Abstract
DHMBA, a novel antioxidant derived from oyster, exhibits dual properties that block oxidative stress by acting as a radical scavenger in various cells. DHMBA has been shown to play a pharmacological role by exerting anti-macrophage and anti-cancer effects. Gallic acid, which is widely distributed in edible plants, exhibits a potent antioxidant activity. In this study, we compared the effects of DHMBA and gallic acid using inflammatory macrophages (RAW264.7 cells) and human lung cancer cells (A549 cells) in vitro. In these cells, we demonstrated that DHMBA at comparatively lower concentrations (1 or 10 µM) significantly affected cell proliferation and stimulated cell death, resulting in a decrease in cell number. Gallic acid at 1 and 10 μM did not affect the proliferation or death of RAW264.7 and A549 cells. DHMBA may be a potent antioxidant that regulates cell function. Despite having the same molecular weight, the chemical structure of DHMBA and gallic acid exerted different effects. Notably, the combined DHMBA and gallic acid with comparatively lower concentrations (1 and 10 μM) showed potent effects in suppressing the proliferation of RAW264.7 and A549 cells. However, this combination did not induce a significant effect on cell death. Thus, the effects of DHMBA were potentiated in the presence of the antioxidant gallic acid. This finding suggests a potential effect induced by the combined antioxidants. This study provides a new strategy for disease prevention using the strong antioxidants DHMBA and gallic acid.
1. Introduction
Oxidative stress occurs when there is an imbalance between two types of molecules, radicals and antioxidants, in the body. Free radicals can damage cells and tissues. Free radicals damage the different parts of cells, including lipids and proteins. Oxidative stress plays a role in the development of many chronic and degenerative conditions, leading to several diseases, including cardiovascular [1,2]. Antioxidants play a crucial role in preventing several diseases associated with inflammation, diabetes, and cancer [1,2]. The novel antioxidant 3,5-dihydroxy-4-methoxybenzyl alcohol (DHMBA) was first isolated and identified from the Pacific oyster Crassostrea gigas [3,4,5,6]. DHMBA exhibits dual properties, blocking oxidative stress by acting as a radical scavenger and exhibiting antioxidant activity in cells [3,4]. Computational chemistry revealed that DHMBA is a potent peroxyl radical scavenger, approximately 15 times stronger than Trolox and 4 orders of magnitude stronger than other known antioxidants, including resveratrol and ascorbic acid [5]. In recent years, increasing evidence has shown that DHMBA plays a protective role in living cells [6,7]. Additionally, DHMBA has been shown to play a novel pharmacological role by inhibiting the activity of adipogenesis [7], inflammatory macrophages and their related osteoclastogenesis in vitro [8], while stimulating osteoblastic bone formation [9]. Furthermore, DHMBA has been shown to exhibit anticancer activity against metastatic prostate cancer cells by targeting various signaling pathways [10]. Thus, DHMBA’s novel antioxidant properties may have pharmacological applications in the prevention and treatment of various diseases.
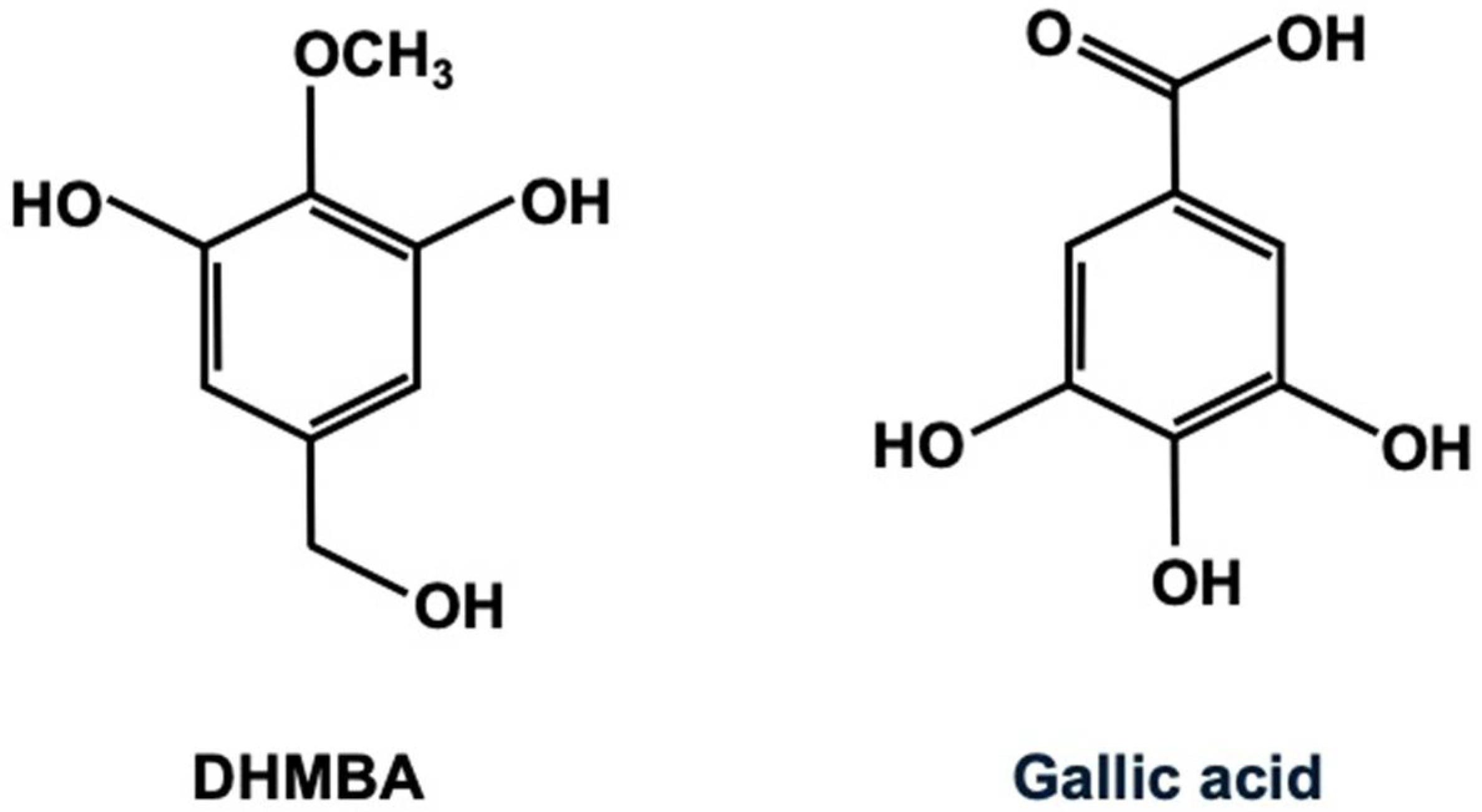
Figure 1 illustrates the chemical structures of the antioxidants DHMBA and gallic acid. The molecular weights of DHMBA and gallic acid are 170.164 and 170.120, respectively, indicating their chemical similarity. Gallic acid (3,4,5-trihydroxybenzoic acid) has potent antioxidant activity and is widely distributed in edible plants [11,12]. It has been shown to exhibit several pharmacological activities, including antioxidant, anti-inflammatory, antineoplastic, and antimicrobial properties. Because of its health benefits in treating various functional disorders associated with oxidative stress, including renal, neurological, hepatic, pulmonary, reproductive, and cardiovascular diseases, gallic acid may be a potential dietary supplement. It is rapidly absorbed and metabolized after oral administration. Additionally, extensive studies have been conducted on gallic acid regarding its applications in the food industry and its health benefits when used as a food additive or dietary supplement.
