Abstract
Major depressive disorder (MDD) is the most common mood disorder among adults. Despite the wide use of pharmacological agents by those with MDD, the evidence indicates that only a small fraction of patients benefits, and many individuals using antidepressant therapy relapse. Side effects are numerous with antidepressants, which can be a factor in patient medication compliance. Along with psychotherapy and fine-tuning lifestyle components, another emerging option in treating MDD is the use of bioactive natural products known as nutraceuticals. We present the scientific findings specific to select nutraceuticals (e.g., omega-3 fatty acids, S-adenosyl-methionine, folate-based compounds, and vitamin D) either as a monotherapy or as adjunctive therapy to a pharmaceutical antidepressant, for treatment of MDD. Many studies demonstrate that nutraceuticals result in a decrease in depressive symptoms with fewer side effects as traditional medications and have the potential to improve the result of antidepressants, especially in individuals experiencing resistance to medication. From a therapeutic perspective, a holistic approach incorporating psychotherapy, pharmacological therapy, and lifestyle factors (inclusive of nutraceutical use) appears most logical and could provide for enhanced treatment efficacy.
1. Introduction
According to the National Alliance of Mental Illness (NAMI), one in five US adults experience mental illness each year, with one in twenty experiencing a serious mental illness [1]. Depression and anxiety account for 8.3% and 19.1% of these illnesses, respectively. In the US, mental health diagnoses increased by close to 40% between 2019 and 2023, with the first year of the COVID-19 pandemic accounting for 25% of this increase [1]. Depression can be situational, seasonal, or chronic and recurring, but early detection and treatment can lead to more favorable treatment outcomes.
Pharmacological interventions for mental disorders such as major depressive disorder (MDD) were introduced in the 1950s [2]. While antidepressants are efficacious, nearly 50% of patients remain unable to attain remission [3]. Approximately 11% of the US population take antidepressants [1], and use of these medications have increased yearly over the last eight years [4]. The increasing prevalence of MDD and use of antidepressants, combined with the less than desirable side effects and remission rate, have given rise to the exploration of alternative treatment options.
Beginning in the mid-1990s, a specific class of nutrients referred to as “nutraceuticals” began gaining value in the adjunctive treatment of psychiatric disorders [5]. Today, the nutraceutical business is flourishing at an aggressive rate and is expected by many to become an essential part of the disease care model. To provide some context, the global nutraceuticals market was valued at USD 353 billion in 2022 and is estimated to reach USD 700 billion by 2030 [5].
Nutraceuticals can affect physiological factors that impact the onset of mood disorders including monoamines and brain-derived neurotrophic factor (BDNF) concentrations, neuroinflammation, oxidative stress, and sleep quality [3]. Due to neurobiological effects, neuroprotection, and enhancing re-uptake of inhibited monoamines, it has been emphasized that certain nutraceuticals enhance the effectiveness of medical therapy and alleviate side effects in managing mood disorders [6].
While there are numerous nutraceuticals that are consumed for their supposed mood-enhancing properties, there is a dearth of research to support this practice. Herein, the scientific findings specific to efficacy of nutraceuticals in addressing symptoms of MDD are discussed. Although multiple nutrients are claimed to have efficacy, very few have been studied in a clinical setting. Therefore, the focus of this review is on those nutrients for which a fair number of clinical evaluations have been performed, including omega-3 fatty acids, vitamin D, S-adenosyl-methionine, and folate-based compounds.
We systematically searched PubMed and Google Scholar for articles from 1999 to 2025. Included studies were published, peer-reviewed, and available in English-language journals. The articles examined nutraceuticals and the possible monotherapy and adjunctive effect with pharmaceutical antidepressants, specific to the effect on major depressive disorder.
2. Overview of Major Depressive Disorder
The Diagnostic and Statistical Manual of Mental Disorders (DSM-5) separates mood disorders into the following two categories: (1) bipolar and related disorders and (2) depressive disorders.
In terms of relevant research, where nutraceuticals are concerned, MDD is the primary mood disorder studied and is the most common in the adult population, with a prevalence of 8.4% in US adults 18 and older [1]. MDD can occur at any age but is most commonly observed in those between the teenage years and the 30s.
MDD encompasses periods of intense sadness, hopelessness, irritability, feelings of emptiness, and isolation or a loss of interest in otherwise pleasurable activities. Additionally, this disorder is often coupled with somatic symptoms, such as sleep and appetite disturbances, fatigue, and aches and pains [7]. Cognitive disturbances may accompany MDD as well. These symptoms can include difficulty concentrating, trouble remembering things, and a decline in problem solving abilities, along with complications when faced with change [8]. Of the individuals with MDD, it is estimated that two-thirds experience suicidal ideation, while 1–2 out of 30 will commit suicide [9]. MDD can be diagnosed, based on the number of symptoms an individual is experiencing, into the following three severity classifications: mild, moderate, or severe. Due to its complexity, successful treatment of MDD can be difficult.
In its infancy, MDD was thought to be caused mainly by abnormalities in neurotransmitters and receptors, such as serotonin, norepinephrine, and dopamine, but is now considered to have a more complex etiology involving biological, genetic, environmental, and psychosocial factors [9]. Data indicate that there is 30–50% chance of heritability, with 100–200 gene loci identified to likely be associated with MDD [7]. Extreme life stress can also contribute to MDD. Along with serotonin, norepinephrine and dopamine, other pathogenic factors can lead to stress-induced changes to the HPA axis, including high levels of glucocorticoids, as well as (thyroid, leptin, and/or estrogen) hormone dysregulation, inflammatory response to oxidative stress (increased inflammatory cytokines including IL-1β, IL-6, IFN-γ, and TNF-α), changes to neurotrophic factors (BDNF and GDNF), inflammasomes, and structural and functional changes in the brain and its communication with other organs (neuroendocrine–immune axis, gut–brain axis, and liver–brain axis).
Suicide is a significant risk with depression and is the mental health condition most frequently associated with MDD. In fact, a major depressive episode is found in 59–87% of suicide victims in the general population. About two-thirds of individuals with MDD contemplate ending their life, and about 10–15% die by suicide [10]. Unfortunately, many individuals cannot afford medication used to combat MDD. Therefore, it would be advantageous to identify more affordable and accessible options, such as nutraceuticals, which can be obtained over the counter [11].
3. Treatments for Major Depressive Disorder
3.1. Pharmaceuticals
Pharmacological treatments for MDD can include selective serotonin reuptake inhibitors (SSRIs), serotonin/norepinephrine reuptake inhibitors (SNRIs), serotonin modulators, tricyclic antidepressants (TCAs), and monoamine oxidase inhibitors (MAOIs) [9]. Other options include mood stabilizers and antipsychotics, which can be used to enhance the effect of an antidepressant [9]. While SSRIs are the most prescribed medication, no single antidepressant will work for everyone. Antidepressants improved symptoms for approximately 20% of treated patients and prevented relapse in 27% [12]. While antidepressants are efficacious, half of users experience side effects, particularly at the beginning of treatment. Among these side effects are digestive issues (e.g., diarrhea, constipation, and nausea), as well as difficulty urinating, headaches, dizziness, vision issues, and insomnia. Severe side effects include broken bones from falls, heart problems, epilepsy, liver damage, and suicide [12,13]. Patients and providers should work together to monitor side effects to determine whether changes in medication are needed [13].
3.2. Psychotherapy
Psychotherapy, much like pharmacotherapy, is tailored to the individual and may require several sessions to see benefits. Cognitive-behavioral therapy and interpersonal therapy are two of the most common therapeutic approaches for MDD [9]. Cognitive-behavioral therapy focuses on challenging negative thoughts and behaviors, while interpersonal therapy aims to improve relationships and communication skills.
While early research found a combination of pharmacological and psychotherapeutic intervention provided the best chance for remission of MDD [14], more recent meta-analyses indicate that these improvements are minor over the individual intervention types [15]. Combination treatment, however, may increase the likelihood of achieving remission and may also support adherence. In addition, continuing to maintain combination treatment may have the benefits of reducing recurrence and improving quality of life [15].
3.3. Lifestyle Factors
First-line antidepressants are not always effective, and alternative treatment options need to be considered [16]. Although treatment-resistant depression is not well defined, research suggests the condition is quite common and more effective treatment regimens are needed [17]. Aside from pharmacological treatment and psychotherapy, patients and clinicians often look to adjust lifestyle factors as a method of improving mental health. Specifically, regular exercise, consumption of a well-balanced and nutrient-dense diet, improved sleep habits, and a robust spiritual life can help to mitigate many symptoms of affective depressive disorders and lessen or negate the need for pharmaceutical treatment.
3.4. Exercise
Exercise can be both an effective monotherapy and adjunctive treatment for MDD [18]. Along with improving psychosocial functioning [19,20], exercise also reduces inflammation, increases production of endorphins, improves sleep, and enhances self-esteem and social support [21]. Greer (2016) found in individuals diagnosed with non-remitted MDD and currently taking antidepressants that low and high dose exercise habits led to improvements in functioning as well as psychosocial and quality of life measures [22].
3.5. Dietary Intake
A healthy, well-balanced, and nutrient-dense diet has been shown to improve symptoms of depression and anxiety and to decrease the risk of developing either. Diets high in trans fatty acids, high in refined carbohydrates, and low in protein have been associated with MDD [23], which might be attributed to pro-inflammatory properties of refined foods [24]. Increasing consumption of vegetables, fruits, fish, and whole grains has been shown to lower the risk of depression [25], and nutritional imbalances with inadequate intake of omega-3 polyunsaturated fats, vitamin D, folate, iron, and zinc increase the risk for depression [16]. In a systematic review by Swainson of five studies examining various diets, the Mediterranean diet showed the most favorable results, with statistically significant reductions in depression and higher remission rates [26]. Ortega found similar results regarding the Mediterranean diet but also stressed that inclusion of a variety of nutrients and consistent adherence is equally important as the type of diet [27]. Finally, a meta-analysis by Lassale (2018) determined that the Mediterranean diet and avoidance of a pro-inflammatory diet resulted in a reduced risk of depression [28].
3.6. Sleep
There is consensus that a reciprocal relationship exists between sleep, depression, and anxiety. Sleep disruptions can activate the inflammatory response, decrease serotonin production, and affect other factors in the pathophysiology of psychiatric conditions [29]. Over time, the longer sleep is interrupted, the greater the risk of developing or prolonging depression and anxiety [30]. For example, Ngyuyen (2022) followed subjects with depression and anxiety long-term and found higher depression and anxiety scores at baseline predicted lower sleep quality, with this trend continuing at both 9 month and 18 month follow-ups [31]. Sleep quality should, therefore, be prioritized in the treatment and mediation of depression and anxiety.
