Abstract
The marine seaweed Undaria pinnatifida belongs to the large group of brown macroalgae (Ochrophyta) and is valued both as a nutritious food and a source of pharmaceutical compounds. It has been widely consumed in East Asia as part of the traditional diet and is generally regarded as a “healthy longevity food.” Consequently, it represents one of the most promising natural sources of biomedicinal and bioactive products. This review aims to synthesize current scientific evidence on the pharmacologically active compounds of U. pinnatifida, emphasizing their mechanisms of action and therapeutic potential in neurodegenerative and chronic diseases. This narrative review is based on a comprehensive literature search of peer-reviewed articles from scientific databases, focusing on studies addressing the pharmacological properties of U. pinnatifida and its major bioactive constituents. Recent research highlights that compounds such as fucoxanthin (a carotenoid), fucosterol (a sterol), fucoidan (a polysaccharide), alginate, and dietary fiber found in U. pinnatifida possess significant potential for developing treatments for conditions including goitre, urinary diseases, scrofula, dropsy, stomach ailments, and hemorrhoids. Moreover, these compounds exhibit remarkable pharmacological properties, including immunomodulation, antitumor, antiviral, antioxidant, antidiabetic, anti-inflammatory, anticoagulant, antithrombotic, and antibacterial activities, all with low toxicity and minimal side effects. Additionally, U. pinnatifida shows promise in the treatment or prevention of neurodegenerative diseases such as Alzheimer’s and Parkinson’s, as well as neuropsychiatric conditions like depression, supported by its antioxidant effects against oxidative stress and neuroprotective activities. Numerous in vitro and in vivo studies have confirmed that U. pinnatifida polysaccharides (UPPs), particularly fucoidans, exhibit significant biological activities. Thus, accumulating evidence positions UPPs as promising therapeutic agents for a variety of diseases.
1. Introduction
The macroalgae Undaria pinnatifida, also known as “sea mustard” (in English), qundaicai (in Chinese) or Wakame (in Japanese), is a notorious marine plant with relevant edible and pharmaceutical applications. Native to China, Japan, Korea, and Russia, this seaweed (from the order Laminariales and family Alariaceae [1]) has since been introduced to nearly every other continent [2,3,4].
U. pinnatifida widespread global consumption is attributable to its high nutritional quality and a wealth of bioactive constituents [3,5,6]. Historically, it has been integrated into the traditional diets of East Asia, particularly China, Japan, and Korea. Simultaneously, it has been widely regarded in numerous Western nations as a ‘longevity food’ or ‘sea vegetable’, with its use spanning a considerable duration [3,7]. Beyond its traditional role as an edible and medicinal alga in East Asia, U. pinnatifida is widely incorporated into health foods and food supplements. Its nutritional value is commendable, containing good amounts of protein, dietary fiber, vitamin A and other vitamins like vitamin B12 and folate, as well as high concentrations of calcium, sodium, potassium, iron, and magnesium [8,9], and traditionally used as a nutritional support during the postnatal recovery phase [10]. Examples of common medicinal uses are for fever and urinary disease treatment and for swelling and lumps [10].
U. pinnatifida belongs to the large group of brown (Ochrophyta), which together with green (Chlorophyta) and red (Rhodophyta) macroalgae are the most promising as biomedicine products. This is due to its structural uniqueness and its functional diversity, as it contains compounds or metabolites of medicinal interest such as phenolic compounds, terpenoids, flavonoids, carotenoids, phytosterols, proteins, fatty acids, alkaloids and polysaccharides [11]. Several in vitro and in vivo research with U. pinnatifida have confirmed that U. pinnatifida polysaccharides (UPPs), particularly fucoidans, have various biological activities [3]. They also present high fiber content and low energy, being very popular among consumers [12,13,14]. Recent scientific evidence shows that compounds such as fucoxanthin (carotenoid), fucosterol (sterol) and fucoidan (polysaccharide) present in these seaweeds have great potential in the development of treatments against various diseases [11]. In fact, in China, these substances have been documented for their application in treating conditions such as goiter, urinary disease, scrofula, dropsy, various stomach ailments, and hemorrhoids [3,15,16,17].
