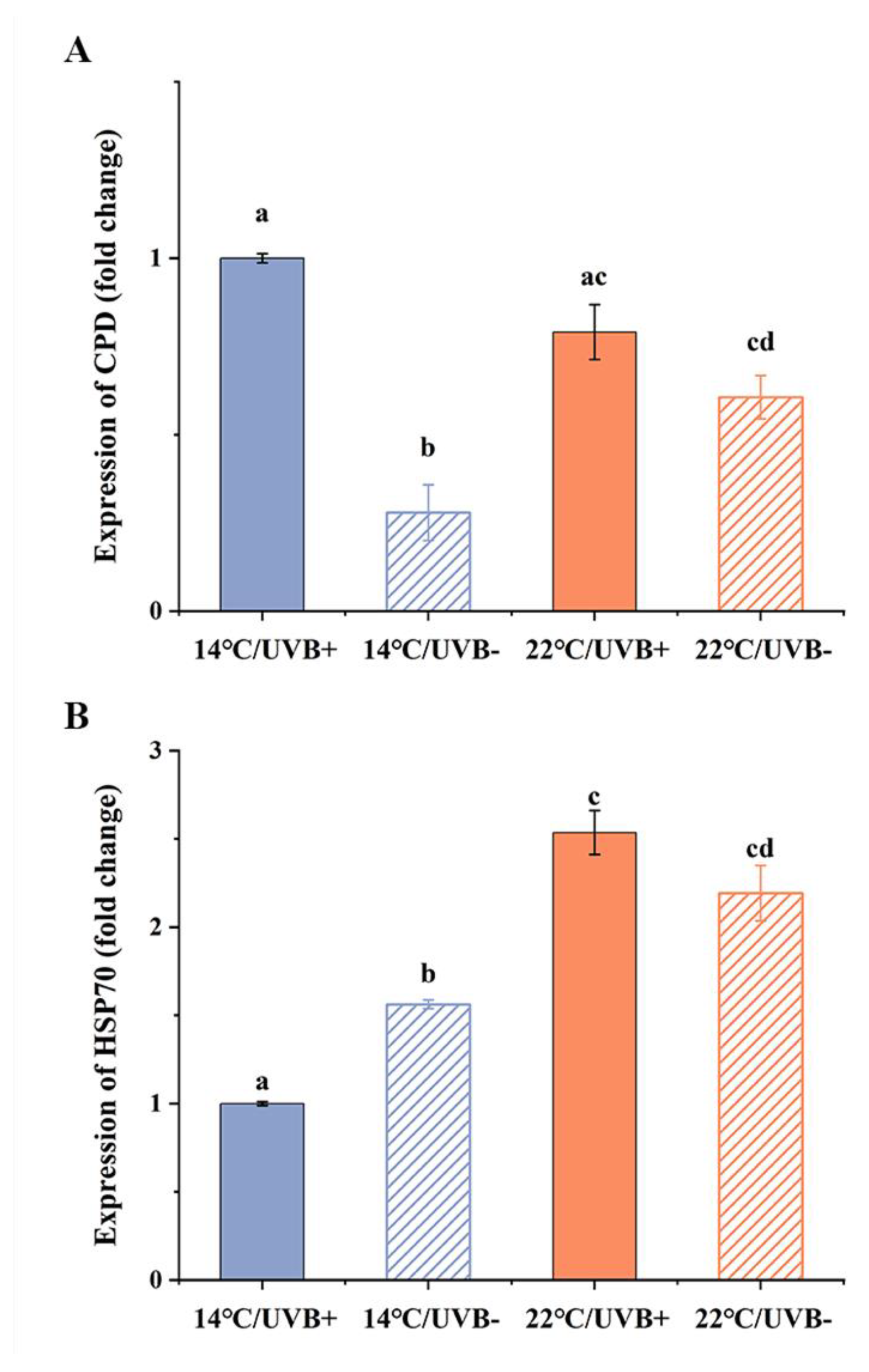
3.6. Gene Expression of CPD Photolyase and HSP70
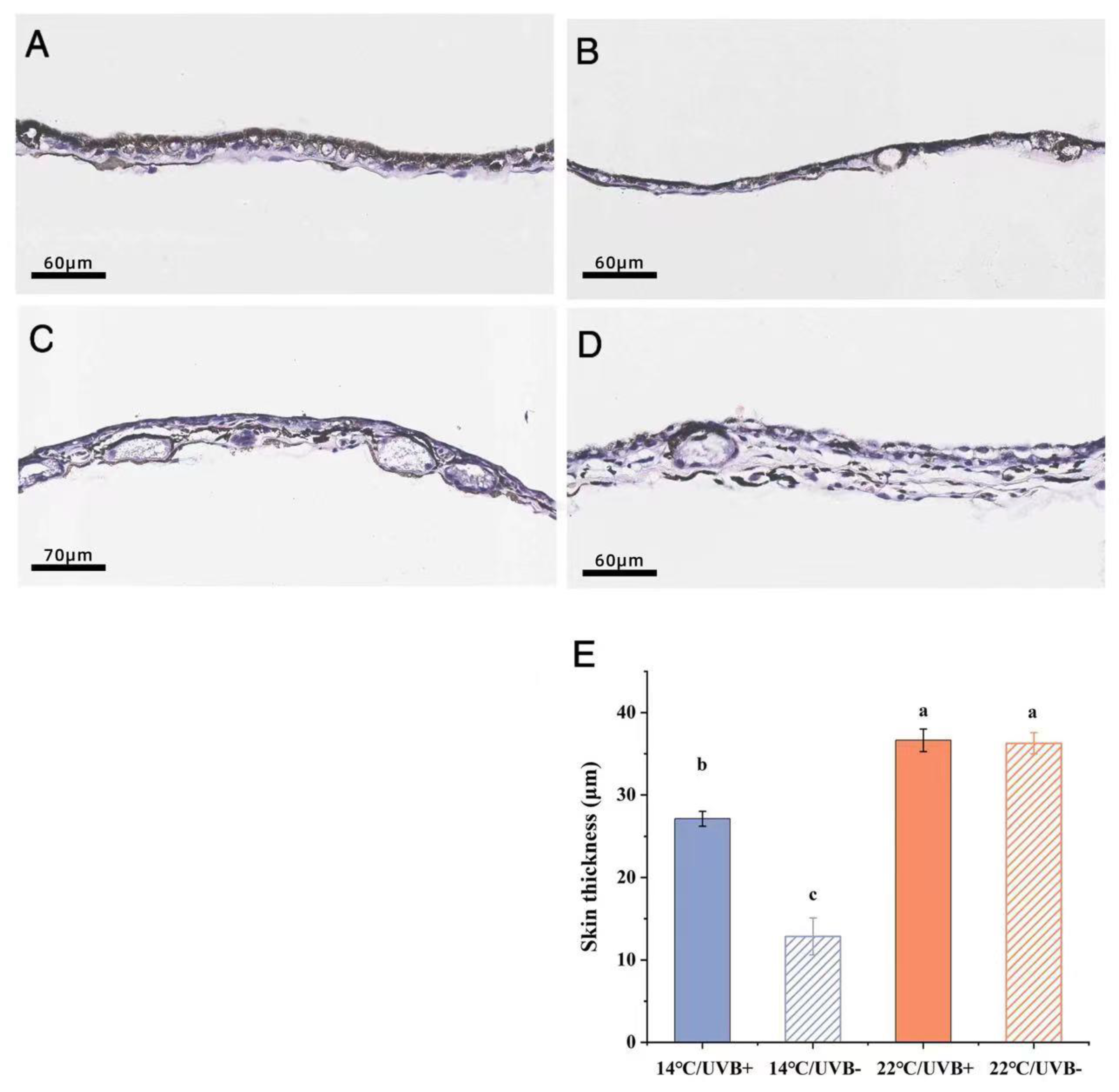
3.7. Skin Section and Body Coloration
4. Discussion
4.1. Thermal Biology
4.2. Development, Survival, and Locomotion
4.3. The Oxidation and Antioxidation Systems
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Parmesan, C. Ecological and evolutionary responses to recent climate change. Annu. Rev. Ecol. Evol. Syst. 2006, 37, 637–669. [Google Scholar] [CrossRef]
- Li, Y.; Cohen, J.M.; Rohr, J.R. Review and synthesis of the effects of climate change on amphibians. Integr. Zool. 2013, 8, 145–161. [Google Scholar] [CrossRef]
- Alton, L.A.; Franklin, C.E. Do high temperatures enhance the negative effects of ultraviolet-B radiation in embryonic and larval amphibians? Biol. Open 2012, 1, 897–903. [Google Scholar] [CrossRef][Green Version]
- Lundsgaard, N.U.; Cramp, R.L.; Franklin, C.E.; Martin, L. Effects of ultraviolet-B radiation on physiology, immune function and survival is dependent on temperature: Implications for amphibian declines. Conserv. Physiol. 2020, 8, coaa002. [Google Scholar] [CrossRef]
- Tejedo, M.; Marangoni, F.; Pertoldi, C.; Richter-Boix, A.; Laurila, A.; Orizaola, G.; Nicieza, A.G.; Álvarez, D.; Gomez-Mestre, I. Contrasting effects of environmental factors during larval stage on morphological plasticity in post-metamorphic frogs. Clim. Res. 2010, 43, 31–39. [Google Scholar] [CrossRef]
- Pintanel, P.; Tejedo, M.; Merino-Viteri, A.; Almeida-Reinoso, F.; Salinas-Ivanenko, S.; López-Rosero, A.C.; Llorente, G.A.; Gutiérrez-Pesquera, L.M. Elevational and local climate variability predicts thermal breadth of mountain tropical tadpoles. Ecography 2022, 2022, e05906. [Google Scholar] [CrossRef]
- Duarte, H.; Tejedo, M.; Katzenberger, M.; Marangoni, F.; Baldo, D.; Beltrán, J.F.; Martí, D.A.; Richter-Boix, A.; Gonzalez-Voyer, A. Can amphibians take the heat? Vulnerability to climate warming in subtropical and temperate larval amphibian communities. Glob. Chang. Biol. 2012, 18, 412–421. [Google Scholar] [CrossRef]
- Angilletta, M.J., Jr.; Niewiarowski, P.H.; Navas, C.A. The evolution of thermal physiology in ectotherms. J. Therm. Biol. 2002, 27, 249–268. [Google Scholar] [CrossRef]
- Wilson, R.S. Consequences of metamorphosis for the locomotor performance and thermal physiology of the newt Triturus cristatus. Physiol. Biochem. Zool. 2005, 78, 967–975. [Google Scholar] [CrossRef][Green Version]