3.7. Spirituality
Studies have shown that prayer (spirituality) can provide protective factors from depression [32]. These practices can decrease activity in the sympathetic nervous system while increasing parasympathetic activity, creating a calm and relaxed state [33]. Additionally, prayer can complement treatment progress, especially when the method is aligned with one’s values and beliefs [34]. Boelens (2012) found that women engaging in six weekly 1 h prayer sessions for one month had decreased symptoms of depression even at a one-year follow-up [35]. Rickhi (2011) conducted an 8-week study with subjects either implementing a home-based spirituality teaching program or being placed on a waitlist that would start the spirituality teaching program at week 9 [36]. At the 8-week mark, depression severity improved significantly more in the active group as did remission rates, which endured throughout the 24-week period. These findings suggest that spirituality or prayer, when tailored to an individual’s beliefs, can be a positive support to other MDD treatment protocols.
4. Nutraceuticals for Treatment of MDD
Doctor Stephen DeFelice (2003) defines a nutraceutical as a food (or part of a food) that provides medical or health benefits, including the prevention and/or treatment of a disease [37]. Nutraceuticals were introduced several decades ago to address nutrient deficiencies associated with mental health, to support the effects of pharmaceutical agents, and to provide options for individuals not yet open to taking medication or those with very minor severities of depression, anxiety, etc. [5]. Although not regulated in the same manner as pharmaceuticals, nutraceuticals typically have far fewer side effects compared to pharmaceutical antidepressants and are thought to be an avenue for titrating off prescribed medications [38]. Despit current evidence showing promise for treating MDD with the limited group of nutraceuticals discussed herein, further clinical research is needed to fully understand their effects, establish optimal dosing, and explore potential combinational usage.
In several trials, the nutraceuticals that have been scientifically tested as an adjunctive therapy for MDD include omega-3, S-adenosylmethionine (SAMe), vitamin D, and L-methylfolate [5,39]. Other agents examined include creatine, curcumin, N-acetyl cysteine (NAC), and pre- and probiotics. However, only a few studies have been conducted, and these nutraceuticals lack consistency in research results.
Juneja (2024) wrote a literature review on creatine supplementation in depression and determined that larger sample sizes, uniformity in methodologies, and further dose–response trials are needed to determine creatine’s effects [40]. Curcumin studies similarly lack adequate sample sizes and randomly controlled trials. Wang’s (2021) meta-analysis found (1) no effect on depressive symptoms, (2) too few small studies included and were underpowered, and (3) the dosage of curcumin in clinically depressed patients was larger than the patients with subclinical depression. Clinical depression has more severe symptoms, and those with mildest clinical effects receive the smallest benefit; (4) all the patients with clinical depression were treated with antidepressants and curcumin at the same time, while patients with subclinical depression were only treated with curcumin [41]. Randomly controlled NAC trials are also limited, and a meta-analysis concluded that NAC supplementation, while promising for its adjunctive use in schizophrenia, lacked efficacy in MDD [42]. Finally, pre- and probiotics have been studied in this area due to findings that the gut microbial profile in individuals with MDD is different compared to healthy individuals, but additional trials are needed to address species, age, diet, and variations in medication use [43]. As previously mentioned, numerous nutraceuticals are touted to benefit mental health, but only a few (omega-3, SAMe, folate, and vitamin D) currently have sufficient scientific support to warrant consideration. These are discussed in more detail below.
4.1. Omega-3 Fatty Acids
Although there are several types of omega-3 fatty acids, the three most prevalent include eicosapentaenoic acid (EPA), docosahexaenoic acid (DHA), and alpha-linolenic acid (ALA). Of these, ALA is considered essential because the body cannot produce ALA and it must be acquired from food or supplemental forms (note: both EPA and DHA can be synthesized from ALA but due to low conversion efficiency, it is recommended that both are consumed). These polyunsaturated fatty acids (PUFAs) are found in fish and other seafood, particularly salmon and mackerel, but are also found in plants such as flax and chia seeds, and they are widely available in supplement form. The Western diet has evolved in such a way that many individuals are highly deficient in omega-3 fatty acids in favor of omega-6 fatty acids [44]. Omega-3s have beneficial antioxidant, anti-inflammatory, and antithrombotic effects. This knowledge has been an impetus behind researching the relationship between omega-3 fatty acids and mood disorders, as depression is associated with a chronic, low-grade inflammatory response, as well as activation of the compensatory anti-inflammatory reflex system [45]. Epidemiologic data implicate omega-3 fatty acid deficiencies in many mental illnesses [44]. A longitudinal study found that the ratio of 6 to 3 PUFAs is positively associated with mood disorders in young individuals at ultra-high risk [46].
EPA and DHA have both been demonstrated to play a role in brain function and development. Total omega-3 fatty acids, as well as EPA and DHA, are lower in individuals with depression [47]. Omega-3 fatty acids have similar functionality to pharmaceutical antidepressants, as both target inflammation and oxidative stress. Antidepressants target inflammation by decreasing levels of pro-inflammatory cytokines, specifically IL-6 and TNF-α, while increasing the levels of the anti-inflammatory cytokine IL-10 [48]. Omega-3s target inflammation through a variety of mechanisms, including leukocyte hemotaxiss, a part of the body’s immune response [49].
4.1.1. Omega-3 Fatty Acids Animal Studies
One mechanism by which omega-3 fatty acids may improve symptoms of MDD is by altering the activation of the hypothalamic–pituitary–adrenal axis (HPA axis). The HPA axis refers to a chemical messenger system in both animals and humans that facilitates and regulates neurobiological response to stimuli in the environment, shifting protective mechanisms and aiding in the body returning to homeostasis [50]. Diet deficiencies of omega-3 fatty acids observed in rodent studies resulted in a decrease in glucocorticoid receptors and elevated corticosterone secretion due to stress; both outcomes were improved by addition of omega-3 fatty acids [51].
Other research has focused on the role that omega-3 fatty acids play in brain function. Rats fed omega-3 PUFA-deficient diets were found to have altered dopaminergic neurotransmission within the frontal cortex, as well as the nucleus accumbens, an area of the brain that plays a vital role in cognitive and affective functioning [52,53,54]. Rats fed diets lacking DHA subsequently experience lower levels of DHA in brain lipids, with various changes in brain function, including changes in learning and memory, responses to stimuli, and nerve growth factor [55].
Alterations in behavior related to depression were also studied [56,57,58]. Of note, DeMar (2006) found that this type of DHA deprivation showed an increase in depression and aggression test scores in male rat pups [56]. These animal studies demonstrate that dietary levels of omega-3s are strongly related with depressive behaviors.
4.1.2. Omega-3 Fatty Acids Monotherapy Clinical Studies
The omega-3 fatty acids EPA and DHA are of scientific interest due to their potential link to psychiatric health. Although they differ in bioactivity, EPA and DHA share anxiolytic and antidepressant properties [59]. Unlike other nutraceuticals, there are a plethora of randomly controlled trials, systematic reviews, and meta-analyses. However, conclusions from these studies are conflicting, and it is likely, as with most nutraceuticals, that certain individuals are responders to treatment while others are not. For example, a meta-analysis suggested that EPA has a greater effect on symptoms than DHA, regardless of use as a monotherapy or adjunctive therapy [60]. In contrast, a separate literature review found very low certainty in the literature and opined that omega-3 fatty acids have no significant effect on MDD [61].
Depression not only manifests emotionally and behaviorally but also physically through inflammatory responses. Mischoulon (2022) conducted a dose-finding study on subjects with MDD, a body mass index > 25 kg/m2, and plasma high-sensitivity C-reactive protein hs-CRP ≥ 3.0 mg/L [62]. Subjects were randomly assigned 1 g, 2 g, or 4 g EPA or a placebo daily for 12 weeks. After 12 weeks, median plasma hs-CRP levels decreased in a dose-dependent manner with the three EPA dosages compared to a minimal decrease in the placebo group. However, no significant changes were found in IL-6 or LPS-stimulated TNF. While all EPA groups displayed sustained improvement in depressive symptoms through the 12 weeks as assessed by the Inventory of Depressive Symptomatology, Clinician-Rated version (IDS-C30), the results showed the highest response rate in the 4 g per day group, with a 64% improvement at 12 weeks. These findings are promising and deserve attention.
Marangell (2003) also studied monotherapy of omega-3 fatty acid using only 2 g DHA daily compared to a placebo. Response was defined a priori as a ≥50% reduction in the score on the Montgomery–Åsberg Depression Rating Scale. Thirty-five participants were evaluable; eighteen received DHA, and seventeen received placebo. The response rates were 27.8% in the DHA group and 23.5% in the placebo group. The difference in response rates among groups did not reach statistical significance [63]. This trial failed to show a significant effect of DHA monotherapy in subjects with major depression at the dosage of 2 g daily. Another consideration that could be clarified in future studies is the potential differences in efficacy for EPA and DHA. EPA exhibited greater efficacy when compared to DHA in [64], but no difference was observed in [65].
4.1.3. Omega-3 Fatty-Acids Adjunctive-Therapy Clinical Studies
While monotherapy use of omega-3 fatty acid shows some promise in alleviating depressive symptoms, it may also be advantageous as an adjunctive therapy to pharmaceutical antidepressants. The Omega-3 Fatty Acid Subcommittee, assembled by the Committee on Research on Psychiatric Treatments of the APA, advises that patients with mood, impulse-control, or psychotic disorders should consume 1 g EPA + DHA per day. A supplement may be useful in patients with mood disorders, with a wide range of dosages (1–9 g per day) [66]. Jazayeri (2008) compared the therapeutic effects in MDD of 1000 mg EPA + fluoxetine placebo, 20 mg fluoxetine + EPA placebo, and a combination of 1000 mg EPA + 20 mg fluoxetine. Depression was evaluated via the Hamilton Depression Rating Scale (HAM-D) and subjects were assessed every two weeks [67]. The results showed the fluoxetine + EPA was significantly better than fluoxetine or EPA individually. Fluoxetine and EPA monotherapies appeared to be equally effective in controlling depressive symptoms. Response rates were a 50%, 56%, and 81% decrease in the fluoxetine, EPA, and combinations groups, respectively. In another adjunctive study, Gertsik (2012) evaluated the effectiveness over 8 weeks of 900 mg EPA + 200 mg DHA + 100 mg other omega-3 fatty acids + 20 g citalopram versus antidepressant monotherapy control, 2 g olive oil + 20 g citalopram [68]. Positively, this study also included a one-week, single-blind, and placebo run-in phase to assess for any placebo effects in the HAM-D scores (used to evaluate changes in depressive symptoms) from baseline testing. The results indicated that beginning at 4 weeks of adjunctive treatment with citalopram and omega-3, a significantly greater reduction in HAM-D scores was found compared to citalopram treatment alone. However, there were no changes in plasma CRP or 24 h urinary cortisol levels over the study period.
