Evaluation of Antioxidant-Rich Fruit Extracts to Improve the Bioactive Compounds of Apple Slices
Abstract
1. Introduction
2. Materials and Methods
2.1. Vegetal Materials and Reagents
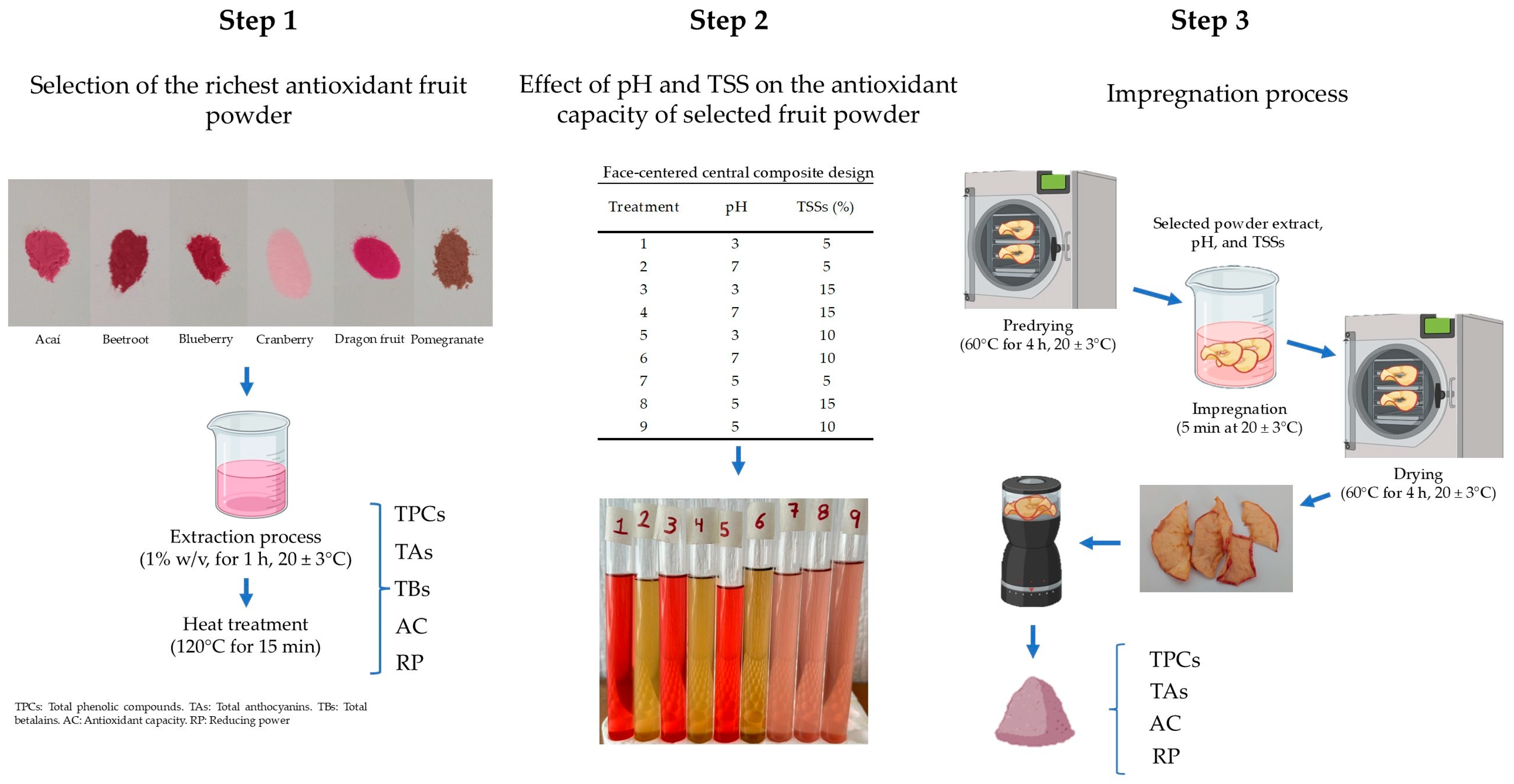
2.2. Evaluation of Bioactive Compounds and Antioxidant Capacity of Antioxidant-Rich Fruit Powder
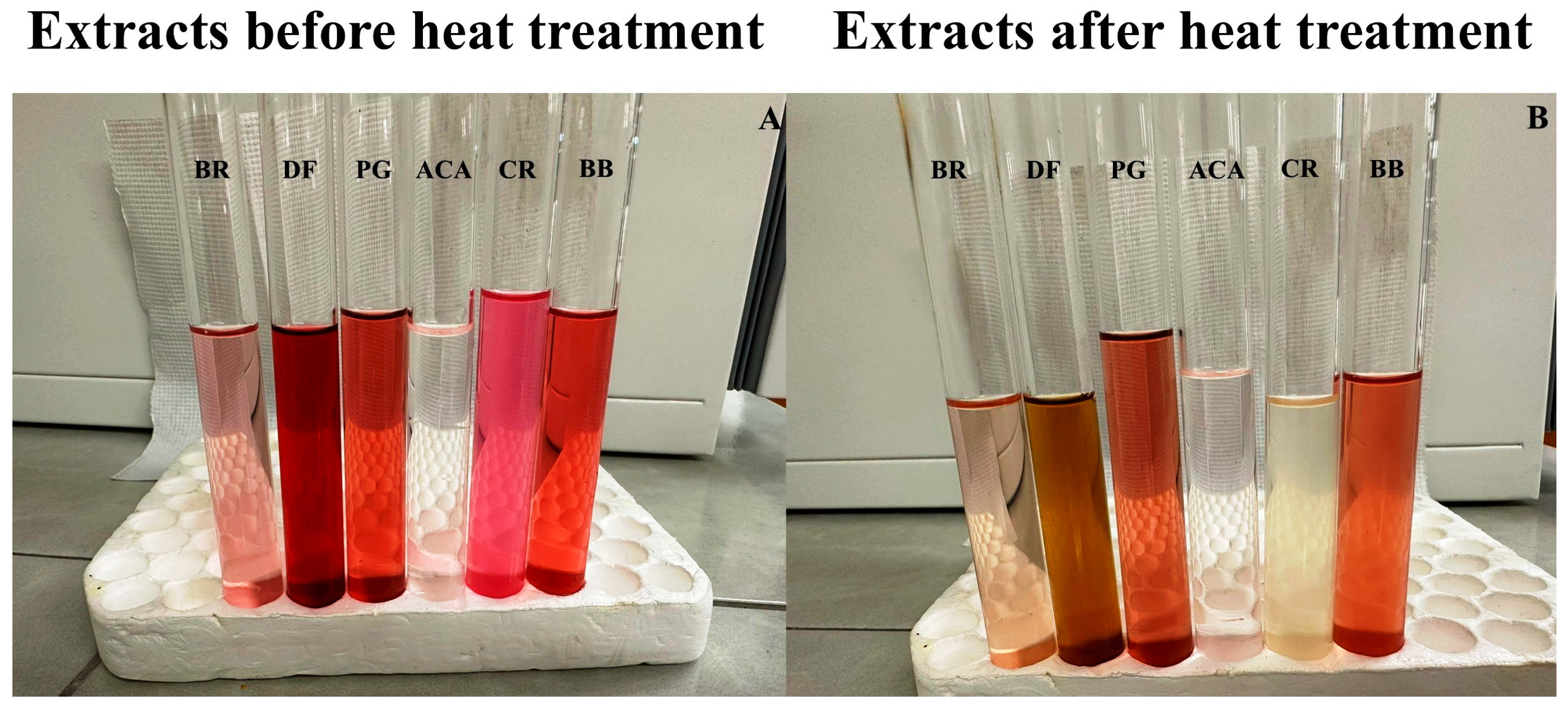
2.3. Effect of Heat Treatment on the Bioactive Compounds and Antioxidant Capacity of Antioxidant-Rich Fruit Powder
2.4. Effect of the pH and TSSs on the Stability of Bioactive Compounds and Antioxidant Capacity of Selected Fruit Extracts
2.5. Impregnation and Drying Processes of Apple Slices
2.6. Total Phenolic Compounds of Antioxidant-Rich Fruit and Apple Powders
2.7. The Total Betalains in Fruit Powders That Contain Them in Their Composition
2.8. The Total Anthocyanins in Fruit Powders That Contain Them in Their Composition
2.9. Antioxidant Capacity (DPPH Assay) of Antioxidant-Rich Fruit and Apple Powders
2.10. Reducing Power of Antioxidant-Rich Fruit and Apple Powders
2.11. Statistical Analysis
3. Results and Discussion
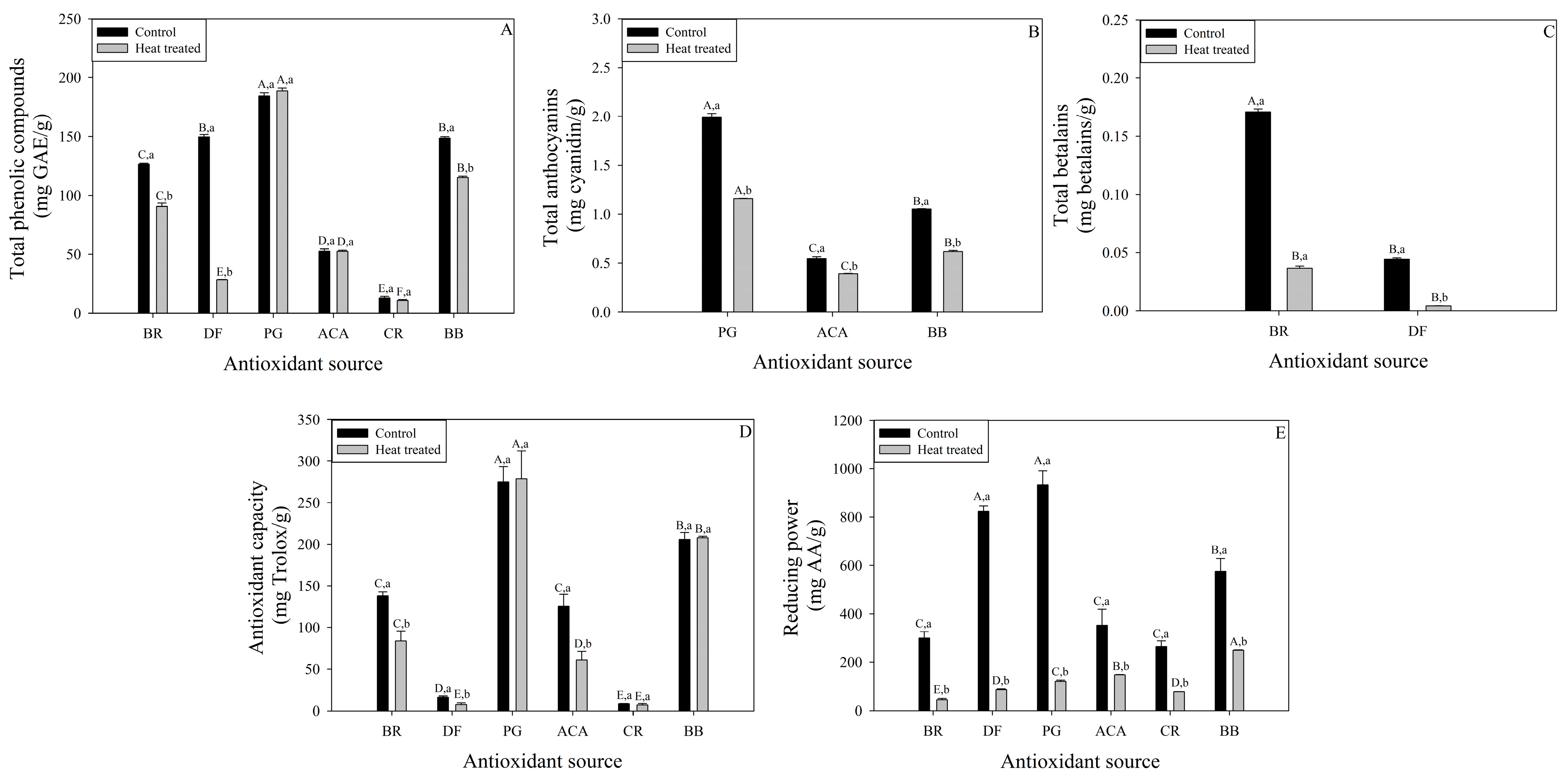
3.1. Bioactive Compounds and Antioxidant Capacity-Rich Fruit Powder
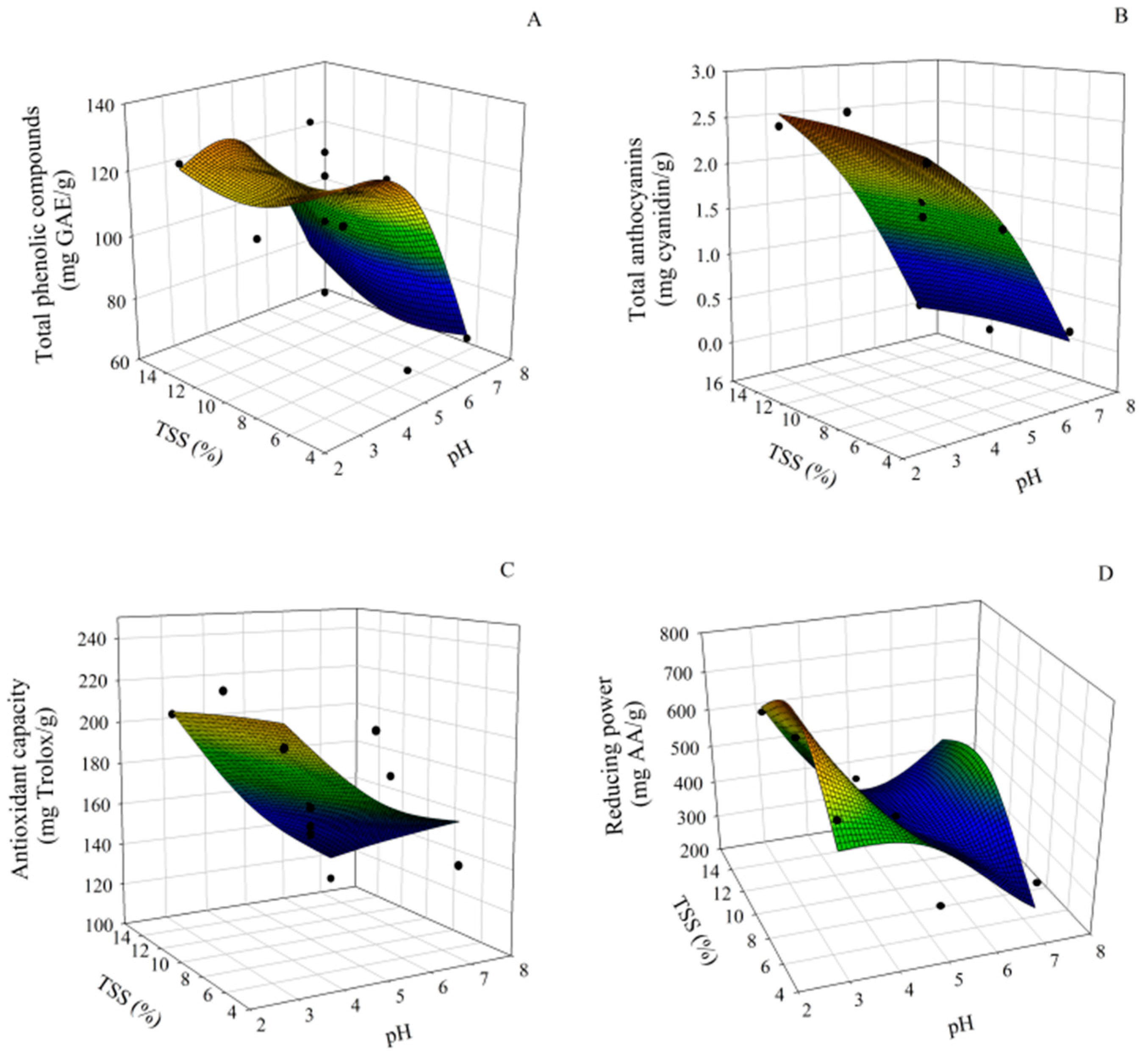
3.2. Effect of pH and TSSs on the Stability of Bioactive Compounds and Antioxidant Capacity of Pomegranate Extract
3.3. Impregnation of Bioactive Compounds from Pomegranate Extracts at Different pH to Apple Slices
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| TPCs | Total phenolic compounds |
| AC | Antioxidant capacity |
| TAs | Total anthocyanins |
| RP | Reduction power |
| BR | Beetroot |
| DF | Dragon fruit |
| PG | Pomegranate |
| CR | Cranberry |
| BB | Blueberry |
| ACA | Acaí |
References