- Mann, J. Tadpoles: The Biology of Anuran Larvae; University of Chicago Press: Chicago, IL, USA, 1999. [Google Scholar]
- Huey, R.B.; Stevenson, R. Integrating thermal physiology and ecology of ectotherms: A discussion of approaches. Am. Zool. 1979, 19, 357–366. [Google Scholar] [CrossRef]
- Smith, K.C. The biological effects of ultraviolet radiation on man, animals, and plants. In Proceedings of the Climatic Impact Assessment Program Survey Conference, Cambridge, MA, USA, 15–16 February 1972; pp. 243–249. [Google Scholar]
- Ghanizadeh Kazerouni, E.; Franklin, C.E.; Seebacher, F. UV-B radiation interacts with temperature to determine animal performance. Funct. Ecol. 2016, 30, 584–595. [Google Scholar] [CrossRef]
- Belhadj Slimen, I.; Najar, T.; Ghram, A.; Dabbebi, H.; Ben Mrad, M.; Abdrabbah, M. Reactive oxygen species, heat stress and oxidative-induced mitochondrial damage. A review. Int. J. Hyperth. 2014, 30, 513–523. [Google Scholar] [CrossRef]
- Cadet, J.; Douki, T.; Ravanat, J.L. Oxidatively generated damage to cellular DNA by UVB and UVA radiation. Photochem. Photobiol. 2015, 91, 140–155. [Google Scholar] [CrossRef]
- Chatgilialoglu, C.; Studer, A. Encyclopedia of Radicals in Chemistry, Biology, and Materials; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2012; Volume 2. [Google Scholar]
- Cockell, C.S.; Knowland, J. Ultraviolet radiation screening compounds. Biol. Rev. 1999, 74, 311–345. [Google Scholar] [CrossRef]
- Rodríguez-Rodríguez, E.J.; Beltrán, J.F.; Márquez, R. Melanophore metachrosis response in amphibian tadpoles: Effect of background colour, light and temperature. Amphib.-Reptil. 2020, 42, 133–140. [Google Scholar] [CrossRef]
- Hofer, R.; Mokri, C. Photoprotection in tadpoles of the common frog, Rana temporaria. J. Photochem. Photobiol. B Biol. 2000, 59, 48–53. [Google Scholar] [CrossRef]
- Thiagarajan, V.; Byrdin, M.; Eker, A.P.; Müller, P.; Brettel, K. Kinetics of cyclobutane thymine dimer splitting by DNA photolyase directly monitored in the UV. Proc. Natl. Acad. Sci. USA 2011, 108, 9402–9407. [Google Scholar] [CrossRef]
- Dosek, A.; Ohno, H.; Acs, Z.; Taylor, A.W.; Radak, Z. High altitude and oxidative stress. Respir. Physiol. Neurobiol. 2007, 158, 128–131. [Google Scholar] [CrossRef]
- Alton, L.A.; Franklin, C.E. Drivers of amphibian declines: Effects of ultraviolet radiation and interactions with other environmental factors. Clim. Chang. Responses 2017, 4, 1–26. [Google Scholar] [CrossRef]