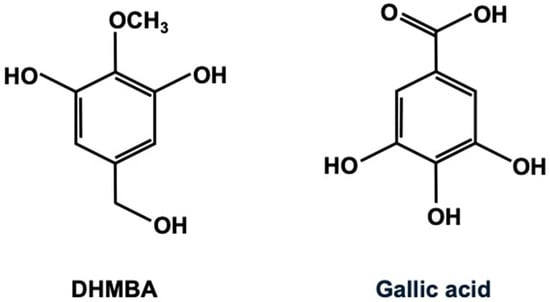
Figure 1.
The chemical structure of 3,5-dihydroxy-4-methoxybenzyl alcohol (DHMBA) and gallic acid (3,4,5-trihydroxybenzoic acid).
This study examined the comparative effects of DHMBA and gallic acid as antioxidants, as well as the combined effects of both antioxidants on their cell growth and death using RAW264.7 macrophages and A549 human lung cancer cells in vitro. Inflammation is a complex biological response of body tissues to harmful stimuli. It is a protective response involving immune cells, blood vessels, and molecular mediators [13]. RAW264.7 mouse macrophages, which are used to model inflammatory conditions in vitro, are monocyte/macrophage-like cells characterized by metabolic and phagocytic functions mediated by macrophages [14]. Meanwhile, lung cancer in humans is classified as either small cell lung cancer (SCLC) or non-small cell lung cancer (NSCLC) [15,16]. Most lung cancers are adenocarcinoma NSCLC, which expresses an aggressive metastatic phenotype. NSCLC patients have a poor prognosis. A549 cells are a human lung cancer cell line that is NSCLC. In the current preliminary study, we demonstrated that, at lower concentrations, DHMBA was more effective than gallic acid at blocking the proliferation and stimulating the death of RAW264.7 cells and A549 cells in vitro, resulting in a decrease in cell number. Interestingly, we found that combining DHMBA and gallic acid potently suppresses cell growth. These results provide valuable insight into the health benefits of antioxidants.
2. Materials and Methods
2.1. Reagents
Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 4.5 g/L glucose, L-glutamine, and sodium pyruvate and antibiotics (100 units/mL penicillin and 100 μg/mL streptomycin; 1% P/S) was purchased from Corning (Mediatech, Inc., Manassas, VA, USA). Fetal bovine serum (FBS) was purchased from Hyclone (Logan, UT, USA). Unless otherwise stated, gallic acid (3,4,5-trihydroxybenzoic acid) and all other reagents were purchased from Sigma-Aldrich (St. Louis, MO, USA). These reagents were diluted with 100% ethanol. Gallic acid dissolved in 100% ethanol was stored at −20 °C until use.
2.2. 3,5-Dihydroxy-4-methoxybenzyl Alcohol
3,5-Dihydroxy-4-methoxybenzyl alcohol (DHMBA) is an amphipathic phenolic compound that was first isolated from the Pacific oyster (Crassostrea gigas) and characterized as an antioxidant [3,4]. Its chemical structure is shown in Figure 1. For this study, the synthesized DHMBA with 100% purity [3] was obtained from Watanabe Oyster Laboratory, Inc. in Hachioji, Tokyo, Japan. The DHMBA was dissolved in 100% ethanol and stored at −20 °C until used. The chemical structure of DHMBA and gallic acid is shown in Figure 1. The molecular formula of DHMBA is C8H10O4, and its molecular weight is 170.164. Gallic acid’s molecular formula is C7H6O5, and its molecular weight is 170.120.
2.3. Cell Culture
RAW264.7 cells are a monocyte/macrophage-like cell line derived from Abelson leukaemia virus-transformed BALB/c mouse cell lines [8,9]. RAW267.4 mouse macrophages were obtained from the American Type Culture Collection (Rockville, MD, USA). The cells were cultured in DMEM supplemented with 10% FBS and 1% P/S.
We also used human lung cancer NSCLC A549 cells obtained from human lung adenocarcinoma. These cells were obtained from the American Type Culture Collection (ATCC; Rockville, MD, USA) and isolated from a 58-year-old male patient with NSCLC. The A549 cells were cultured in DMEM (with high glucose, 4.5 g/L) containing 10% FBS and 1%P/S.
2.4. Cell Treatment
To determine the comparative effects of DHMBA and gallic acid on cell growth, A549 cells or RAW264.7 cells (1 × 105/mL per well in 24-well plates) were cultured in DMEM containing 10% FBS and 1% P/S with either vehicle control (1% ethanol as a final concentration) or with DHMBA (0.1, 1, 10, 100, or 250 μM) or gallic acid (0.1, 1, 10, 100, or 250 μM) for 3 days after reaching subconfluence, in a water-saturated atmosphere containing 5% CO2 and 95% air at 37 °C [9,10]. In another experiment, to determine the effects of the mixture of DHMBA and gallic acid on the growth of RAW264.7 cells or A549 cells, cells were cultured in DMEM (containing 10% FBS and 1% P/S) in the presence of either vehicle (1% ethanol), DHMBA (1 μM or 10 μM) with gallic acid (1 μM or 10 μM) for 3 days.
To determine the comparative effects of DHMBA or gallic acid on cell death, RAW264.7 cells or A549 cells (1 × 105/mL per well in 24-well plates) were cultured in DMEM containing 10% FBS and 1% P/S for 3 days. Once the cells reached subconfluence, they were cultured in DMEM (containing 10% FBS and 1% P/S) in the presence of either vehicle (1% ethanol), DHMBA (0.1, 1, 10, 100, or 250 μM), or gallic acid (0.1, 1, 10, 100, or 250 μM) for an additional 24 h [9,10]. In other experiments, to determine the effects of the mixture of DHMBA and gallic acid on RAW264.7 or A549 cell death, the cells were cultured for an additional 24 h in DMEM (containing 10% FBS and 1% P/S) in the presence of either vehicle (1% ethanol), 1 μM or 10 μM DHMBA with 1 μM or 10 μM gallic acid.