- d’Astous, A.; Labrecque, J. The impact of responsible food packaging perceptions on naturalness and healthiness inferences, and consumer buying intentions. Foods 2021, 10, 2366. [Google Scholar] [CrossRef] [PubMed]
- Spence, C. On the manipulation, and meaning(s), of color in food: A historical perspective. J. Food Sci. 2023, 88, A5–A20. [Google Scholar] [CrossRef] [PubMed]
- Cömert, E.D.; Mogol, B.A.; Gökmen, V. Relationship between color and antioxidant capacity of fruits and vegetables. Curr. Res. Food Sci. 2020, 2, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Spence, C.; Motoki, K.; Petit, O. Factors influencing the visual deliciousness/eye-appeal of food. Food Qual. Prefer. 2022, 102, 104672. [Google Scholar] [CrossRef]
- Paakki, M.; Sandell, M.; Hopia, A. Visual attractiveness depends on colorfulness and color contrasts in mixed Salads. Food Qual. Prefer. 2019, 76, 81–90. [Google Scholar] [CrossRef]
- Ghosh, S.; Sarkar, T.; Das, A.; Chakraborty, R. Natural colorants from plant pigments and their encapsulation: An emerging window for the food industry. LWT-Food Sci. Technol. 2022, 153, 112527. [Google Scholar] [CrossRef]
- Pandiselvam, R.; Mitharwal, R.; Rani, P.; Shanker, M.A.; Kumar, A.; Aslam, R.; Barut, Y.T.; Kothakota, A.; Rustagi, S.; Bhati, D.; et al. The influence of non-thermal technologies on color pigments of food materials: An updated review. Curr. Res. Food Sci. 2023, 6, 100529. [Google Scholar] [CrossRef] [PubMed]
- González de Mejía, E.; Zhang, Q.; Penta, K.; Eroglu, A.; Lila, M.A. The colors of health: Chemistry, bioactivity, and market demand for colorful foods and natural food sources of colorants. Annu. Rev. Food Sci. Technol. 2020, 11, 145–182. [Google Scholar] [CrossRef] [PubMed]