- Kafash, A.; Ashrafi, S.; Ohler, A.; Yousefi, M.; Malakoutikhah, S.; Koehler, G.; Schmidt, B.R. Climate change produces winners and losers: Differential responses of amphibians in mountain forests of the Near East. Glob. Ecol. Conserv. 2018, 16, e00471. [Google Scholar] [CrossRef]
- Cordier, J.M.; Lescano, J.N.; Ríos, N.E.; Leynaud, G.C.; Nori, J. Climate change threatens micro-endemic amphibians of an important South American high-altitude center of endemism. Amphib.-Reptil. 2020, 41, 233–243. [Google Scholar] [CrossRef]
- Morison, S.A.; Cramp, R.L.; Alton, L.A.; Franklin, C.E. Cooler temperatures slow the repair of DNA damage in tadpoles exposed to ultraviolet radiation: Implications for amphibian declines at high altitude. Glob. Chang. Biol. 2020, 26, 1225–1234. [Google Scholar] [CrossRef]
- McCaffery, R.M.; Maxell, B.A. Decreased winter severity increases viability of a montane frog population. Proc. Natl. Acad. Sci. USA 2010, 107, 8644–8649. [Google Scholar] [CrossRef]
- Herman, J.R. Global increase in UV irradiance during the past 30 years (1979–2008) estimated from satellite data. J. Geophys. Res. Atmos. 2010, 115. [Google Scholar] [CrossRef]
- Kuang, X.; Jiao, J.J. Review on climate change on the Tibetan Plateau during the last half century. J. Geophys. Res. Atmos. 2016, 121, 3979–4007. [Google Scholar] [CrossRef]
- Zhu, X.; Wang, W.; Fraedrich, K. Future climate in the Tibetan Plateau from a statistical regional climate model. J. Clim. 2013, 26, 10125–10138. [Google Scholar] [CrossRef][Green Version]
- Jie, H.; Peng, P.; Tao, Z.; Yi, N.Z.; Fei, M.; Lu, X.; Ming, M.; Juan, W.; Long, T.X.; Qiang, C. Influence of high temperatures and heat wave on thermal biology, locomotor performance and antioxidant system of high-altitude frog Nanorana pleskei endemic to Qinghai-Tibet Plateau. Front. Ecol. Evol. 2021, 831. [Google Scholar]
- Oyamaguchi, H.M.; Vo, P.; Grewal, K.; Do, R.; Erwin, E.; Jeong, N.; Tse, K.; Chen, C.; Miyake, M.; Lin, A. Thermal sensitivity of a Neotropical amphibian (Engystomops pustulosus) and its vulnerability to climate change. Biotropica 2018, 50, 326–337. [Google Scholar] [CrossRef]
- Menke, M.E.; Claussen, D.L. Thermal acclimation and hardening in tadpoles of the bullfrog, Rana catesbeiana. J. Therm. Biol. 1982, 7, 215–219. [Google Scholar] [CrossRef]
- Peskin, A.V.; Winterbourn, C.C. A microtiter plate assay for superoxide dismutase using a water-soluble tetrazolium salt (WST-1). Clin. Chim. Acta 2000, 293, 157–166. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free. Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Kruger, A.; Morin, P.J. Predators Induce Morphological Changes in Tadpoles of Hyla andersonii. Copeia 2020, 108, 316–325. [Google Scholar] [CrossRef]