The research reviewed here has strong indications that EPA, as an adjunctive therapy, is promising, but dosing could be dependent on individual factors, such as length of time since diagnosis of depression, inflammatory status, and current intake of omega-3. When referencing the cited articles, dosages vary between 0.9 g and 9 g per day, both in monotherapy and adjunctive uses. It could also be suggested that the addition of a placebo run-in in studies could be greatly useful in clarifying results.
4.2. S-Adenosylmethionine (SAMe)
SAMe is a compound found naturally in the body that acts as a methyl donor in the production and breakdown of chemicals, such as serotonin, dopamine, noradrenaline, and melatonin, within the brain [69]. Higher concentrations are found in the liver and the adrenal and pineal glands [70]. SAMe is not readily found in food sources and, therefore, not technically a nutraceutical by definition. However, SAMe is marketed and sold in the United States as a dietary supplement and has previously been described as a nutraceutical in previous research. Disruption of the methylation cycle leads to lowered levels of SAMe and elevated homocysteine, which negatively affects the balance of the neurotransmitter systems [71]. This imbalance contributes to depressive symptoms by increasing oxidative stress and homocysteine levels [71]. Unlike many nutraceuticals that have solely been researched as an adjunctive treatment, SAMe has been studied as a monotherapy for MDD, specifically in individuals considered non-responders to traditional antidepressants. It is important to note that SAMe may exacerbate mania symptoms in bipolar disorder and should, therefore, be avoided, even during depressive episodes [72].
4.2.1. SAMe Animal Studies
There have been limited animal studies to date on the effects of SAMe on depression. Tillman (2019) studied the effects of SAMe in the Flinders Sensitive Line rat model, which shares similarities with humans in terms of depression and reduced liver SAMe concentrations, and found that SAMe did not exhibit antidepressant effects [73].
Benelli (1999) studied anhedonic behavior in castrated rats and whether different dosages of SAMe were as effective a treatment as imipramine, which is known to increase levels of serotonin and norepinephrine in the brain [74]. Anhedonia is a classic symptom of depression defined by lack of interest, enjoyment, or pleasure. This was measured by sucrose intake, the open-field test (OFT), and the place preference test. After three weeks of exposure to chronic mild stress (modifications of light/dark cycle, food and water availability, and housing conditions), sucrose consumption was reduced and animals were assigned to one of the following five treatments: (1) 100 mg SAMe, (2) 200 mg SAMe, (3) 300 mg SAMe, (4) 10 mg imipramine (a tricyclic antidepressant), or (5) 1 mL vehicle. Increased sucrose consumption (a measure of anhedonia) was observed following injection of SAMe, as well as a rapid reversal of anhedonia-like behavior demonstrated by the OFT and place-preference testing. Due to its role in serotonin production and breakdown, some researchers believe the baseline serotonin levels may be a determining factor in the effect SAMe has, postulating that this nutraceutical may be quicker acting and more potent when serotonin levels are low.
4.2.2. SAMe-Monotherapy Clinical Studies
Sarris (2014) compared individuals randomly assigned to SAMe (1600–3200 mg/day) as a monotherapy, escitalopram (10–20 mg/day), or placebo for 12 weeks after a wash out period [75]. Depression was measured using the Hamilton Depression Rating Scale (HAMD-17) at baseline and then again every two weeks during treatment. SAMe outperformed escitalopram and placebo in reducing the HAMD-17 score, although significance was not reached. At the 12-week endpoint, remission rates were 34% for SAMe, 23% for escitalopram, and 6% for placebo.
In a more recent study, 800 mg SAMe per day was compared to placebo in an 8-week double-blind, randomized trial [76]. Despite a high placebo response rate of 53%, SAMe had a greater reduction from baseline to week 8 in the Montgomery-Åsberg Depression Rating Scale (MADRS), though significance was not reached. These studies elucidate that even at varying doses, SAMe has not been shown to be an effectual monotherapy for MDD.
4.2.3. SAMe-Adjunctive-Therapy Clinical Studies
Sarris (2018) also conducted an 8-week study for SAMe adjunctive therapy in patients diagnosed with MDD [77]. The study compared 800 mg SAMe with antidepressant to a placebo with antidepressant. Outcomes were measured using the MADRS and analyzing one-carbon cycle nutrients, pertinent single-nucleotide polymorphisms (SNPs), and BDNF. The results showed a significant overall reduction in MADRS scores over time, but no significant between-group difference was observed, nor were significant differences found for any secondary measures.
In an earlier study, Papakostas (2010) evaluated a higher dose of SAMe (800 mg/twice daily) for 6 weeks as an adjunct therapy to antidepressants with patients considered non-responders to serotonin reuptake inhibitors (SRIs) [70]. When compared to placebo + SRI, the SAMe + SRI group demonstrated a higher HAM-D response along with higher remission rates. Response rates for SAMe treatment versus placebo were 36.1% versus 17.6%, respectively and remission rates were 25.8% versus 11.7%, respectively.
While SAMe does not have notable effects as a monotherapy, it does show an ability to decrease depressive symptoms as an adjunctive therapy to antidepressants Considering the span of dosages in the cited studies, which were between 100 mg and 3200 mg, future research may benefit by looking more into baseline levels of SAMe prior to beginning the intervention.
4.3. Folate
Folate is a B-vitamin and essential micronutrient necessary in the synthesis of dopamine, serotonin, and epinephrine. Lower levels of folate have been found in mental health disorders such as schizophrenia and MDD and may also contribute to the severity of such disorders [71]. Folate deficiency is associated with a metabolic imbalance resulting in decreased levels of SAMe; thus, these two nutraceuticals are often taken together. Along with its tie to mental disorders, folate deficiency has also been linked to resistance to pharmaceutical agents [78].
4.3.1. Folate Animal Studies
In a 6-week study, Zhou (2020) compared the following four groups of rats: (1) control, (2) chronic unpredictable mild stress (CUMS), (3) CUMS + folic acid, and (4) CUMS + citalopram [79]. The results showed a significant increase in serum folate and a reduction in serum homocysteine in the CUMS + folic group; citalopram did not influence these. Sucrose preference test (SPT), exercise distance, and standing times were all improved in the CUMS + folic acid group, but CUMS + citalopram group performed better. Monoamine neurotransmitters (5-HT, dopamine, norepinephrine) were not affected in either the folic acid or CUMS group, but all three were higher in the citalopram group. Folic acid significantly decreased IL-6 in serum and brain tissue, as well as the level of BDNF in serum but not brain tissue. No significant difference between folic acid and CUMS groups in b-EP was found, leading researchers to express the need for more studies on the relationship between folic acid and b-EP.
Réus (2018) conducted a rodent study that looked at treating depression stemming from both early- and late-life stress [80]. Early-life stress was brought on by maternal deprivation (MD) and late-life stress was induced using the chronic mild stress (CMS) protocol. Rats were treated with either folic acid, NAC, or omega-3 fatty acids. Depressive behavior was assessed by evaluating immobility in the forced swimming test (FST)and oxidative stress in the brain was also measured. The results showed a decrease in immobility time in stressed rats treated with folic acid compared to untreated stressed rats. Rats subjected to MD and treated with NAC, folic acid, and omega-3s showed a decrease in immobility time compared with MD rats treated with water. Malondialdehyde (MDA), a biomarker of lipid-specific oxidative stress, was reduced in the stressed rats treated with folic acid when compared to untreated stressed rats.
4.3.2. Folate-Monotherapy Clinical Studies
Low folate levels have been associated with depression and lengthened durations due to its role in the production of serotonin, dopamine, and norepinephrine. Studies on folate as a monotherapy are rare, but Shelton (2013) performed a study focused on both monotherapy and adjunctive therapy with folate [81]. Of the 52 subjects in the monotherapy group, there was a 7.7-point decrease in the Patient Health Questionnaire (PHQ-9), with nearly two-thirds of subjects reporting a positive response to treatment and half reporting remission in their depressive symptoms. Reynolds (2015) conducted a study on folate as a monotherapy, and although the sample was small, n = 8, found that this group responded with a mean reduction in MADRS scoring of 45% [82]. Response was defined as a 25% reduction in MADRS scoring, concluding a positive outcome.
4.3.3. Folate-Adjunctive-Therapy Clinical Studies
Not only are low folate levels correlated with depression, but individuals with decreased folate are less likely to respond to traditional antidepressants. Coppen and Bailey (2000) designed a 10-week study comparing 500 mg folic acid + 20 mg fluoxetine to placebo + 20 mg fluoxetine in groups of men and women [83]. Depressive symptoms were measured at baseline and every 2 weeks using the Hamilton Rating Scale (HRS), and plasma folate was measured at baseline, 4 weeks, and 10 weeks. All groups experienced a decrease in HRS scores, with the women in the folic acid + fluoxetine group having the most significant decrease. All patients in the folic acid + fluoxetine group had significantly increased levels of plasma folate.
Ginsberg (2011) compared two dosages of folic acid paired with an antidepressant and a control group of subjects using antidepressant monotherapy [84]. Subjects had a primary diagnosis of MDD and had been on their usual antidepressant for at least 60 days. The Clinical Global Impression-Severity scale (CGI-S) was used to evaluate the response to pharmacologic medication. In subjects with less severe depression, results showed 18.5% of the folic acid + antidepressant group experienced an improvement in CGI-S scores, compared to 7.04% of the monotherapy group. In those with more severe depression, 40% of the folic acid + antidepressant group saw an improvement in CGI-S scores, compared to 16.3% in the monotherapy group.
As with the previous nutraceuticals mentioned, folate produces positive results in depressive symptoms when used as an adjunctive treatment, but individuals with less severe symptoms do not experience the same robust effects. Considering the relationship between folate and SAMe, it would be worthwhile to study the pairing of these agents. Dosages in the cited studies range widely, from 7.5 mg to 500 mg of folate.