The cells attached to the dish were counted after culturing, as described in the “Cell Counting” section.
2.5. Cell Counting
After culturing, the cells were detached from each well by adding a sterile solution of 0.05% trypsin plus EDTA in Ca2+/Mg2+-free PBS (0.1 mL per well; Thermo Fisher Scientific, Waltham, MA, USA) with incubation for 2 min at 37 °C as previously reported [7,8,9,10]. Then, 0.9 mL of DMEM containing 10% FBS and 1% P/S was added to each well. The medium containing the suspended cells (0.1 mL) was mixed with 0.1 mL of 0.5% trypan blue staining solution to distinguish live cells from dead cells. Viable cells were counted under a microscope (Olympus MTV-3) using a hemocytometer (Sigma-Aldrich, St. Louis, MO, USA) and a cell counter (Line Seiki H-102P, Tokyo, Japan). Cells stained dark blue or light blue by trypan blue staining were not counted. For each well of the culture plate, the two counts were taken from the same suspended cells and averaged. The total number of cells counted per well is shown in the corresponding figure bar. Results are presented as mean ± SD from a total of 8 wells with different cell preparations.
2.6. Statistical Analysis
The data are presented as the mean ± standard deviation (SD). Statistical significance was estimated using GraphPad InStat version 3 for Windows XP (GraphPad Software Inc., La Jolla, CA, USA). A Student’s t-test was used to calculate the statistical significance between the two groups. Multiple comparisons were performed using one-way analysis of variance (ANOVA) with Tukey–Kramer multiple comparison post hoc test for parametric data. A p-value of less than 0.05 was considered statistically significant.
3. Results
3.1. Comparative Effects of DHMBA and Gallic Acid on the Growth and Death of Mouse Macrophage RAW264.7 Cells
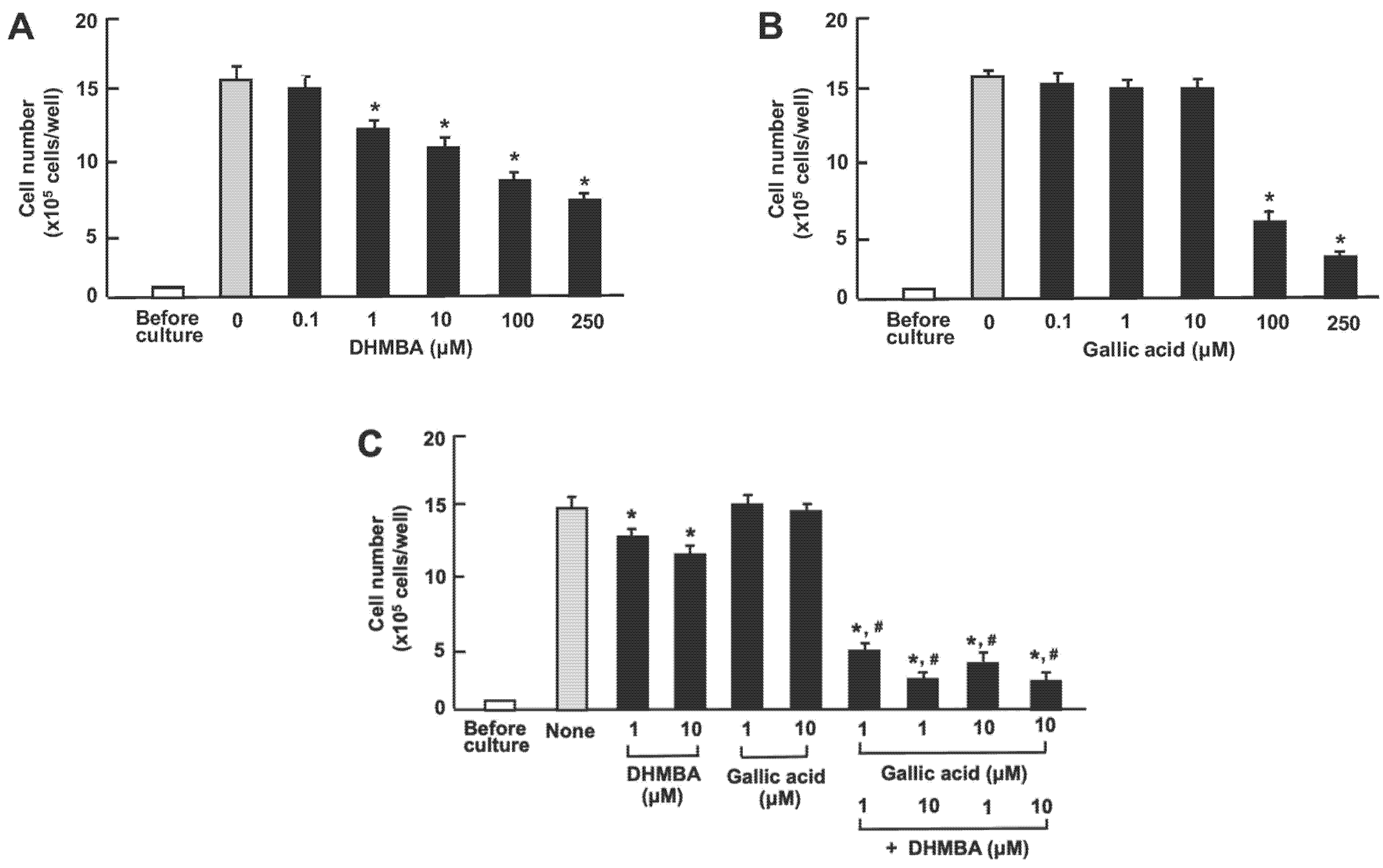
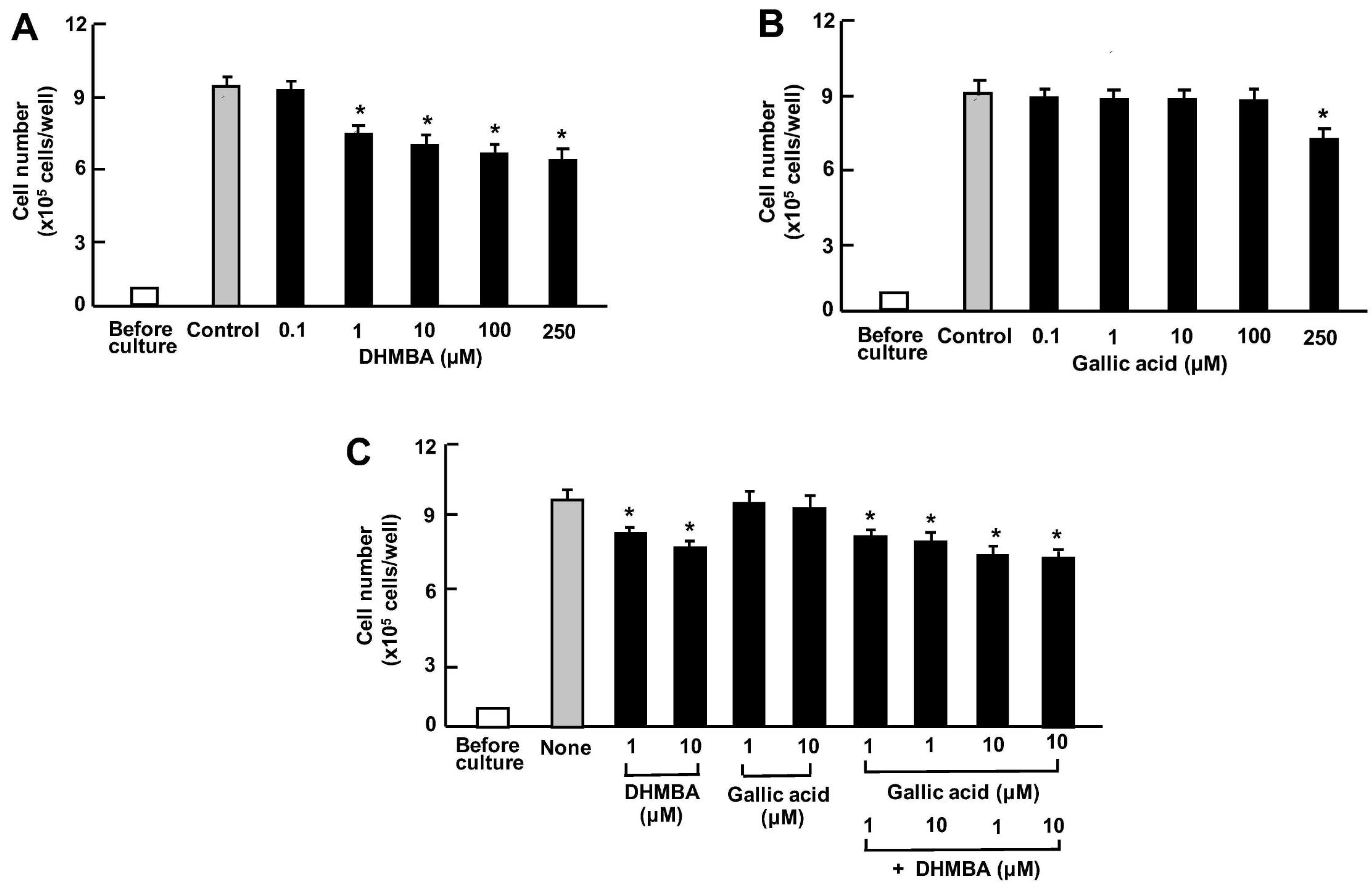