- Pfeiffer, W. Die verbreitung der schreckreaktion bei kaulquappen und die herkunft des schreckstoffes. Z. Für Vgl. Physiol. 1966, 52, 79–98. [Google Scholar] [CrossRef]
- Van Uitregt, V.O.; Wilson, R.S.; Franklin, C.E. Cooler temperatures increase sensitivity to ultraviolet B radiation in embryos and larvae of the frog Limnodynastes peronii. Glob. Chang. Biol. 2007, 13, 1114–1121. [Google Scholar] [CrossRef]
- Bodensteiner, B.L.; Agudelo-Cantero, G.A.; Arietta, A.A.; Gunderson, A.R.; Muñoz, M.M.; Refsnider, J.M.; Gangloff, E.J. Thermal adaptation revisited: How conserved are thermal traits of reptiles and amphibians? J. Exp. Zool. Part A Ecol. Integr. Physiol. 2021, 335, 173–194. [Google Scholar] [CrossRef] [PubMed]
- Dupré, R.K.; Petranka, J.W. Ontogeny of Temperature Selection in Larval Amphibians. Copeia 1985, 1985, 462–467. [Google Scholar] [CrossRef]
- Sørensen, J.G.; Pekkonen, M.; Lindgren, B.; Loeschcke, V.; Laurila, A.; Merilä, J. Complex patterns of geographic variation in heat tolerance and Hsp70 expression levels in the common frog Rana temporaria. J. Therm. Biol. 2009, 34, 49–54. [Google Scholar] [CrossRef]
- Sørensen, J.G.; Kristensen, T.N.; Loeschcke, V. The evolutionary and ecological role of heat shock proteins. Ecol. Lett. 2003, 6, 1025–1037. [Google Scholar] [CrossRef]
- Heikkila, J.J.; Ohan, N.; Tam, Y.; Ali, A. Heat shock protein gene expression during Xenopus development. Cell. Mol. Life Sci. CMLS 1997, 53, 114–121. [Google Scholar] [CrossRef]
- Azambuja, G.; Martins, I.K.; Franco, J.L.; dos Santos, T.G. Effects of mancozeb on heat Shock protein 70 (HSP70) and its relationship with the thermal physiology of Physalaemus henselii (Peters, 1872) tadpoles (Anura: Leptodactylidae). J. Therm. Biol. 2021, 98, 102911. [Google Scholar] [CrossRef]
- McMillan, D.M.; Irschick, D.J.; Rees, B.B. Geographic Variation in the Effects of Heat Exposure on Maximum Sprint Speed and Hsp70 Expression in the Western Fence Lizard Sceloporus occidentalis. Physiol. Biochem. Zool. 2011, 84, 573–582. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Tang, S.; Mayer, G.D.; Cobb, G.P.; Maul, J.D. Interactive effects of ultraviolet-B radiation and pesticide exposure on DNA photo-adduct accumulation and expression of DNA damage and repair genes in Xenopus laevis embryos. Aquat. Toxicol. 2015, 159, 256–266. [Google Scholar] [CrossRef] [PubMed]