- Guevara, M.; Tejera, E.; Granda-Albija, M.G.; Iturralde, G.; Chisaguano-Tonato, M.; Granda-Albuja, S.; Jaramillo-Vivanco, T.; Giampieri, F.; Battino, M.; Alvarez-Suarez, J.M. Chemical composition and antioxidant activity of the main fruits consumed in the western coastal region of Ecuador as a source of health-promoting compounds. Antioxidants 2019, 8, 387. [Google Scholar] [CrossRef] [PubMed]
- Prieto-Santiago, V.; Cavia, M.M.; Alonso-Torre, S.R.; Carrillo, C. Relationship between color and betalain content in different thermally treated beetroot products. J. Food Sci. Technol. 2020, 57, 3305–3313. [Google Scholar] [CrossRef] [PubMed]
- Rojas-Bravo, M.; Rojas-Zenteno, E.G.; Hernández-Carranza, P.; Ávila-Sosa, R.; Aguilar-Sánchez, R.; Ruiz-López, I.I.; Ochoa-Velasco, C.E. A potential application of mango (Mangifera indica L. cv Manila) peel powder to increase the total phenolic compounds and antioxidant capacity of edible films and coatings. Food Bioprocess Technol. 2019, 12, 1584–1592. [Google Scholar] [CrossRef]
- Wojdyło, A.; Oszmiański, J. Antioxidant activity modulated by polyphenol contents in apple and leaves during fruit development and ripening. Antioxidants 2020, 9, 567. [Google Scholar] [CrossRef] [PubMed]
- Kidón, M.; Grabowska, J. Bioactive compounds, antioxidant activity, and sensory qualities of red-fleshed apples dried by different methods. LWT-Food Sci. Technol. 2021, 136, 110302. [Google Scholar] [CrossRef]
- Aguirre-García, M.; Hernández-Carranza, P.; Cortés-Zavaleta, O.; Ruiz-Espinosa, H.; Ochoa-Velasco, C.E.; Ruiz-López, I.I. Mass transfer analysis of bioactive compounds in apple wedges impregnated with beetroot juice: A 3D modelling approach. J. Food Eng. 2020, 282, 110003. [Google Scholar] [CrossRef]
- Hernández-Carranza, P.; Heredia-Soberanes, K.; Ruiz-López, I.I.; Ochoa-Velasco, C.E. Effect of impregnation-osmodehydration with Hibiscus sabdariffa extracts on the bioactive compounds and sensory acceptance of apple wedges: Fresh, convective dried, and stored. J. Food Process. Preserv. 2022, 46, e17110. [Google Scholar] [CrossRef]