First, we examined the impact of DHMBA and gallic acid on the growth of the mouse RAW264.7 macrophage in vitro. The cells were cultured for 3 days after reaching subconfluence, in the presence of either vehicle (1% ethanol), DHMBA (0.1, 1, 10, 100, or 250 μM) (Figure 2A), or gallic acid (0.1, 1, 10, 100, or 250 μM) (Figure 2B). RAW264.7 cell growth was suppressed by culturing with DHMBA (1, 10, 100, or 250 μM) or gallic acid (100 or 250 μM), indicating DHMBA’s more potent effect compared to gallic acid. We also determined the combined effects of DHMBA (1 or 10 μM) or gallic acid (1 or 10 μM) on cell growth after culturing for 3 days (Figure 2C). A significant decrease in cell growth was observed with the combined use of DHMBA (1 or 10 μM) and gallic acid (1 or 10 μM), which alone did not have a significant effect (Figure 2C). Thus, the combined DHMBA and gallic acid at comparatively lower concentrations were found to potently suppress the growth of RAW264.7 cells in vitro. Compared with the untreated control and vehicle controls presented in the Figure, these cell numbers did not change.
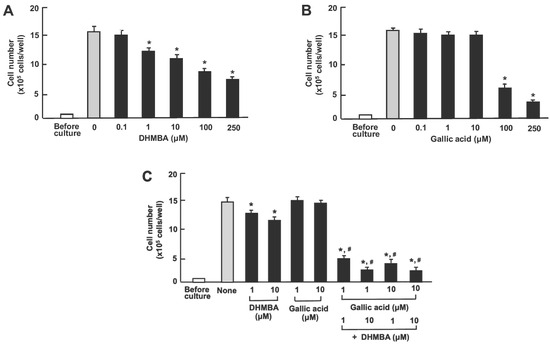
Figure 2.
Effects of DHMBA and gallic acid on the growth of mouse macrophage RAW264.7 cells in vitro. (A,B): Cells were cultured for 3 days in the presence of either vehicle only, DHMBA (A), or gallic acid (B) at a final concentration of 0.1, 1, 10, 100, or 250 µM, respectively. (C) Cells were cultured for 3 days in the presence of either vehicle, DHMBA (1 or 10 µM), or gallic acid (1 or 10 µM) with or without combined DHMBA or gallic acid. After culturing, the number of cells attached to the dish was counted. Data are presented as the mean ± SD of 8 wells with different cell preparations. Compared with the untreated control and vehicle controls (presented in the figure), these cell numbers did not change. * p < 0.001 versus the control group (grey bar). # p < 0.001 versus the DHMBA group or gallic acid group. 1-way ANOVA, Tukey–Kramer post-test.