- Kültz, D. Molecular and evolutionary basis of the cellular stress response. Annu. Rev. Physiol. 2005, 67, 225–257. [Google Scholar] [CrossRef] [PubMed]
- Hayes, T.; Falso, P.; Gallipeau, S.; Stice, M. The cause of global amphibian declines: A developmental endocrinologist’s perspective. J. Exp. Biol. 2010, 213, 921–933. [Google Scholar] [CrossRef]
- Häkkinen, J.; Pasanen, S.; Kukkonen, J.V. The effects of solar UV-B radiation on embryonic mortality and development in three boreal anurans (Rana temporaria, Rana arvalis and Bufo bufo). Chemosphere 2001, 44, 441–446. [Google Scholar] [CrossRef]
- Bancroft, B.A.; Baker, N.J.; Searle, C.L.; Garcia, T.S.; Blaustein, A.R. Larval amphibians seek warm temperatures and do not avoid harmful UVB radiation. Behav. Ecol. 2008, 19, 879–886. [Google Scholar] [CrossRef]
- Kern, P.; Cramp, R.L.; Franklin, C.E. Temperature and UV-B-insensitive performance in tadpoles of the ornate burrowing frog: An ephemeral pond specialist. J. Exp. Biol. 2014, 217, 1246–1252. [Google Scholar] [CrossRef]
- Broomhall, S.D.; Osborne, W.S.; Cunningham, R.B. Comparative effects of ambient ultraviolet-B radiation on two sympatric species of Australian frogs. Conserv. Biol. 2000, 14, 420–427. [Google Scholar] [CrossRef]
- Middleton, E.M.; Herman, J.R.; Celarier, E.A.; Wilkinson, J.W.; Carey, C.; Rusin, R.J. Evaluating ultraviolet radiation exposure with satellite data at sites of amphibian declines in Central and South America. Conserv. Biol. 2001, 15, 914–929. [Google Scholar] [CrossRef]
- Blaustein, A.R.; Romansic, J.M.; Kiesecker, J.M.; Hatch, A.C. Ultraviolet radiation, toxic chemicals and amphibian population declines. Divers. Distrib. 2003, 9, 123–140. [Google Scholar] [CrossRef]
- Seebacher, F.; Grigaltchik, V.S. Embryonic developmental temperatures modulate thermal acclimation of performance curves in tadpoles of the frog Limnodynastes peronii. PLoS ONE 2014, 9, e106492. [Google Scholar] [CrossRef]
- Berchtold, M.W.; Brinkmeier, H.; Muntener, M. Calcium ion in skeletal muscle: Its crucial role for muscle function, plasticity, and disease. Physiol. Rev. 2000, 80, 1215–1265. [Google Scholar] [CrossRef]
- Bateson, P.; Gluckman, P.; Hanson, M. The biology of developmental plasticity and the Predictive Adaptive Response hypothesis. J. Physiol. 2014, 592, 2357–2368. [Google Scholar] [CrossRef]
- Bayeva, M.; Ardehali, H. Mitochondrial dysfunction and oxidative damage to sarcomeric proteins. Curr. Hypertens. Rep. 2010, 12, 426–432. [Google Scholar] [CrossRef]