4.3.4. Other B Vitamins
While much research has focused on folate alone, it has been put forth that a combination strategy to vitamin B use may be more effective due to the differing actions of the B vitamins, suggesting that other B vitamins may also play an important role in mitigating the pathology of depression [85]. In one RCT, vitamin B6 plus magnesium was found to reduce depression scores from moderate to normal over the course of 8 weeks [86], and a large cross-sectional study found an association between lower B6 intake and increased risk of depression and anxiety [87]. Vitamin B6 has also been found to reduce self-reported anxiety and induced a trend toward reduced depression, which aligns with these two mental health conditions considering they are intrinsically related and overlap in GABAergic neurochemistry [88]. B vitamins may improve MDD via actions in neurotransmittors, as well as decreasing levels of inflammatory cytokines [89,90]. In addition to folate (vitamin B9), deficiencies of vitamins B6 (pyridoxine) and B12 (cobalamin) have previously been associated with various neurological disorders including depression [91]. Combination B6, B9, B12 enhanced antidepressive response of individuals on citalopram over 1 year compared to a placebo, as determined by Montgomery–åsberg Depression Rating Scale (MADRS) [92]. In a systematic review of 20 randomly controlled trials, the combination of folic acid, B1, B12, and vitamin D significantly decreased depressive scale scores by enhancing the efficacy of antidepressants and was also found to be effective as a monotherapy [93]. In one longitudinal study of adults over the age of 50, it was observed that those with a deficient-low B12 status had a 51% increased likelihood of developing depressive symptoms over 4 years [94]. A relationship was also found between a healthy dietary pattern and a reduced risk of depression via increased serum levels of folate and vitamin B12 [95]. Additionally, consuming high levels of B8 (particularly for women) has been associated with lower likelihood of depression [91]. However, conflicting literature also exists. In a systematic review and meta-analysis, there was found to be no effect of vitamin supplementation on depressive symptoms; neither as adjunctive nor monotherapy [96]. More research is needed to determine whether B vitamins in addition to folate or combinations of B vitamins could provide a therapeutic response for MDD.
4.4. Vitamin D
While vitamin D has a known role in calcium metabolism, it may also be involved in brain and nervous system health and disease [97]. Reduced levels of vitamin D have also been reported in many psychiatric disorders, namely depression and anxiety. Touted as a neuro hormone, vitamin D has two main forms, D2 (ergocalciferol) and D3 (cholecalciferol). It is involved in the synthesis, release, and regulation of neurotransmitters such as serotonin and may also affect other hormones such as oxytocin, although supporting research is scarce. Vitamin D3 has been shown to be more active and superior to D2 in raising and maintaining serum vitamin D levels [98]. Vitamin D3 is available naturally in many food sources such as eggs and fatty fish, while vitamin D2 is mainly found in mushrooms [99]. However, getting an adequate amount through food can be difficult, and many individuals do not obtain the needed sunlight exposure (especially considering the use of high SPF sunscreens) to allow for the conversion of vitamin D through the skin. Thus, vitamin D supplements are viewed by many as beneficial. However, it is not clear whether adjunctive treatment is effective in alleviating depressive symptoms or contributing to remission [100].
Reduced levels of vitamin D have been reported in many psychiatric disorders, namely depression and anxiety. Vitamin D has a known role in calcium metabolism, but it also affects brain and nervous system health and disease [97].
The relationship of vitamin D and seasonal effects on mental health, specifically depression in the form of seasonal affective disorder is also a topic of interest [101]. Seasonal affective disorder differs from MDD in that it originates in fall or winter months when sun exposure is lower, and typically remits as the season changes to spring or summer [102].
4.4.1. Vitamin D Animal Studies
Vitamin D supplementation (100–10,000 IU daily) improved anxiety and depression in rats exposed to unpredictable chronic mild stress (UCMS), as assessed via the following three behavioral tests: OFT, elevated plus maze (EPM), and FST. Brain tissue oxidation (MDA) and neuroinflammation (IL-6) were also decreased following UCMS in the rats treated with vitamin D compared to those that were not [103]. In a separate study, anhedonic behavior assessed via SPT was lower in male rats undergoing CMS that were injected with 10,000 IU vitamin D daily compared to CMS and no treatment, indicating decreased depression. However, the study saw no improvement to anxiety during OFT with treatment [104].
Al-Ramadhan (2023) explored vitamin D as both a monotherapy and adjunctive therapy [105]. Alterations in behavioral markers of depression were assessed using the following five experimental groups: (1) control group, (2) the CUMS group, (3) the CUMS group receiving vitamin D (10 mg/kg), (4) the CUMS group receiving fluoxetine (5 mg/kg), and (5) the CUMS group receiving both vitamin D and fluoxetine. Baseline testing was performed in behavioral tests (EPM, FST, OFT, tail-suspension test (TST), and SPT). After three weeks, significant changes were observed in vitamin D + CUMS group in the EPM and TST. In both the vitamin D + fluoxetine + CUMS and the vitamin D + CUMS groups, the vitamin D treatments had antidepressant effects in the FST, OFT, and SPT tests. In summary, this study found that a combination of fluoxetine and vitamin D yielded better results than either agent on its own.
4.4.2. Vitamin-D-Monotherapy Clinical Studies
In an 8-week double-blind study on the effects of high-dose vitamin D on mild to moderate depression, subjects were assigned either vitamin D3 (50,000 IU/2 weeks) or placebo and were assessed pre- and post-treatment using the Beck Depression Inventory (BDI-II) and blood analysis [106]. At 8 weeks, serum concentrations of vitamin D increased significantly in the vitamin D group by approximately 40 nmol/L compared to placebo approximately 5 nmol/L. BDI-II scores decreased in both groups; however, the vitamin D group was significantly lower than placebo. There were no significant differences between treatment and placebo for oxytocin, serotonin, intact parathyroid hormone post-treatment. These findings suggest that while vitamin D improved mood, it did not impact hormones or neurotransmitters.
In a 6-month female only study, Choukri (2018) studied the effect of vitamin D on depressive symptoms, anxiety, flourishing, and positive and negative mood during the winter months in New Zealand [107]. Subjects were randomly assigned either 50,000 IU of vitamin D or placebo once per month. The vitamin D group showed a significant increase in serum vitamin D. Serum vitamin D levels in the placebo group declined over the study, which was expected due to seasonal changes. No significant differences, however, were found between the vitamin D and placebo groups in any outcome measures of depression assessed using the Center for Epidemiologic Studies Depression Scale (CES-D), the Hospital Anxiety and Depression Scale (HADS), and the Flourishing Scale.
4.4.3. Vitamin D Adjunctive-Therapy Clinical Studies
In an 8-week study, Khoraminya (2013) assessed vitamin D adjunctive-therapeutic effects using the 24-item Hamilton Depression Rating Scale (HAM-D) and 21-item Beck Depression Inventory (BDI) post-treatment with either 1500 IU vitamin D daily + 20 mg fluoxetine or 20 mg fluoxetine + placebo in patients with MDD [108]. The results showed that serum 25(OH)D increased significantly in the vitamin D group following treatment, and depression severity improved significantly more in the vitamin D group from the fourth week of treatment.
In a recent double-blind, randomized controlled study with MDD patients, Zhao (2024) used 1600 IU vitamin D as an adjunctive therapy to existing antidepressant regimens on clinical symptoms, as well as its potential preservation effects on brain structural and functional connectivity [109]. Following 7 months, serum vitamin D levels increased from baseline to follow-up in the group receiving vitamin D with no change in the placebo group. Clinical symptoms in both groups improved scores on the Hamilton Rating Scale for Depression (HAM-D) and Hamilton Rating Scale for Anxiety (HAMA), although no significant group-by-time interactions were observed. White-matter integrity and functional network connectivity results revealed decreased fractional anisotropy (FA), a quantitative biomarker of white-matter integritys in the right inferior fronto-occipital fascisulus (IFOF) of the placebo group with no change in the vitamin D group. There was a significant group-by-time interaction effect on functional connectivity between the right frontoparietal network (rFPN) and medial vestibular nucleus (mVN). This study found that adjunctive vitamin D improved clinical symptoms of depression and shows the potential to protect white matter in the brain.
The studies reviewed here indicate that vitamin D is a promising adjunctive therapy to antidepressants, but, as with the prior supplements reviewed in this paper, treatment as a monotherapy agent does not have significant effects on depressive symptoms. Dosages in the cited papers ranged widely, from 100 IU daily of vitamin D to 50,000 IU over the course of 2 weeks. Considering such a large dosage range, future research may focus on determining a more tightly defined dose range, as well as treatment period.
4.5. Summary of Nutraceuticals for MDD
When considering the collective data summarized in Table 1, it appears that select nutraceuticals may share similar mechanisms of action as available pharmaceutical treatments used for MDD. The research to date suggests that nutraceuticals may be more efficacious as an adjunct to pharmaceutical therapies than as monotherapies. Omega-3 fatty acids were shown to be effective in reducing depressive symptoms, both as a monotherapy and adjunct to pharmaceutical therapies; however, EPA was found to be more efficacious when compared to DHA. When used alone, SAMe did not have significant positive results in depression symptoms but can decrease symptoms when used as an adjunctive therapy, and the effect can be more robust in individuals with low serotonin levels. Folate as a monotherapy decreased scores on depression scales and decreased depressive symptoms as an adjunctive therapy. B vitamins and vitamin D both carry conflicting results as monotherapies, but both show promise in improving symptoms and enhancing the effectiveness of pharmaceutical interventions.

Table 1.
Relevant clinical trials of nutraceuticals as a monotherapy and/or adjunct therapy for depression.
Positively, the studies reviewed include both animal and human clinical trials; however, the body of research on the subject of nutraceuticals in the treatment of MDD remains sparce and is faced with many limitations. For example, there has been a span of severities of MDD and variations in nutraceutical intervention with regard to nutrient type and dosage, as well as length of intervention, and, therefore, no continuity of length of study treatment or definition of follow-up results. Studies typically ranged from 8 weeks to several months, and, while it may be challenging and costly to do so, future work should consider following subjects for greater than 6 months. It may also be advantageous to consider the lifestyle factors of subjects or incorporating these into studies along with nutraceutical intervention. This could possibly address how, or if, the current lifestyle factors of individuals affect the efficacy of these supplements. Future studies could also benefit by categorizing by sex, age, length of time an individual has suffered with MDD, and severity of MDD.
More research is needed in order for nutraceuticals to be accepted into standard therapeutic practice, specifically as adjunctive therapy with antidepressants. Studies utilizing higher doses of select ingredients are typically met with positive results, therefore there is likely a need to determine dosing recommendations as we see in clinical pharmacokinetic studies. Ideally, nutraceuticals need to be assessed with the same tangible efficacy metrics and attentiveness as are done with FDA approved pharmaceuticals. This would better allow individuals and health professionals to make scientifically based recommendations based on patient needs.