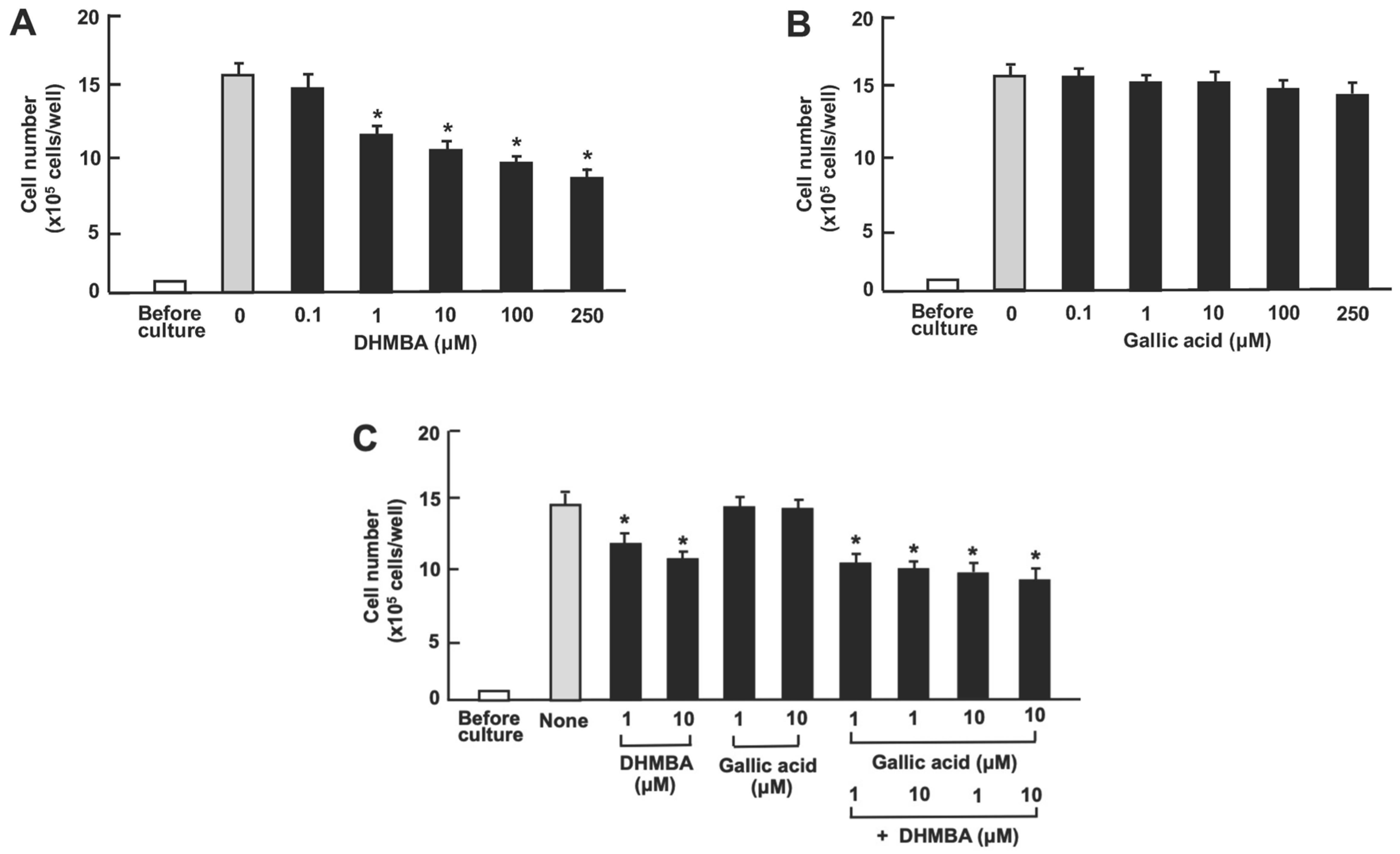
Next, we examined the impact of DHMBA and gallic acid on the viability of RAW264.7 mouse macrophages in vitro. The cells were cultured for 3 days after reaching subconfluence. Then, they were cultured further with either vehicle (1% ethanol), DHMBA (0.1, 1, 10, 100, or 250 μM) (Figure 3A), or gallic acid (0.1, 1, 10, 100, or 250 μM) (Figure 3B). RAW264.7 cell growth was suppressed by culturing with DHMBA (1, 10, 100, or 250 μM). Culturing with gallic acid (0.1, 1, 10, 100, or 250 μM) did not significantly affect cell death (Figure 3B). Furthermore, we examined the combined effects of DHMBA (1 or 10 μM) or gallic acid (1 or 10 μM) on cell death at subconfluence 24 h after adding combined compounds (Figure 3C). The stimulatory effects of DHMBA (1 or 10 μM) on cell death were not potentiated by the addition of gallic acid (1 or 10 μM) (Figure 3C).
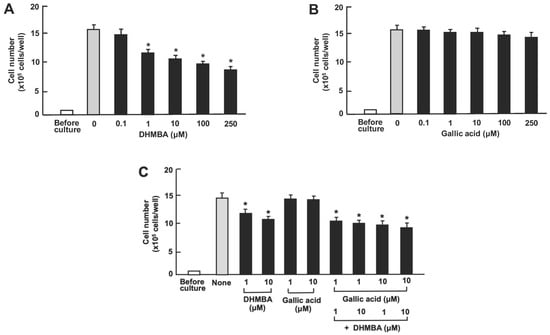
Figure 3.
Effects of DHMBA and gallic acid on the death of mouse macrophage RAW264.7 cells in vitro. (A,B): Cells were cultured for 3 days and then cultured for an additional 24 h in the presence of DHMBA (A), or gallic acid (B) at final concentrations of 0.1, 1, 10, 100, or 250 µM, respectively. (C) The cells were cultured for 3 days and then cultured for an additional 24 h in the presence of either vehicle, DHMBA (1 or 10 µM), or gallic acid (1 or 10 µM), a combination of DHMBA (1 or 10 µM) and gallic acid (1 or 10 µM). After incubation, the number of cells attached to the dish was counted. Data are presented as the mean ± SD of 8 wells with different cell preparations. Compared with the untreated control and vehicle controls (presented in the Figure), these cell numbers did not change. * p < 0.001 versus the control group (grey bar). 1-way ANOVA, Tukey–Kramer post-test.