- Dinçer, C. Modeling of hibiscus anthocyanins transport to apple tissue during ultrasound-assisted vacuum impregnation. J. Food Process. Preserv. 2022, 46, e15886. [Google Scholar] [CrossRef]
- Castagnini, J.M.; Betoret, N.; Betoret, E.; Fito, P. Vacuum impregnation and air drying temperature effect on individual anthocyanins and antiradical capacity of blueberry juice included into an apple matrix. LWT-Food Sci. Technol. 2015, 64, 1289–1296. [Google Scholar] [CrossRef]
- Tappi, S.; Tylewicz, U.; Romani, S.; Rosa, M.; Rizzi, F.; Rocculi, P. Study on the quality and stability of minimally processed apples impregnated with green tea polyphenols during storage. Innov. Food Sci. Emerg. Technol. 2017, 39, 148–155. [Google Scholar] [CrossRef]
- Yilmaz, F.M.; Bilek, S.E. Natural colorant enrichment of apple tissue with black carrot concentrate using vacuum impregnation. Int. J. Food Sci. Technol. 2017, 52, 1508–1516. [Google Scholar] [CrossRef]
- Nawirska-Olszańska, A.; Pasławska, M.; Stepień, B.; Oziembłowski, M.; Sala, K.; Smorowska, A. Effect of vacuum impregnation with apple-pear juice on content of bioactive compounds and antioxidant activity of dried chokeberry fruit. Foods 2020, 9, 108. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Marti, M.; Jafari, S.M.; Rashidinejad, A.; Xiao, J.; Simal-Gandara, J. An overview of different food bioactive ingredients. In Handbook of Food Bioactive Ingredients. Properties and Applications, 3rd ed.; Jafari, S.M., Rashidinejad, A., Simal-Gandara, J., Eds.; Springer: Cham, Switzerland, 2023; Volume 11, p. 24. [Google Scholar]