4.6. Prebiotics, Probiotics, and Symbiotics
Although a relatively new player in the world of nutraceuticals and dietary supplements, agents that impact the gut microbiome have become extremely popular in recent years, with the microbiome being another potential therapeutic target for MDD, as it interacts with the brain via the gut–brain axis. Many studies have identified a significant relationship between the gut microbiome and MDD, with differences in beta diversity and increased Proteobacteria and Enterobacteriaceae, in particular [43,110]. The gut microbiome, particularly during dysbiosis, may disturb multiple potential MDD pathways including neurotransmitters, stress response in the HPA axis, brain-derived neurotrophic factor (BDNF) levels, and inflammatory cytokine levels [43], as seen in Figure 1. Supplementing prebiotics (nondigestible sugars metabolized by certain microorganisms in the gut microbiome), probiotics (specific strains of microorganisms such as Lactobacillus and Bifidobacteria), or symbiotics (combination of pre- and probiotics) may ameliorate the negative conditions contributing to MDD. Multiple meta-analyses have shown improvements in depression scores, principally for probiotics, with varying degrees for probiotics and symbiotics when compared to placebo [43,110,111]. Despite these findings, there is insufficient data on how these agents perform as a mono- or adjunct therapy for the treatment of MDD in comparison to pharmaceuticals, and, therefore, no recommendation is being made at this time.
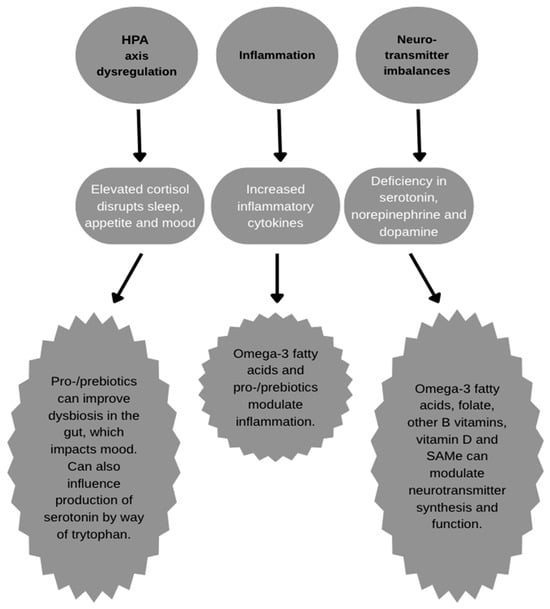
Figure 1.
Actions of nutraceuticals on depression pathology.
5. Conclusions
MDD is a serious mental disorder affecting a substantial part of the population and prevalence is on the rise. Accepted treatments include psychotherapy and antidepressants, but the latter carry many undesirable side effects, and some individuals find they cannot tolerate them. Lifestyle changes, such as exercise, diet, sleep, and spirituality, could also be first-line defense strategies, as well as preventive measures. Nutraceuticals can provide support for MDD, with many nutrients incorporated into the diet through food sources, with increases in dosing more easily provided in supplemental form. Nutraceuticals have the most potential as adjunctive agents to antidepressants in improving depressive symptoms and can possibly lower inflammatory biomarkers. From a therapeutic perspective, a holistic approach inclusive of lifestyle modification (spiritual growth, adequate sleep, exercise, and nutrient-dense diet), psychotherapy, pharmaceutical intervention, and nutraceutical support should be considered.
Author Contributions
Conceptualization, A.D. and R.J.B.; literature review, A.D.; writing—original draft preparation, A.D.; writing—review and editing, A.D., J.P. and R.J.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
R.J.B. has received research support from dietary supplement companies and has served as a consultant to dietary-supplement companies. Other authors declare no conflicts of interest related to this work.
Abbreviations
The following abbreviations are used in this manuscript:
| ALA | Alpha-linoleic acid |
| BDI | Beck Depression Inventory |
| BDNF | Brain-derived neurotrophic factor |
| CES-D | Center for Epidemiologic Studies Depression Scale |
| CUMS | Chronic unpredictable mild stress |
| DHA | Docosahexaenoic acid |
| DSM-5 | Diagnostic and Statistical Manual of Mental Disorders |
| EPA | Eicosapentaenoic acid |
| EPM | Elevated plus maze |
| FA | Fractional anisotropy |
| FST | Forced swim test |
| HADS | Hospital Anxiety Depression Scale |
| HAM-D | Hamilton Depression Rating Scale |
| HPA | Hypothalamic–pituitary–adrenal |
| HRS | Hamilton Rating Scale |
| hs-CRP | High-sensitivity C-reactive protein |
| IDS-C30 | Inventory of Depressive Symptomatology, Clinician-Rated |
| IFOF | Inferior fronto-occipital fascisulus |
| IU | International units |
| MADRS | Montgomery–Åsberg Depression Rating Scale |
| MAOI | Monoamine oxidase inhibitor |
| MD | Maternal deprivation |
| MDD | Major depressive disorder |
| mVN | Medial vestibular nucleus |
| NAC | N-acetyl cysteine |
| OFT | Open-field test |
| PHQ-9 | Patient Health Questionnaire |
| PUFA | Polyunsaturated fatty acid |
| rFPN RCT | Right frontoparietal network Randomized controlled trial |
| SAMe | S-adenosylmethionine |
| SNP | Single-nucleotide polymorphism |
| SNRI | Serotonin/norepinephrine reuptake inhibitor |
| SPT | Sucrose preference test |
| SSRI | Selective serotonin reuptake inhibitor |
| TCA | Tricyclic antidepressant |
| TST | Tail-suspension test |
| UCMS | Unpredictable chronic mild stress |
References
- Mental Health By the Numbers. Available online: https://www.nami.org/about-mental-illness/mental-health-by-the-numbers/ (accessed on 8 November 2024).
- López-Muñoz, F.; Alamo, C. Monoaminergic Neurotransmission: The History of the Discovery of Antidepressants from 1950s until Today. Curr. Pharm. Des. 2009, 15, 1563–1586. [Google Scholar] [CrossRef]
- Mechlińska, A.; Wiglusz, M.S.; Słupski, J.; Włodarczyk, A.; Cubała, W.J. Exploring the Relationship between Mood Disorders and Coexisting Health Conditions: The Focus on Nutraceuticals. Brain Sci. 2023, 13, 1262. [Google Scholar] [CrossRef]
- Burns, C. Antidepressant Prescribing Increases by 35% in Six Years. Available online: https://pharmaceutical-journal.com/article/news/antidepressant-prescribing-increases-by-35-in-six-years (accessed on 3 December 2024).
- Thakuria, H.; Medhi, D. Use of Nutraceuticals in Psychiatry. Acad. Bull. Ment. Health 2024, 2, 24–28. [Google Scholar] [CrossRef]
- Makkar, R.; Behl, T.; Bungau, S.; Zengin, G.; Mehta, V.; Kumar, A.; Uddin, M.S.; Ashraf, G.M.; Abdel-Daim, M.M.; Arora, S.; et al. Nutraceuticals in Neurological Disorders. Int. J. Mol. Sci. 2020, 21, 4424. [Google Scholar] [CrossRef] [PubMed]
- Cui, L.; Li, S.; Wang, S.; Wu, X.; Liu, Y.; Yu, W.; Wang, Y.; Tang, Y.; Xia, M.; Li, B. Major Depressive Disorder: Hypothesis, Mechanism, Prevention and Treatment. Signal Transduct. Target. Ther. 2024, 9, 30. [Google Scholar] [CrossRef] [PubMed]
- Lam, R.W.; Kennedy, S.H.; McIntyre, R.S.; Khullar, A. Cognitive Dysfunction in Major Depressive Disorder: Effects on Psychosocial Functioning and Implications for Treatment. Can. J. Psychiatry 2014, 59, 649–654. [Google Scholar] [CrossRef]
- Bains, N.; Abdijadid, S. Major Depressive Disorder. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Gonda, X.; Fountoulakis, K.N.; Kaprinis, G.; Rihmer, Z. Prediction and Prevention of Suicide in Patients with Unipolar Depression and Anxiety. Ann. Gen. Psychiatry 2007, 6, 23. [Google Scholar] [CrossRef]
- Pompili, M.; Serafini, G.; Innamorati, M.; Ambrosi, E.; Giordano, G.; Girardi, P.; Tatarelli, R.; Lester, D. Antidepressants and Suicide Risk: A Comprehensive Overview. Pharmaceuticals 2010, 3, 2861–2883. [Google Scholar] [CrossRef] [PubMed]
- Depression: Learn More—How Effective Are Antidepressants? In InformedHealth.org [Internet]; Institute for Quality and Efficiency in Health Care (IQWiG): Cologne, Germany, 2024.
- Antidepressants: Selecting One That’s Right for You. Available online: https://www.mayoclinic.org/diseases-conditions/depression/in-depth/antidepressants/art-20046273 (accessed on 22 October 2024).