3.2. Comparative Effects of DHMBA and Gallic Acid on the Growth and Death of Human Lung Cancer A549 Cells
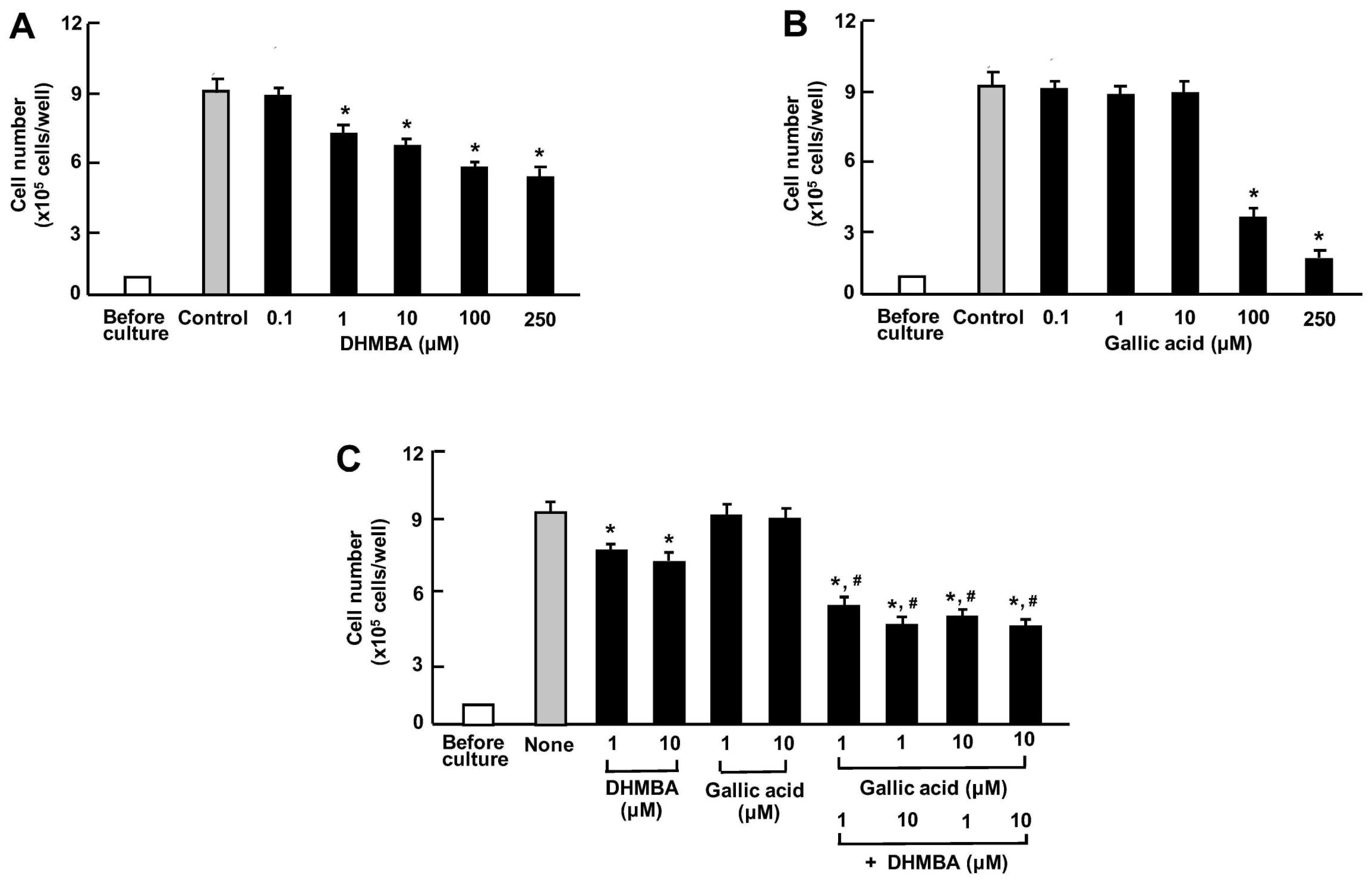
We compared the effects of DHMBA and gallic acid on the growth of human lung cancer A549 cells in vitro. The cells were cultured for 3 days after reaching subconfluence in the presence of either vehicle (1% ethanol), DHMBA (0.1, 1, 10, 100, or 250 μM) (Figure 4A), or gallic acid (0.1, 1, 10, 100, or 250 μM) (Figure 4B). A549 cell growth was suppressed by culturing with DHMBA (1, 10, 100, or 250 μM) or gallic acid (100 or 250 μM), indicating a stronger effect of DHMBA compared to gallic acid. Furthermore, we examined the combined effect of DHMBA (1 or 10 μM) or gallic acid (1 or 10 μM) on cell growth after culturing for 3 days (Figure 4C). A significant decrease in cell growth was observed with the combined use of DHMBA (1 or 10 μM) and gallic acid (1 or 10 μM), which alone did not have a significant effect (Figure 4C). Thus, the combined DHMBA and gallic acid at comparatively lower concentrations were found to potently suppress A549 cells growth.
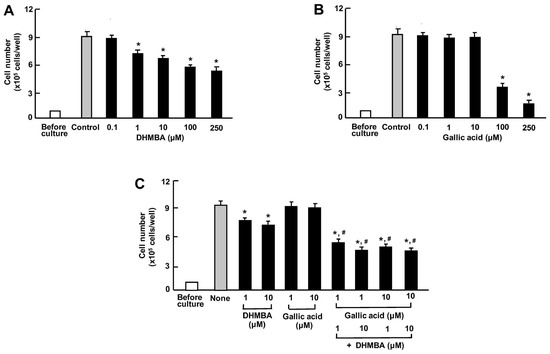
Figure 4.
Effects of DHMBA and gallic acid on the growth of human lung cancer A549 cells in vitro. (A,B): Cells were cultured for 3 days after reaching subconfluence in the presence of either vehicle, DHMBA (A), or gallic acid (B) at final concentrations of 0.1, 1, 10, 100, or 250 µM, respectively. (C) Cells were cultured for 3 days in the presence of either vehicle, DHMBA (1 or 10 µM), or gallic acid (1 or 10 µM) with or without combined DHMBA and gallic acid. After incubation, the number of cells attached to the dish was counted. Data are presented as the mean ± SD of 8 wells with different cell preparations. * p < 0.001 versus the control group (grey bar). # p < 0.001 versus the DHMBA or gallic acid alone group. 1-way ANOVA, Tukey–Kramer post-test.
Furthermore, we compared the effects of DHMBA or gallic acid on A549 cell death in vitro. The cells were cultured for 3 days after reaching subconfluence. Then, they were cultured further with either vehicle (1% ethanol), DHMBA (0.1, 1, 10, 100, or 250 μM) (Figure 5A), or gallic acid (0.1, 1, 10, 100, or 250 μM) (Figure 5B). The growth of A549 cells was suppressed by culturing them with DHMBA (1, 10, 100, or 250 μM). Culturing with gallic acid (0.1, 1, 10, or 100 μM) did not significantly affect cell death (Figure 5B). Furthermore, we determined the combined effects of DHMBA (1 or 10 μM) or gallic acid (1 or 10 μM) on cell death at subconfluence 24 h after adding the compounds (Figure 5C). The stimulatory effects of DHMBA (1 or 10 μM) on cell death were not potentiated by the addition of gallic acid (1 or 10 μM) (Figure 5C).
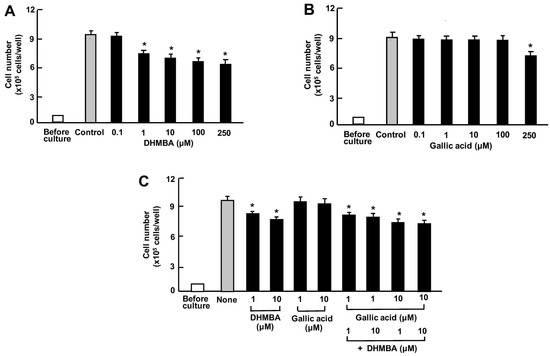
Figure 5.