- Essid, I.; Tajine, S.; Gharbi, S.; Bellagha, S. Use of pomegranate peel and artichoke leaf extracts to improve the quality of marinated sardine (Sardinella aurita) fillets. J. Food Sci. Technol. 2020, 57, 713–722. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Carranza, P.; Ávila-Sosa, R.; Guerrero-Beltrán, J.A.; Navarro-Cruz, A.R.; Corona-Jiménez, E.; Ochoa-Velasco, C.E. Optimization of antioxidant compounds extraction from fruit byproducts: Apple pomace, orange and banana peel. J. Food Process. Preserv. 2016, 40, 103–115. [Google Scholar] [CrossRef]
- Aparicio-Fernández, X.; Vega-Ahuatzin, A.; Ochoa-Velasco, C.E.; Cid-Pérez, S.; Hernández-Carranza, P.; Ávila-Sosa, R. Physical and antioxidant characterization of edible films added with red prickly pear (Opuntia ficus-indica L.) cv. San Martín peel and/or its aqueous extracts. Food Bioprocess Technol. 2018, 11, 368–379. [Google Scholar] [CrossRef]
- Vega-Arroy, J.D.; Ruiz-Espinosa, H.; Luna-Guevara, J.J.; Luna-Guevara, M.L.; Hernández-Carranza, P.; Ávila-Sosa, R.; Ochoa-Velasco, C.E. Effect of solvents and extraction methods on total anthocyanins, phenolic compounds and antioxidant capacity of Renealmia alpinia (Rottb.) maas peel. Czech J. Food Sci. 2017, 35, 456–465. [Google Scholar] [CrossRef]
- Dorman, H.J.D.; Koşar, M.; Kahlos, K.; Holm, Y.; Hiltunen, R. Antioxidant properties and composition of aqueous extracts from Mentha species, hybrids, varieties, and cultivars. J. Agric. Food Chem. 2003, 51, 4563–4569. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Yuan, Z. Anthocyanins from pomegranate (Punica granatum L.) and their role in antioxidant capacities in vitro. Chem. Biodivers. 2021, 18, e2100399. [Google Scholar] [CrossRef] [PubMed]
- Pirzadeh, M.; Caporaso, N.; Rauf, A.; Shariati, M.A.; Yessimbekov, Z.; Khan, M.U.; Imran, M.; Mubarak, M.S. Pomegranate as a source of bioactive constituents: A review on their characterization, properties and applications. Crit. Rev. Food Sci. Nutr. 2021, 61, 982–999. [Google Scholar] [CrossRef] [PubMed]