- Seebacher, F.; Kazerouni, E.G.; Franklin, C.E. Ultraviolet B radiation alters movement and thermal selection of zebrafish (Danio rerio). Biol. Lett. 2016, 12, 20160258. [Google Scholar] [CrossRef]
- Ghanizadeh Kazerouni, E.; Franklin, C.E.; Seebacher, F. UV-B exposure reduces locomotor performance by impairing muscle function but not mitochondrial ATP production. J. Exp. Biol. 2016, 219, 96–102. [Google Scholar]
- Suzuki, N.; Mittler, R. Reactive oxygen species and temperature stresses: A delicate balance between signaling and destruction. Physiol. Plant. 2006, 126, 45–51. [Google Scholar] [CrossRef]
- Meng, X.-l.; Liu, P.; Li, J.; Gao, B.-Q.; Chen, P. Physiological responses of swimming crab Portunus trituberculatus under cold acclimation: Antioxidant defense and heat shock proteins. Aquaculture 2014, 434, 11–17. [Google Scholar] [CrossRef]
- Regueira, E.; Dávila, C.; Hermida, G.N. Morphological Changes in Skin Glands during Development in Rhinella arenarum (Anura: Bufonidae). Anat. Rec. 2016, 299, 141–156. [Google Scholar] [CrossRef]
- Brattstrom, B.H.; Lawrence, P. The rate of thermal acclimation in anuran amphibians. Physiol. Zool. 1962, 35, 148–156. [Google Scholar] [CrossRef]
- Thomas, M.; Raharivololoniaina, L.; Glaw, F.; Vences, M.; Vieites, D.R. Montane tadpoles in Madagascar: Molecular identification and description of the larval stages of Mantidactylus elegans, Mantidactylus madecassus, and Boophis laurenti from the Andringitra Massif. Copeia 2005, 2005, 174–183. [Google Scholar] [CrossRef]
- Licht, L.E.; Grant, K.P. The effects of ultraviolet radiation on the biology of amphibians. Am. Zool. 1997, 37, 137–145. [Google Scholar] [CrossRef]
- Kim, D.S.; Park, S.H.; Kwon, S.B.; Joo, Y.H.; Youn, S.W.; Sohn, U.D.; Park, K.C. Temperature regulates melanin synthesis in melanocytes. Arch. Pharm. Res. 2003, 26, 840–845. [Google Scholar] [CrossRef]
- Blaustein, A.R.; Belden, L.K. Amphibian defenses against ultraviolet-B radiation. Evol. Dev. 2003, 5, 89–97. [Google Scholar] [CrossRef]
- MacFadyen, E.J.; Williamson, C.E.; Grad, G.; Lowery, M.; Jeffrey, W.H.; Mitchell, D.L. Molecular response to climate change: Temperature dependence of UV-induced DNA damage and repair in the freshwater crustacean Daphnia pulicaria. Glob. Change Biol. 2004, 10, 408–416. [Google Scholar] [CrossRef]
- Demas, G.; Nelson, R. Ecoimmunology; Oxford University Press: Oxford, UK, 2012. [Google Scholar]



| Group | Developmental Stage | Body Mass (g) | Total Body Length (mm) | Tail Length (mm) | Tail Height (mm) |