Effects of DHMBA and gallic acid on the death of human lung cancer A549 cells in vitro. (A,B): Cells were cultured for 3 days and then cultured for an additional 24 h in the presence of DHMBA (A), or gallic acid (B) with final concentrations of 0.1, 1, 10, 100, or 250 µM, respectively. (C) The cells were cultured for 3 days and then cultured for an additional 24 h in the presence of either vehicle, DHMBA (1 or 10 µM), gallic acid (1 or 10 µM), or a combination of DHMBA (1 or 10 µM) and gallic acid (1 or 10 µM). After incubation, the number of cells attached to the dish was counted. Data are presented as the mean ± SD of 8 wells with different cell preparations. * p < 0.001 versus the control group (grey bar). 1-way ANOVA, Tukey–Kramer post-test.
4. Discussion
It is well known that antioxidants play a crucial role in preventing disease, including those associated with inflammation, diabetes, and cancer [1,2]. DHMBA is a potent, novel antioxidant that can block oxidative stress by acting as a radical scavenger in various cells [3,4]. Recently, it has been demonstrated that DHMBA plays a novel pharmacological role by inhibiting the activity of inflammatory macrophages and cancer cells [8,10]. Meanwhile, gallic acid, which is widely distributed in edible plants, exhibits potent antioxidant properties [11,12]. Gallic acid has been shown to have several pharmacological activities, and it may be a potential dietary supplement due to its health benefits [11,12]. The molecular weights of DHMBA and gallic acid are identical, indicating their chemical similarity. In the current study, we compared the effects of DHMBA and gallic acid on RAW264.7 mouse macrophages and A549 human lung cancer cells in vitro. In these model cells, we demonstrated that, at a comparatively lower concentration, DHMBA exerted a more potent effect on suppressed proliferation and stimulated cell death than gallic acid, resulting in a decrease in cell number. DHMBA may be effective than gallic acid in the modeled cells used, supporting the view that this compound is a potent antioxidant that regulates cellular function. As shown in Figure 1, it is hypothesized that the methoxy group plays an important role in cell regulation. DHMBA may have pharmacological effects in various diseases [7,8,9,10].
Notably, we found that comparatively lower concentrations of the combined antioxidants DHMBA and gallic acid, at 1 or 10 µM, exerted a potent effect in suppressing the proliferation of RAW264.7 macrophages and A549 lung cancer cells, suggesting a synergistic effect. However, the combined effect was not potent enough to induce cell death, although DHMBA significantly stimulated the death of both cell types in the presence of gallic acid. Further research is needed to elucidate the mechanism by which the combination of DHMBA and gallic acid exhibits a synergistic effect on suppressing cell growth. DHMBA (10 μM) reduced the levels of key proteins that promote cell growth, including Ras, PI3K, Akt, MAPK, and mTOR in prostate cancer PC-3 cells [10]. Additionally, DHMBA increased the levels of tumor suppressors p53, p21, Rb, and regucalcin [10]. Gallic acid has been shown to exert anti-cancer effects [17,18,19,20]. At higher concentrations than 100 μM, gallic acid has been shown to suppress cancer progression by inducing cell cycle arrest and apoptosis in A549 lung cancer cells via the PI3K/AKT pathway, which is involved in cell proliferation signalling in vitro [20]. The combined effects of DHMBA and gallic acid on cell proliferation may be mediated by signalling pathways in cells. Mechanistic studies remain to be conducted.
A comparatively lower concentration of the combination of DHMBA and gallic acid was found to have a synergistic effect on the growth of RAW264.7 macrophages and A549 lung cancer cells in vitro. It is currently unknown whether this effect occurs in activated macrophages under inflammatory conditions. DHMBA has been shown to inhibit the proliferation of macrophages stimulated with lipopolysaccharide, which is associated with inflammation [8,9]. We speculate that the proliferation of activated macrophages is inhibited by the combination of DHMBA and gallic acid.
DHMBA exhibits anticancer effects in several human cancer cell lines in vitro [10]. The combination of DHMBA and gallic acid has been found to have a synergistic effect on A549 lung cancer cells. Furthermore, the combination of DHMBA and gallic acid potently suppress lung cancer carcinogenesis in vivo at lower doses. This remains to be elucidated in animal studies. Several cancer drugs have toxic side effects. The combination of the strong antioxidant DHMBA and gallic acid may be an effective cancer treatment.
In conclusion, this preliminary study compared the effects of DHMBA and gallic acid, both of which are strong antioxidants with a similar structure, on proliferation and cell death using modelled cells (RAW264.7 macrophages or A549 human lung cancer cells) in vitro. DHMBA was found to be more potent than gallic acid in terms of cell proliferation and death. Interestingly, we found that DHMBA, at comparatively lower concentrations, exerted synergistic effects on suppressed proliferation and stimulated cell death of these cells, resulting in decreased cell numbers.
Author Contributions
M.Y.: Conceptualization, data curation, formal analysis, funding acquisition, investigation, methodology, project management, resources, software, supervision, validation, visualization, writing—original draft, writing—review and editing. K.Y.: Research, resources, writing—review and editing. E.M.: Research, resources, writing—review and editing. H.W.: Research, resources, writing—review and editing. M.W.: Fundraising, research, project, management, administration, resources, supervision, validation, visualisation, writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported in part by funds from the University of Hawaii Cancer Center (M.Y.) and the Foundation of Watanabe Oyster Laboratory (M.W.). We did not have a grant number.
Institutional Review Board Statement
This article does not include any human or animal studies performed by any of the authors. All experimental protocols used databases or in vitro cell culture.
Data Availability Statement
The datasets used in this study are available from the corresponding author upon reasonable request.
Conflicts of Interest
Authors Kenji Yoshiike, Emiko Miki, Hideaki Watanabe and Mitsugu Watanabe were employed by the company Watanabe Oyster Laboratory Co., Ltd. Mitsugu Watanabe received research grants from The Foundation of Watanabe Oyster Laboratory. The funding sponsor had no role in the design of the study; in the collection, analyses, or inter-pretation of data; in the writing of the manuscript; or in the decision to publish the results. All authors declare that they have no known competing financial interests or personal relationships that could be perceived as influencing the work reported in this paper.