- Mena, P.; Vegara, S.; Martí, N.; García-Viguera, C.; Saura, D.; Valero, M. Changes on indigenous microbiota, colour, bioactive compounds and antioxidant activity of pasteurised pomegranate juice. Food Chem. 2013, 141, 2122–2129. [Google Scholar] [CrossRef] [PubMed]
- Masci, A.; Coccia, A.; Lendaro, E.; Mosca, L.; Paolicelli, P.; Cesa, S. Evaluation of different extraction methods from pomegranate whole fruit or peels and the antioxidant and antiproliferative activity of the polyphenolic fraction. Food Chem. 2016, 202, 59–69. [Google Scholar] [CrossRef] [PubMed]
- Rababah, T.M.; Banat, F.; Rababah, A.; Ereifej, K.; Yang, W. Optimization of extraction conditions of total phenolics, antioxidant activities, and anthocyanin of oregano, thyme, terebinth, and pomegranate. J. Food Sci. 2010, 75, C626–C632. [Google Scholar] [CrossRef] [PubMed]
- Gil, M.I.; Tomás-Barberán, A.; Hess-Pierce, B.; Holcroft, D.; Kader, A.A. Antioxidant activity of pomegranate juice and its relationship with phenolic composition and processing. J. Agric. Food Chem. 2000, 48, 4581–4589. [Google Scholar] [CrossRef] [PubMed]
- Jiang, T.; Mao, Y.; Sui, L.; Yang, N.; Li, S.; Zhu, Z.; Wang, C.; Yin, S.; He, J.; He, Y. Degradation of anthocyanins and polymeric color formation during heat treatment of purple sweet potato extract at different pH. Food Chem. 2019, 274, 460–470. [Google Scholar] [CrossRef] [PubMed]
- Enaru, B.; Dretcanu, G.; Pop, T.D.; Stănilă, A.; Diaconeasa, Z. Anthocyanins: Factors affecting their stability and degradation. Antioxidants 2021, 10, 1967. [Google Scholar] [CrossRef] [PubMed]