- Picardi, A.; Gaetano, P. Psychotherapy of Mood Disorders. Clin. Pract. Epidemiol. Ment. Health 1970, 10, 140. [Google Scholar] [CrossRef]
- Dunlop, B.W. Evidence-Based Applications of Combination Psychotherapy and Pharmacotherapy for Depression. Focus 2016, 14, 156–173. [Google Scholar] [CrossRef]
- Kunugi, H. Depression and Lifestyle: Focusing on Nutrition, Exercise, and Their Possible Relevance to Molecular Mechanisms. Psychiatry Clin. Neurosci. 2023, 77, 420. [Google Scholar] [CrossRef]
- Oliveira-Maia, A.J.; Bobrowska, A.; Constant, E.; Ito, T.; Kambarov, Y.; Luedke, H.; Mulhern-Haughey, S.; Holt, C. von Treatment-Resistant Depression in Real-World Clinical Practice: A Systematic Literature Review of Data from 2012 to 2022. Adv. Ther. 2023, 41, 34. [Google Scholar] [CrossRef]
- Carek, P.J.; Laibstain, S.E.; Carek, S.M. Exercise for the Treatment of Depression and Anxiety. Int. J. Psychiatry Med. 2011, 41, 15–28. [Google Scholar] [CrossRef]
- Blumenthal, J.A.; Rozanski, A. Exercise as a Therapeutic Modality for the Prevention and Treatment of Depression. Prog. Cardiovasc. Dis. 2023, 77, 50–58. [Google Scholar] [CrossRef] [PubMed]
- Schuch, F.B.; Stubbs, B. The Role of Exercise in Preventing and Treating Depression. Curr. Sports Med. Rep. 2019, 18, 299–304. [Google Scholar] [CrossRef]
- Hossain, M.N.; Lee, J.; Choi, H.; Kwak, Y.-S.; Kim, J. The Impact of Exercise on Depression: How Moving Makes Your Brain and Body Feel Better. Phys. Act. Nutr. 2024, 28, 43–51. [Google Scholar] [CrossRef]
- Greer, T.L.; Trombello, J.M.; Rethorst, C.D.; Carmody, T.J.; Jha, M.K.; Liao, A.; Grannemann, B.D.; Chambliss, H.O.; Church, T.S.; Trivedi, M.H. Improvements in psychosocial functioning and health-related quality of life following exercise augmentation in patients with treatment response but nonremitted major depressive disorder: Results from the tread study. Depress. Anxiety 2016, 33, 870–881. [Google Scholar] [CrossRef]
- Oh, J.; Yun, K.; Chae, J.-H.; Kim, T.-S. Association Between Macronutrients Intake and Depression in the United States and South Korea. Front. Psychiatry 2020, 11, 207. [Google Scholar] [CrossRef] [PubMed]
- Marx, W.; Lane, M.; Hockey, M.; Aslam, H.; Berk, M.; Walder, K.; Borsini, A.; Firth, J.; Pariante, C.M.; Berding, K.; et al. Diet and Depression: Exploring the Biological Mechanisms of Action. Mol. Psychiatry 2021, 26, 134–150. [Google Scholar] [CrossRef] [PubMed]
- Akbaraly, T.N.; Brunner, E.J.; Ferrie, J.E.; Marmot, M.G.; Kivimaki, M.; Singh-Manoux, A. Dietary Pattern and Depressive Symptoms in Middle Age. Br. J. Psychiatry 2009, 195, 408. [Google Scholar] [CrossRef]
- Swainson, J.; Reeson, M.; Malik, U.; Stefanuk, I.; Cummins, M.; Sivapalan, S. Diet and Depression: A Systematic Review of Whole Dietary Interventions as Treatment in Patients with Depression. J. Affect. Disord. 2023, 327, 270–278. [Google Scholar] [CrossRef]
- Ortega, M.A.; Fraile-Martínez, Ó.; García-Montero, C.; Alvarez-Mon, M.A.; Lahera, G.; Monserrat, J.; Llavero-Valero, M.; Gutiérrez-Rojas, L.; Molina, R.; Rodríguez-Jimenez, R.; et al. Biological Role of Nutrients, Food and Dietary Patterns in the Prevention and Clinical Management of Major Depressive Disorder. Nutrients 2022, 14, 3099. [Google Scholar] [CrossRef]
- Lassale, C.; Batty, G.D.; Baghdadli, A.; Jacka, F.; Sánchez-Villegas, A.; Kivimäki, M.; Akbaraly, T. Healthy Dietary Indices and Risk of Depressive Outcomes: A Systematic Review and Meta-Analysis of Observational Studies. Mol. Psychiatry 2018, 24, 965. [Google Scholar] [CrossRef]
- Manosso, L.M.; Duarte, L.A.; Martinello, N.S.; Mathia, G.B.; Réus, G.Z. Circadian Rhythms and Sleep Disorders Associated to Major Depressive Disorder: Pathophysiology and Therapeutic Opportunities. CNS Neurol. Disord.-Drug Targets 2023, 23, 1085–1100. [Google Scholar] [CrossRef]
- Jackson, M.L.; Sztendur, E.M.; Diamond, N.T.; Byles, J.E.; Bruck, D. Sleep Difficulties and the Development of Depression and Anxiety: A Longitudinal Study of Young Australian Women. Arch. Womens Ment. Health 2014, 17, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, V.V.; Zainal, N.H.; Newman, M.G. Why Sleep Is Key: Poor Sleep Quality Is a Mechanism for the Bidirectional Relationship between Major Depressive Disorder and Generalized Anxiety Disorder Across 18 Years. J. Anxiety Disord. 2022, 90, 102601. [Google Scholar] [CrossRef] [PubMed]
- Dein, S.; Cook, C.C.H.; Koenig, H. Religion, Spirituality, and Mental Health: Current Controversies and Future Directions. J. Nerv. Ment. Dis. 2012, 200, 852. [Google Scholar] [CrossRef] [PubMed]
- Upenieks, L. Unpacking the Relationship Between Prayer and Anxiety: A Consideration of Prayer Types and Expectations in the United States. J. Relig. Health 2023, 62, 1810–1831. [Google Scholar] [CrossRef] [PubMed]
- Kim, N.-Y.; Huh, H.-J.; Chae, J.-H. Effects of Religiosity and Spirituality on the Treatment Response in Patients with Depressive Disorders. Compr. Psychiatry 2015, 60, 26–34. [Google Scholar] [CrossRef]
- Boelens, P.A.; Reeves, R.R.; Replogle, W.H.; Koenig, H.G. The Effect of Prayer on Depression and Anxiety: Maintenance of Positive Influence One Year after Prayer Intervention. Int. J. Psychiatry Med. 2012, 43, 85–98. [Google Scholar] [CrossRef]
- Rickhi, B.; Moritz, S.; Reesal, R.; Xu, T.J.; Paccagnan, P.; Urbanska, B.; Liu, M.F.; Ewing, H.; Toews, J.; Gordon, J.; et al. A Spirituality Teaching Program for Depression: A Randomized Controlled Trial. Int. J. Psychiatry Med. 2011, 42, 315–329. [Google Scholar] [CrossRef]
- Nutraceutical-Definition and Introduction. Available online: https://www.researchgate.net/publication/9005014_Nutraceutical_-Definition_and_Introduction (accessed on 3 December 2024).
- Alvarez-Mon, M.A.; Ortega, M.A.; García-Montero, C.; Fraile-Martinez, O.; Monserrat, J.; Lahera, G.; Mora, F.; Rodriguez-Quiroga, A.; Fernandez-Rojo, S.; Quintero, J.; et al. Exploring the Role of Nutraceuticals in Major Depressive Disorder (MDD): Rationale, State of the Art and Future Prospects. Pharmaceuticals 2021, 14, 821. [Google Scholar] [CrossRef]
- Sarris, J.; Murphy, J.; Mischoulon, D.; Papakostas, G.I.; Fava, M.; Berk, M.; Ng, C.H. Adjunctive Nutraceuticals for Depression: A Systematic Review and Meta-Analyses. Am. J. Psychiatry 2016, 173, 575–587. [Google Scholar] [CrossRef]
- Juneja, K.; Bhuchakra, H.P.; Sadhukhan, S.; Mehta, I.; Niharika, A.; Thareja, S.; Nimmakayala, T.; Sahu, S. Creatine Supplementation in Depression: A Review of Mechanisms, Efficacy, Clinical Outcomes, and Future Directions. Cureus 2024, 16, e71638. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, Q.; Huang, H.; Liu, Z. The Efficacy and Acceptability of Curcumin for the Treatment of Depression or Depressive Symptoms: A Systematic Review and Meta-Analysis. J. Affect. Disord. 2021, 282, 242–251. [Google Scholar] [CrossRef]
- Zheng, W.; Li, W.; Qi, H.; Xiao, L.; Sim, K.; Ungvari, G.S.; Lu, X.-B.; Huang, X.; Ning, Y.-P.; Xiang, Y.-T. Adjunctive Folate for Major Mental Disorders: A Systematic Review. J. Affect. Disord. 2020, 267, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Alli, S.R.; Gorbovskaya, I.; Liu, J.C.W.; Kolla, N.J.; Brown, L.; Müller, D.J. The Gut Microbiome in Depression and Potential Benefit of Prebiotics, Probiotics and Synbiotics: A Systematic Review of Clinical Trials and Observational Studies. Int. J. Mol. Sci. 2022, 23, 4494. [Google Scholar] [CrossRef]
- Gören, J.L.; Tewksbury, A.T. The Use of Omega-3 Fatty Acids in Mental Illness. J. Pharm. Pract. 2011, 24, 452–471. [Google Scholar] [CrossRef] [PubMed]
- Berk, M.; Williams, L.J.; Jacka, F.N.; O’Neil, A.; Pasco, J.A.; Moylan, S.; Allen, N.B.; Stuart, A.L.; Hayley, A.C.; Byrne, M.L.; et al. So Depression Is an Inflammatory Disease, but Where Does the Inflammation Come From? BMC Med. 2013, 11, 200. [Google Scholar] [CrossRef] [PubMed]
- Berger, M.E.; Smesny, S.; Kim, S.-W.; Davey, C.G.; Rice, S.; Sarnyai, Z.; Schlögelhofer, M.; Schäfer, M.R.; Berk, M.; McGorry, P.D.; et al. Omega-6 to Omega-3 Polyunsaturated Fatty Acid Ratio and Subsequent Mood Disorders in Young People with at-Risk Mental States: A 7-Year Longitudinal Study. Transl. Psychiatry 2017, 7, e1220. [Google Scholar] [CrossRef] [PubMed]
- Lin, P.-Y.; Huang, S.-Y.; Su, K.-P. A Meta-Analytic Review of Polyunsaturated Fatty Acid Compositions in Patients with Depression. Biol. Psychiatry 2010, 68, 140–147. [Google Scholar] [CrossRef]