|---|---|---|---|---|---|
| Initial period | 26 (25–27) | 0.033 ± 0.001 | 14.401 ± 0.158 | 8.063 ± 0.088 | 2.620 ± 0.045 |
| 14 °C/UVBR | 31 (30–32) d | 0.153 ± 0.004 c | 23.458 ± 0.273 d | 13.398 ± 0.174 c | 4.388 ± 0.178 b |
| 14 °C/UVBR-free | 34 (32–34) c | 0.195 ± 0.005 b | 24.909 ± 0.304 c | 13.869 ± 0.150 c | 4.65 ± 0.084 a |
| 22 °C/UVBR | 38 (37–39) b | 0.232 ± 0.003 a | 27.899 ± 0.251 b | 16.023 ± 0.184 b | 4.74 ± 0.065 a |
| 22 °C/UVB-free | 39 (38–41) a | 0.235 ± 0.004 a | 28.817 ± 0.214 a | 16.917 ± 0.176 a | 4.783 ± 0.052 a |
| Group | CTmax (°C) | CTmin (°C) | ΔCT (°C) | Tpre (°C) | AT (°C) | LTM (°C) |
|---|---|---|---|---|---|---|
| 14 °C/UVBR | 30.51 ± 0.80 c | 4.28 ± 0.17 c | 26.24 | 18.14 ± 0.30 c | 26.66 ± 0.18 c | 38.35 ± 0.09 c |
| 14 °C/UVBR-free | 31.88 ± 0.74 b | 3.08 ± 0.12 d | 28.80 | 18.12 ± 0.27 c | 28.80 ± 0.28 b | 38.72 ± 0.13 c |
| 22 °C/UVBR | 36.02 ± 0.62 a | 8.69 ± 0.20 a | 27.33 | 23.08 ± 0.45 b | 28.81 ± 0.27 b | 40.60 ± 0.27 a |
| 22 °C/UVB-free | 36.09 ± 0.65 a | 7.03 ± 0.27 b | 29.06 | 25.06 ± 0.19 a | 30.43 ± 0.35 a | 39.98 ± 0.24 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, X.; Xi, L.; Niu, Z.; Jia, L.; Bai, Y.; Wang, H.; Ma, M.; Chen, Q. Does a Moderately Warming Climate Compensate for the Negative Effects of UV-B Radiation on Amphibians at High Altitudes? A Test of Rana kukunoris Living on the Qinghai–Tibetan Plateau. Biology 2022, 11, 838. https://doi.org/10.3390/biology11060838
Tang X, Xi L, Niu Z, Jia L, Bai Y, Wang H, Ma M, Chen Q. Does a Moderately Warming Climate Compensate for the Negative Effects of UV-B Radiation on Amphibians at High Altitudes? A Test of Rana kukunoris Living on the Qinghai–Tibetan Plateau. Biology. 2022; 11(6):838. https://doi.org/10.3390/biology11060838
Chicago/Turabian StyleTang, Xiaolong, Lu Xi, Zhiyi Niu, Lun Jia, Yucheng Bai, Huihui Wang, Miaojun Ma, and Qiang Chen. 2022. "Does a Moderately Warming Climate Compensate for the Negative Effects of UV-B Radiation on Amphibians at High Altitudes? A Test of Rana kukunoris Living on the Qinghai–Tibetan Plateau" Biology 11, no. 6: 838. https://doi.org/10.3390/biology11060838
APA StyleTang, X., Xi, L., Niu, Z., Jia, L., Bai, Y., Wang, H., Ma, M., & Chen, Q. (2022). Does a Moderately Warming Climate Compensate for the Negative Effects of UV-B Radiation on Amphibians at High Altitudes? A Test of Rana kukunoris Living on the Qinghai–Tibetan Plateau. Biology, 11(6), 838. https://doi.org/10.3390/biology11060838