- Wahyuningsih, S.E.; Wulandari, L.; Wartono, W.; Munawaroh, H.; Ramelan, A.H. The effect of pH and color stability of anthocyanin on food colorant. IOP Conf. Ser. Mater. Sci. Eng. 2016, 193, 012047. [Google Scholar] [CrossRef]
- Li, S.; Wang, X.; Zhang, X.; Zhanf, H.; Li, S.; Zhou, J.; Fan, L. Interactions between zein and anthocyanins at different pH: Structural characterization, binding mechanism and stability. Food Res. Int. 2023, 166, 112552. [Google Scholar] [CrossRef] [PubMed]
- Vinod, B.R.; Asrey, R.; Sethi, S.; Menaka, M.; Kumar, N.; Shivaswamy, G. Recent advaces in vacuum impregnation of fruits and vegetables processing: A concise review. Heliyon 2024, 10, e28023. [Google Scholar] [CrossRef] [PubMed]
- Saleena, P.; Jayashree, E.; Anees, K. A comprehensive review on vacuum impregnation: Mechanism, applications and prospects. Food Bioprocess Technol. 2024, 17, 1434–1447. [Google Scholar] [CrossRef]

| TPCs | TAs|TBs | AC | RP | |
|---|---|---|---|---|
| TPCs a | 1 | 0.591 †/0.888 ‡ | 0.625/0.967 | 0.808/0.872 |
| TAs b|TBs c | 1 | 0.904/0.931 | 0.629/0.975 | |
| AC d | 1 | 0.403/0.898 | ||
| RP e | 1 | |||
| Treatment | pH | TSSs (%) | TPCs b (mg GAE/g) | TAs c (mg Cyanidin/g) | AC d (mg Trolox/g) | RP e (mg AA/g) |
|---|---|---|---|---|---|---|
| 1 | 3 | 5 | 125.70 ± 0.92 a | 2.27 ± 0.01 b | 203.44 ± 4.18 ab | 510.70 ± 4.48 a |
| 2 | 7 | 5 | 69.20 ± 6.32 b | 0.26 ± 0.05 d | 142.52 ± 11.47 cd | 265.74 ± 2.56 c |
| 3 | 3 | 15 | 118.61 ± 3.22 a | 2.39 ± 0.10 b | 203.62 ± 3.84 ab | 614.85 ± 2.31 bc |
| 4 | 7 | 15 | 82.04 ± 3.66 b | 0.05 ± 0.04 e | 110.23 ± 28.81 d | 453.94 ± 4.99 bc |
| 5 | 3 | 10 | 118.49 ± 3.77 a | 2.65 ± 0.13 a | 220.89 ± 3.97 a | 718.57 ± 4.00 bc |
| 6 | 7 | 10 | 67.77 ± 2.77 c | 0.02 ± 0.03 e | 174.04 ± 17.99 bc | 497.48 ± 2.31 bc |
| 7 | 5 | 5 | 123.47 ± 9.40 a | 1.50± 0.06 c | 207.57 ± 1.34 ab | 466.10 ± 1.11 bc |
| 8 | 5 | 15 | 120.81 ± 3.60 a | 1.70 ± 0.03 c | 163.30 ± 12.43 c | 344.63 ± 4.62 bc |