- Patel, S.; Keating, B.A.; Dale, R.C. Anti-Inflammatory Properties of Commonly Used Psychiatric Drugs. Front. Neurosci. 2023, 16, 1039379. [Google Scholar] [CrossRef]
- Calder, P.C. Omega-3 Fatty Acids and Inflammatory Processes: From Molecules to Man. Biochem. Soc. Trans. 2017, 45, 1105–1115. [Google Scholar] [CrossRef]
- Glover, D. Hypothalamic-Pituitary-Adrenal Axis. In Encyclopedia of Autism Spectrum Disorders; Volkmar, F.R., Ed.; Springer: New York, NY, USA, 2013; pp. 1530–1535. ISBN 978-1-4419-1698-3. [Google Scholar]
- Zhou, L.; Xiong, J.-Y.; Chai, Y.-Q.; Huang, L.; Tang, Z.-Y.; Zhang, X.-F.; Liu, B.; Zhang, J.-T. Possible Antidepressant Mechanisms of Omega-3 Polyunsaturated Fatty Acids Acting on the Central Nervous System. Front. Psychiatry 2022, 13, 933704. [Google Scholar] [CrossRef] [PubMed]
- Chalon, S.; Delion-Vancassel, S.; Belzung, C.; Guilloteau, D.; Leguisquet, A.-M.; Besnard, J.-C.; Durand, G. Dietary Fish Oil Affects Monoaminergic Neurotransmission and Behavior in Rats12. J. Nutr. 1998, 128, 2512–2519. [Google Scholar] [CrossRef] [PubMed]
- Delion, S.; Chalon, S.; Hérault, J.; Guilloteau, D.; Besnard, J.-C.; Durand, G. Chronic Dietary α-Linolenic Acid Deficiency Alters Dopaminergic and Serotoninergic Neurotransmission in Rats. J. Nutr. 1994, 124, 2466–2476. [Google Scholar] [CrossRef]
- Zimmer, L.; Delion-Vancassel, S.; Durand, G.; Guilloteau, D.; Bodard, S.; Besnard, J.-C.; Chalon, S. Modification of Dopamine Neurotransmission in the Nucleus Accumbens of Rats Deficient in n–3 Polyunsaturated Fatty Acids. J. Lipid Res. 2000, 41, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Sinclair, A.; Begg, D.; Mathai, M.; Weisinger, R. Omega 3 Fatty Acids and the Brain: Review of Studies in Depression. Asia Pac. J. Clin. Nutr. 2007, 16 (Suppl. l), 391–397. [Google Scholar] [PubMed]
- DeMar, J.C.; Ma, K.; Bell, J.M.; Igarashi, M.; Greenstein, D.; Rapoport, S.I. One Generation of N-3 Polyunsaturated Fatty Acid Deprivation Increases Depression and Aggression Test Scores in Rats. J. Lipid Res. 2006, 47, 172–180. [Google Scholar] [CrossRef]
- Gonzales, E.; Barrett, D.W.; Shumake, J.; Gonzalez-Lima, F.; Lane, M.A. Omega-3 Fatty Acids Improve Behavioral Coping to Stress in Multiparous Rats. Behav. Brain Res. 2015, 279, 129–138. [Google Scholar] [CrossRef]
- Weiser, M.J.; Wynalda, K.; Norman Salem, J.; Butt, C.M. Dietary DHA during Development Affects Depression-like Behaviors and Biomarkers That Emerge after Puberty in Adolescent Rats. J. Lipid Res. 2015, 56, 151. [Google Scholar] [CrossRef]
- Kelaiditis, C.F.; Gibson, E.L.; Dyall, S.C. Effects of Long-Chain Omega-3 Polyunsaturated Fatty Acids on Reducing Anxiety and/or Depression in Adults; A Systematic Review and Meta-Analysis of Randomised Controlled Trials. Prostaglandins Leukot Essent Fat. Acids 2023, 192, 102572. [Google Scholar] [CrossRef]
- Liao, Y.; Xie, B.; Zhang, H.; He, Q.; Guo, L.; Subramanieapillai, M.; Fan, B.; Lu, C.; McIntyre, R.S. Efficacy of Omega-3 PUFAs in Depression: A Meta-Analysis. Transl. Psychiatry 2019, 9, 190. [Google Scholar] [CrossRef]
- Appleton, K.M.; Voyias, P.D.; Sallis, H.M.; Dawson, S.; Ness, A.R.; Churchill, R.; Perry, R. Omega-3 Fatty Acids for Depression in Adults. Cochrane Database Syst. Rev. 2021, 11, CD004692. [Google Scholar] [CrossRef]
- Mischoulon, D.; Dunlop, B.W.; Kinkead, B.; Schettler, P.J.; Lamon-Fava, S.; Rakofsky, J.J.; Nierenberg, A.A.; Clain, A.J.; Crowe, T.M.; Wong, A.; et al. Omega-3 Fatty Acids for Major Depressive Disorder With High Inflammation: A Randomized Dose-Finding Clinical Trial. J. Clin. Psychiatry 2022, 83, 42432. [Google Scholar] [CrossRef]
- Marangell, L.B.; Martinez, J.M.; Zboyan, H.A.; Kertz, B.; Kim, H.F.S.; Puryear, L.J. A Double-Blind, Placebo-Controlled Study of the Omega-3 Fatty Acid Docosahexaenoic Acid in the Treatment of Major Depression. Am. J. Psychiatry 2003, 160, 996–998. [Google Scholar] [CrossRef]
- Mozaffari-Khosravi, H.; Yassini-Ardakani, M.; Karamati, M.; Shariati-Bafghi, S.-E. Eicosapentaenoic Acid versus Docosahexaenoic Acid in Mild-to-Moderate Depression: A Randomized, Double-Blind, Placebo-Controlled Trial. Eur. Neuropsychopharmacol. 2013, 23, 636–644. [Google Scholar] [CrossRef] [PubMed]
- Mischoulon, D.; Nierenberg, A.A.; Schettler, P.J.; Kinkead, B.L.; Fehling, K.; Martinson, M.A.; Rapaport, M.H. A Double-Blind, Randomized Controlled Clinical Trial Comparing Eicosapentaenoic Acid Versus Docosahexaenoic Acid for Depression. J. Clin. Psychiatry 2014, 76, 4830. [Google Scholar] [CrossRef] [PubMed]
- Nasir, M.; Bloch, M.H. Trim the Fat: The Role of Omega-3 Fatty Acids in Psychopharmacology. Ther. Adv. Psychopharmacol. 2019, 9, 2045125319869791. [Google Scholar] [CrossRef] [PubMed]
- Jazayeri, S.; Tehrani-Doost, M.; Keshavarz, S.A.; Hosseini, M.; Djazayery, A.; Amini, H.; Jalali, M.; Peet, M. Comparison of Therapeutic Effects of Omega-3 Fatty Acid Eicosapentaenoic Acid and Fluoxetine, Separately and in Combination, in Major Depressive Disorder. Aust. N. Z. J. Psychiatry 2008, 42, 192–198. [Google Scholar] [CrossRef]
- Gertsik, L.; Poland, R.E.; Bresee, C.; Rapaport, M.H. Omega-3 Fatty Acid Augmentation of Citalopram Treatment for Patients with Major Depressive Disorder. J. Clin. Psychopharmacol. 2012, 32, 61–64. [Google Scholar] [CrossRef] [PubMed]
- Galizia, I.; Oldani, L.; Macritchie, K.; Amari, E.; Dougall, D.; Jones, T.N.; Lam, R.W.; Massei, G.J.; Yatham, L.N.; Young, A.H. S-adenosyl Methionine (SAMe) for Depression in Adults. Cochrane Database Syst. Rev. 2016, 2016, CD011286. [Google Scholar] [CrossRef]
- Papakostas, G.I.; Mischoulon, D.; Shyu, I.; Alpert, J.E.; Fava, M. S-Adenosyl Methionine (SAMe) Augmentation of Serotonin Reuptake Inhibitors for Antidepressant Nonresponders with Major Depressive Disorder: A Double-Blind, Randomized Clinical Trial. Am. J. Psychiatry 2010, 167, 942–948. [Google Scholar] [CrossRef]
- Liwinski, T.; Lang, U.E. Folate and Its Significance in Depressive Disorders and Suicidality: A Comprehensive Narrative Review. Nutrients 2023, 15, 3859. [Google Scholar] [CrossRef]
- S-Adenosyl-L-Methionine (SAMe): In Depth. Available online: https://www.nccih.nih.gov/health/sadenosyllmethionine-same-in-depth (accessed on 14 November 2024).
- Tillmann, S.; Happ, D.F.; Mikkelsen, P.F.; Geisel, J.; Wegener, G.; Obeid, R. Behavioral and Metabolic Effects of S-Adenosylmethionine and Imipramine in the Flinders Sensitive Line Rat Model of Depression. Behav. Brain Res. 2019, 364, 274–280. [Google Scholar] [CrossRef]
- Benelli, A.; Filaferro, M.; Bertolini, A.; Genedani, S. Influence of S-Adenosyl-L-Methionine on Chronic Mild Stress-Induced Anhedonia in Castrated Rats. Br. J. Pharmacol. 1999, 127, 645–654. [Google Scholar] [CrossRef] [PubMed]
- Sarris, J.; Papakostas, G.I.; Vitolo, O.; Fava, M.; Mischoulon, D. S-Adenosyl Methionine (SAMe) versus Escitalopram and Placebo in Major Depression RCT: Efficacy and Effects of Histamine and Carnitine as Moderators of Response. J. Affect. Disord. 2014, 164, 76–81. [Google Scholar] [CrossRef]
- Sarris, J.; Murphy, J.; Stough, C.; Mischoulon, D.; Bousman, C.; MacDonald, P.; Adams, L.; Nazareth, S.; Oliver, G.; Cribb, L.; et al. S-Adenosylmethionine (SAMe) Monotherapy for Depression: An 8-Week Double-Blind, Randomised, Controlled Trial. Psychopharmacology 2020, 237, 209–218. [Google Scholar] [CrossRef]
- Sarris, J.; Byrne, G.J.; Bousman, C.; Stough, C.; Murphy, J.; MacDonald, P.; Adams, L.; Nazareth, S.; Oliver, G.; Cribb, L.; et al. Adjunctive S-Adenosylmethionine (SAMe) in Treating Non-Remittent Major Depressive Disorder: An 8-Week Double-Blind, Randomized, Controlled Trial. Eur. Neuropsychopharmacol. 2018, 28, 1126–1136. [Google Scholar] [CrossRef]
- Altaf, R.; Gonzalez, I.; Rubino, K.; Nemec, E.C. Folate as Adjunct Therapy to SSRI/SNRI for Major Depressive Disorder: Systematic Review & Meta-Analysis. Complement. Ther. Med. 2021, 61, 102770. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Cong, Y.; Liu, H. Folic Acid Ameliorates Depression-like Behaviour in a Rat Model of Chronic Unpredictable Mild Stress. BMC Neurosci. 2020, 21, 1. [Google Scholar] [CrossRef]