References
- Rosa, A.; Pujia, A.M.; Arcuri, C. The Protective Role Antioxidant of Vitamin C in the Prevention of oral Disease: A Scoping Review of Current Literature. Eur. J. Dent. 2024, 18, 965–970. [Google Scholar] [CrossRef] [PubMed]
- Asplund, K. Antioxidant vitamins in the prevention of cardiovascular disease: A systematic review. J. Intern. Med. 2002, 251, 372–392. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, M.; Fuda, H.; Jin, S.; Sakurai, T.; Ohkawa, F.; Hui, S.-P.; Takeda, S.; Watanabe, T.; Koike, T.; Chiba, H. Isolation and Characterization of a Phenolic Antioxidant from the Pacific Oyster (Crassostrea gigas). J. Agric. Food Chem. 2012, 60, 830–835. [Google Scholar] [CrossRef] [PubMed]
- Fuda, H.; Watanabe, M.; Hui, S.-P.; Joko, S.; Okabe, H.; Jin, S.; Takeda, S.; Miki, E.; Watanabe, T.; Chiba, H. Anti-apoptotic effects of novel phenolic antioxidant isolated from the Pacific oyster (Crassostrea gigas) on cultured human hepatocytes under oxidative stress. Food Chem. 2015, 176, 226–233. [Google Scholar] [CrossRef] [PubMed]
- Villuendas-Rey Y, Alvarer-Idaboy JR, Galano A:Assessing the protective activity of a recently discovered phenolic com-poundagainst oxidative stress using computational chemistry. J. Chem. Inf. Model. 2015, 55, 2552–2561. [CrossRef] [PubMed]
- Ho, H.-J.; Aoki, N.; Wu, Y.-J.; Gao, M.-C.; Sekine, K.; Sakurai, T.; Chiba, H.; Watanabe, H.; Watanabe, M.; Hui, S.-P. A Pacific Oyster-Derived Antioxidant, DHMBA, Protects Renal Tubular HK-2 Cells against Oxidative Stress via Reduction of Mitochondrial ROS Production and Fragmentation. Int. J. Mol. Sci. 2023, 24, 10061. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, M.; Yoshiike, K.; Watanabe, H.; Watanabe, M. The Marine Factor 3,5-Dihydroxy-4-methoxybenzyl Alcohol Represses Adipogenesis in Mouse 3T3-L1 Adipocytes In Vitro: Regulating Diverse Signaling Pathways. Nutraceuticals 2023, 3, 366–379. [Google Scholar] [CrossRef]
- Yamaguchi, M.; Yoshiike, K.; Watanabe, H.; Watanabe, M. The Marine Factor 3,5-dihydroxy-4-methoxybenzyl Alcohol Suppresses Cell Growth, Inflammatory Cytokine Production, and NF-κB Signaling-enhanced Osteoclastogenesis in In vitro Mouse Macrophages RAW264.7 Cells. Curr. Mol. Med. 2024, 24, 813–825. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, M.; Yoshiike, K.; Watanabe, H.; Watanabe, M. The marine factor 3,5-dihydroxy-4-methoxybenzyl alcohol prevents the impairment of mineralization via TNF-α signaling in mouse osteoblastic MC3T3-E1 cells: Implication of macrophage activation. Chem. Biol. Interact. 2024, 390, 110871. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, M.; Yoshiike, K.; Watanabe, H.; Watanabe, M. The marine factor 3,5-dihydroxy-4-methoxybenzyl alcohol suppresses growth, migration and invasion and stimulates death of metastatic human prostate cancer cells: Targeting diverse signaling processes. Anti-Cancer Drugs 2022, 33, 424–436. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Z.; Guan, H.; Zhao, X.; Xie, Q.; Xie, Z.; Cai, F.; Dang, R.; Li, M.; Wang, C. Dietary gallic acid as an antioxidant: A review of its food industry applications, health benefits, bioavailability, nano-delivery systems, and drug interactions. Food Res. Int. 2024, 180, 114068. [Google Scholar] [CrossRef] [PubMed]
- Harwansh, R.K.; Deshmukh, R.; Shukla, V.P.; Khunt, D.; Prajapati, B.G.; Rashid, S.; Ali, N.; Elossaily, G.M.; Suryawanshi, V.K.; Kumar, A. Recent Advancements in Gallic Acid-Based Drug Delivery: Applications, Clinical Trials, and Future Directions. Pharmaceutics 2024, 16, 1202. [Google Scholar] [CrossRef] [PubMed]
- Maldonado, R.F.; Sá-Correia, I.; Valvano, M.A. Lipopolysaccharide modification in Gram-negative bacteria during chronic infection. FEMS Microbiol. Rev. 2016, 40, 480–493. [Google Scholar] [CrossRef] [PubMed]
- Abdelhamid, R.E.; Sluka, K.A. ASICs Mediate Pain and Inflammation in Musculoskeletal Diseases. Physiology 2015, 30, 449–459. [Google Scholar] [CrossRef] [PubMed]
- Slomka, J.; Berthou, H.; Mansuet-Lupo, A.; Blons, H.; Fabre, E.; Lerner, I.; Rance, B.; Alifano, M.; Chapron, J.; Birsen, G.; et al. Clinical and molecular characteristics associated with high PD-L1 expression in EGFR-mutated lung adenocarcinoma. PLoS ONE 2024, 19, e0307161. [Google Scholar] [CrossRef] [PubMed]
- Sampat, P.J.; Cortese, A.; Goodman, A.; Ghelani, G.H.; Mix, M.D.; Graziano, S.; Basnet, A. Treatment of brain metastases from non-small cell lung cancer: Preclinical, clinical, and translational research. Front. Oncol. 2024, 14, 1411432. [Google Scholar] [CrossRef] [PubMed]
- Hu, Q.; Yuan, B.; Xiao, H.; Zhao, L.; Wu, X.; Rakariyatham, K.; Zhong, L.; Han, Y.; Kimatu, B.M.; Yang, W. Polyphenols-rich extract from Pleurotus eryngii with growth inhibitory of HCT116 colon cancer cells and anti-inflammatory function in RAW264.7 cells. Food Funct. 2018, 9, 1601–1611. [Google Scholar] [CrossRef] [PubMed]
- Park, K.H.; Yin, J.; Yoon, K.H.; Hwang, Y.J.; Lee, M.W. Antiproliferative Effects of New Dimeric Ellagitannin from Cornus alba in Prostate Cancer Cells Including Apoptosis-Related S-Phase Arrest. Molecules 2016, 21, 137. [Google Scholar] [CrossRef] [PubMed]
- Meng, R.; Zhang, Z. Gallic Acid Inhibits the Proliferation and Migration of Ovarian Cancer Cells via Inhibition of the PI3K-AKT Pathway and Promoting M1-Like Macrophage Polarization. Anal. Cell. Pathol. 2025, 2025, 3880719. [Google Scholar] [CrossRef] [PubMed]
- Ko, E.-B.; Jang, Y.-G.; Kim, C.-W.; Go, R.-E.; Lee, H.K.; Choi, K.-C. Gallic Acid Hindered Lung Cancer Progression by Inducing Cell Cycle Arrest and Apoptosis in A549 Lung Cancer Cells via PI3K/Akt Pathway. Biomol. Ther. 2022, 30, 151–161. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).