| 9 | 5 | 10 | 118.12 ± 4.45 a | 1.70 ± 0.04 c | 163.30 ± 12.91 c | 390.23 ± 5.47 b |
| Compounds | β0 | β1 | β2 | β3 | β4 | β5 | R2 |
|---|---|---|---|---|---|---|---|
| TPCs a | 97.03 | 35.60 | −8.78 | −5.26 | 0.32 | 0.50 | 0.96 |
| TAs b | 1.89 | 0.30 | 0.08 | −0.08 | −0.00 | −0.01 | 0.99 |
| AC c | 299.57 | −36.32 | 2.20 | 2.77 | −0.03 | −0.81 | 0.72 |
| RP d | 1348.77 | −424.15 | 45.03 | 35.90 | −2.49 | 2.10 | 0.74 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hernández-Carranza, P.; Avila-Zarco, K.C.; del Carmen Beristain-Bauza, S.; Ramírez-López, C.; Ruiz-López, I.I.; Ochoa-Velasco, C.E. Evaluation of Antioxidant-Rich Fruit Extracts to Improve the Bioactive Compounds of Apple Slices. Nutraceuticals 2025, 5, 18. https://doi.org/10.3390/nutraceuticals5030018
Hernández-Carranza P, Avila-Zarco KC, del Carmen Beristain-Bauza S, Ramírez-López C, Ruiz-López II, Ochoa-Velasco CE. Evaluation of Antioxidant-Rich Fruit Extracts to Improve the Bioactive Compounds of Apple Slices. Nutraceuticals. 2025; 5(3):18. https://doi.org/10.3390/nutraceuticals5030018
Chicago/Turabian StyleHernández-Carranza, Paola, Katya Chantal Avila-Zarco, Silvia del Carmen Beristain-Bauza, Carolina Ramírez-López, Irving Israel Ruiz-López, and Carlos Enrique Ochoa-Velasco. 2025. "Evaluation of Antioxidant-Rich Fruit Extracts to Improve the Bioactive Compounds of Apple Slices" Nutraceuticals 5, no. 3: 18. https://doi.org/10.3390/nutraceuticals5030018
APA StyleHernández-Carranza, P., Avila-Zarco, K. C., del Carmen Beristain-Bauza, S., Ramírez-López, C., Ruiz-López, I. I., & Ochoa-Velasco, C. E. (2025). Evaluation of Antioxidant-Rich Fruit Extracts to Improve the Bioactive Compounds of Apple Slices. Nutraceuticals, 5(3), 18. https://doi.org/10.3390/nutraceuticals5030018