- Réus, G.Z.; Maciel, A.L.; Abelaira, H.M.; de Moura, A.B.; de Souza, T.G.; dos Santos, T.R.; Darabas, A.C.; Parzianello, M.; Matos, D.; Abatti, M.; et al. ω-3 and Folic Acid Act against Depressive-like Behavior and Oxidative Damage in the Brain of Rats Subjected to Early- or Late-Life Stress. Nutrition 2018, 53, 120–133. [Google Scholar] [CrossRef]
- Shelton, R.C.; Manning, J.S.; Barrentine, L.W.; Tipa, E.V. Assessing Effects of L-Methylfolate in Depression Management: Results of a Real-World Patient Experience Trial. Prim. Care Companion CNS Disord. 2013, 15, 23902. [Google Scholar] [CrossRef]
- Reynolds, E.; Crellin, R.; Bottiglieri, T.; Laundy, M.; Toone, B.; Carney, M. Methylfolate as Monotherapy in Depression. A Pilot Randomised Controlled Trial. J. Neurol. Psychol. 2015, 3, 5. [Google Scholar] [CrossRef]
- Coppen, A.; Bailey, J. Enhancement of the Antidepressant Action of Fluoxetine by Folic Acid: A Randomised, Placebo Controlled Trial. J. Affect. Disord. 2000, 60, 121–130. [Google Scholar] [CrossRef]
- Ginsberg, L.D.; Oubre, A.Y.; DaouD, Y.A. L-Methylfolate Plus SSRI or SNRI from Treatment Initiation Compared to SSRI or SNRI Monotherapy in a Major Depressive Episode. Innov. Clin. Neurosci. 2011, 8, 19–28. [Google Scholar] [PubMed]
- Kennedy, D. B Vitamins and the Brain: Mechanisms, Dose and Efficacy—A Review. Nutrients 2016, 8, 68. [Google Scholar] [CrossRef] [PubMed]
- Noah, L.; Dye, L.; Bois De Fer, B.; Mazur, A.; Pickering, G.; Pouteau, E. Effect of Magnesium and Vitamin B6 Supplementation on Mental Health and Quality of Life in Stressed Healthy Adults: Post-hoc Analysis of a Randomised Controlled Trial. Stress Health 2021, 37, 1000–1009. [Google Scholar] [CrossRef] [PubMed]
- Kafeshani, M.; Feizi, A.; Esmaillzadeh, A.; Keshteli, A.H.; Afshar, H.; Roohafza, H.; Adibi, P. Higher Vitamin B6 Intake Is Associated with Lower Depression and Anxiety Risk in Women but Not in Men: A Large Cross-Sectional Study. Int. J. Vitam. Nutr. Res. 2019, 90, 484–492. [Google Scholar] [CrossRef]
- Field, D.T.; Cracknell, R.O.; Eastwood, J.R.; Scarfe, P.; Williams, C.M.; Zheng, Y.; Tavassoli, T. High-Dose Vitamin B6 Supplementation Reduces Anxiety and Strengthens Visual Surround Suppression. Hum. Psychopharmacol. Clin. Exp. 2022, 37, e2852. [Google Scholar] [CrossRef]
- Mikkelsen, K.; Stojanovska, L.; Apostolopoulos, V. The Effects of Vitamin B in Depression. Curr. Med. Chem. 2016, 23, 4317–4337. [Google Scholar] [CrossRef] [PubMed]
- Mikkelsen, K.; Stojanovska, L.; Prakash, M.; Apostolopoulos, V. The Effects of Vitamin B on the Immune/Cytokine Network and Their Involvement in Depression. Maturitas 2017, 96, 58–71. [Google Scholar] [CrossRef]
- Mahdavifar, B.; Hosseinzadeh, M.; Salehi-Abargouei, A.; Mirzaei, M.; Vafa, M. Dietary Intake of B Vitamins and Their Association with Depression, Anxiety, and Stress Symptoms: A Cross-Sectional, Population-Based Survey. J. Affect. Disord. 2021, 288, 92–98. [Google Scholar] [CrossRef]
- Almeida, O.P.; Ford, A.H.; Hirani, V.; Singh, V.; vanBockxmeer, F.M.; McCaul, K.; Flicker, L. B Vitamins to Enhance Treatment Response to Antidepressants in Middle-Aged and Older Adults: Results from the B-VITAGE Randomised, Double-Blind, Placebo-Controlled Trial. Br. J. Psychiatry 2014, 205, 450–457. [Google Scholar] [CrossRef]
- Borges-Vieira, J.G.; Cardoso, C.K.S. Efficacy of B-Vitamins and Vitamin D Therapy in Improving Depressive and Anxiety Disorders: A Systematic Review of Randomized Controlled Trials. Nutr. Neurosci. 2023, 26, 187–207. [Google Scholar] [CrossRef]
- Laird, E.J.; O’Halloran, A.M.; Molloy, A.M.; Healy, M.; Hernandez, B.; O’Connor, D.M.A.; Kenny, R.A.; Briggs, R. Low Vitamin B12 but Not Folate Is Associated with Incident Depressive Symptoms in Community-Dwelling Older Adults: A 4-Year Longitudinal Study. Br. J. Nutr. 2023, 130, 268–275. [Google Scholar] [CrossRef]
- Khosravi, M.; Sotoudeh, G.; Amini, M.; Raisi, F.; Mansoori, A.; Hosseinzadeh, M. The Relationship between Dietary Patterns and Depression Mediated by Serum Levels of Folate and Vitamin B12. BMC Psychiatry 2020, 20, 63. [Google Scholar] [CrossRef] [PubMed]
- Markun, S.; Gravestock, I.; Jäger, L.; Rosemann, T.; Pichierri, G.; Burgstaller, J.M. Effects of Vitamin B12 Supplementation on Cognitive Function, Depressive Symptoms, and Fatigue: A Systematic Review, Meta-Analysis, and Meta-Regression. Nutrients 2021, 13, 923. [Google Scholar] [CrossRef]
- Bičíková, M.; Dušková, M.; Vítků, J.; Kalvachová, B.; Řípová, D.; Mohr, P.; Stárka, L. Vitamin D in Anxiety and Affective Disorders. Physiol. Res. 2015, 64, S101–S103. [Google Scholar] [CrossRef]
- Albarri, E.M.A.; Alnuaimi, A.S.; Abdelghani, D. Effectiveness of Vitamin D2 Compared with Vitamin D3 Replacement Therapy in a Primary Healthcare Setting: A Retrospective Cohort Study. Qatar Med. J. 2022, 2022, 29. [Google Scholar] [CrossRef]
- Office of Dietary Supplements—Vitamin D. Available online: https://ods.od.nih.gov/factsheets/VitaminD-HealthProfessional/ (accessed on 13 November 2024).
- Aucoin, M.; Cooley, K.; Anand, L.; Furtado, M.; Canzonieri, A.; Fine, A.; Fotinos, K.; Chandrasena, R.; Klassen, L.J.; Epstein, I.; et al. Adjunctive Vitamin D in the Treatment of Non-Remitted Depression: Lessons from a Failed Clinical Trial. Complement. Ther. Med. 2018, 36, 38–45. [Google Scholar] [CrossRef]
- Jahan-Mihan, A.; Stevens, P.; Medero-Alfonso, S.; Brace, G.; Overby, L.K.; Berg, K.; Labyak, C. The Role of Water-Soluble Vitamins and Vitamin D in Prevention and Treatment of Depression and Seasonal Affective Disorder in Adults. Nutrients 2024, 16, 1902. [Google Scholar] [CrossRef] [PubMed]
- Galima, S.V.; Vogel, S.R.; Kowalski, A.W. Seasonal Affective Disorder: Common Questions and Answers. Am. Fam. Physician 2020, 102, 668–672. [Google Scholar]
- Bakhtiari-Dovvombaygi, H.; Izadi, S.; Zare Moghaddam, M.; Hashemzehi, M.; Hosseini, M.; Azhdari-Zarmehri, H.; Dinpanah, H.; Beheshti, F. Beneficial Effects of Vitamin D on Anxiety and Depression-like Behaviors Induced by Unpredictable Chronic Mild Stress by Suppression of Brain Oxidative Stress and Neuroinflammation in Rats. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2021, 394, 655–667. [Google Scholar] [CrossRef]
- Sedaghat, K.; Yousefian, Z.; Vafaei, A.A.; Rashidy-Pour, A.; Parsaei, H.; Khaleghian, A.; Choobdar, S. Mesolimbic Dopamine System and Its Modulation by Vitamin D in a Chronic Mild Stress Model of Depression in the Rat. Behav. Brain Res. 2019, 356, 156–169. [Google Scholar] [CrossRef]
- Al-Ramadhan, F.R.; Abulmeaty, M.M.A.; Alquraishi, M.; Razak, S.; Alhussain, M.H. Effect of Vitamin D3 on Depressive Behaviors of Rats Exposed to Chronic Unpredictable Mild Stress. Biomedicines 2023, 11, 2112. [Google Scholar] [CrossRef]
- Kaviani, M.; Nikooyeh, B.; Zand, H.; Yaghmaei, P.; Neyestani, T.R. Effects of Vitamin D Supplementation on Depression and Some Involved Neurotransmitters. J. Affect. Disord. 2020, 269, 28–35. [Google Scholar] [CrossRef]
- Choukri, M.A.; Conner, T.S.; Haszard, J.J.; Harper, M.J.; Houghton, L.A. Effect of Vitamin D Supplementation on Depressive Symptoms and Psychological Wellbeing in Healthy Adult Women: A Double-Blind Randomised Controlled Clinical Trial. J. Nutr. Sci. 2018, 7, e23. [Google Scholar] [CrossRef] [PubMed]
- Khoraminya, N.; Tehrani-Doost, M.; Jazayeri, S.; Hosseini, A.; Djazayery, A. Therapeutic Effects of Vitamin D as Adjunctive Therapy to Fluoxetine in Patients with Major Depressive Disorder. Aust. N. Z. J. Psychiatry 2013, 47, 271–275. [Google Scholar] [CrossRef]
- Zhao, W.; Zhu, D.; Shen, Y.; Zhang, Y.; Chen, T.; Cai, H.; Zhu, J.; Yu, Y. The Protective Effect of Vitamin D Supplementation as Adjunctive Therapy to Antidepressants on Brain Structural and Functional Connectivity of Patients with Major Depressive Disorder: A Randomized Controlled Trial. Psychol. Med. 2024, 54, 2403–2413. [Google Scholar] [CrossRef] [PubMed]
- Moshfeghinia, R.; Nemati, H.; Ebrahimi, A.; Shekouh, D.; Karami, S.; Eraghi, M.M.; Mohagheghzadeh, H.; Hunter, J.; Pasalar, M. The Impact of Probiotics, Prebiotics, and Synbiotics on Depression and Anxiety Symptoms of Patients with Depression: A Systematic Review and Meta-Analysis. J. Psychiatr. Res. 2025, 188, 104–116. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Chen, B.; Zhang, J.; Dong, J.; Ma, J.; Zhang, Y.; Jin, K.; Lu, J. Effect of Prebiotics, Probiotics, Synbiotics on Depression: Results from a Meta-Analysis. BMC Psychiatry 2023, 23, 477. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).