Polysaccharides are abundant constituents of U. pinnatifida, representing a significant class of biologically active biomacromolecules [18]. It is well-established that the diverse pharmacological activities of polysaccharides are directly linked to their unique structural features, with their various biological effects strongly correlating with their specific chemical compositions and structural characteristics. Consequently, numerous studies have explored the structure-activity relationships of UPPs.
Pharmacologists and chemists are increasingly focusing on the pharmacological activities and biofunctional properties of UPPs. This growing interest is driven by their rich array of efficacious ingredients, associated beneficial effects, and highly diversified structural characteristics [3,19,20]. It’s widely recognized that the pharmacological activity of polysaccharides derived from natural sources is intimately associated with their chemical attributes, including monosaccharide composition, glycosidic linkages, molecular weights, and conformational features [3,21]. Over recent decades, accumulating evidence has demonstrated that polysaccharides isolated from U. pinnatifida, primarily fucoidan, alginate, and dietary fiber [3,22,23,24], exhibit remarkable and diverse pharmacological properties. These include immunomodulatory, antitumor, antiviral, antioxidant, antidiabetic, anti-inflammatory, anticoagulant, antithrombotic, and antibacterial effects. In favour, these beneficial properties are often observed with lower toxicity and fewer side effects [3,25,26,27,28,29,30,31]. The inherent cell wall polysaccharides obtained from U. pinnatifida have been thoroughly and extensively researched [3,32]. Furthermore, UPPs are extensively utilised in the synthetic biomaterials, functional food, and pharmaceutical industries because of their low toxicity, effectiveness, and biocompatibility [3,33,34].
This narrative review is based on a comprehensive literature search of peer-reviewed articles sourced from databases including PubMed, Scopus, and Web of Science, focusing on studies related to the pharmacological properties of Undaria pinnatifida and its major bioactive compounds.
Therefore, the aim of this review is to comprehensively synthesise current scientific evidence on the pharmacologically active compounds derived from U. pinnatifida. By examining their structural characteristics, biological activities, and mechanisms of action, this work seeks to highlight the therapeutic potential of these compounds in the prevention and treatment of neurodegenerative and other chronic diseases, thereby supporting future research directions and the development of novel integrative therapeutic strategies.
2. U. pinnatifida Compounds and Applications
2.1. U. pinnatifida Polysaccharides
This section focuses on the pharmacological activities of polysaccharides from U. pinnatifida, including fucoidan and alginate. These compounds exhibit diverse therapeutic effects such as antioxidant, anti-inflammatory, antitumour, and neuroprotective actions. Their mechanisms of action and biomedical relevance are presented below.
2.1.1. Fucoidan (Figure 1)
Fucoidan demonstrates diverse pharmacological actions. It primarily acts as a secondary antioxidant by neutralising hydroperoxides and inhibiting oxidative damage. In addition to its antioxidant activity, fucoidan exhibits enzyme inhibitory effects against MAO-A, MAO-B, AChE, and BuChE, supporting its potential in modulating neurotransmitter balance in neurodegenerative and neuropsychiatric conditions [35,36]. Given its low toxicity [30] and multifunctional biological profile, including anti-inflammatory, anticoagulant, antiviral, and antitumour properties, fucoidan is of growing interest as a candidate for both pharmaceutical applications and functional food development [6,9,37]. Its inhibitory effects on microglial activation and NF-κB signaling are depicted in Figure 2.
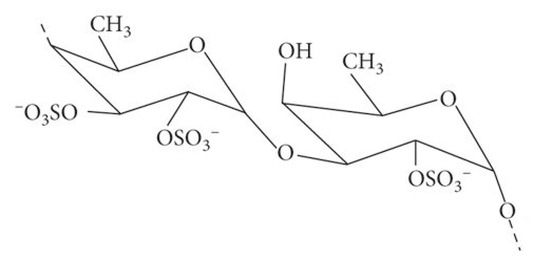
Figure 1.
Fucoidan is a polymer of fucan sulfate composed primarily of 1,2-linked L-fucose-4-sulfate units (as depicted). In some instances, it also contains 1,3- or 1,4-linked fucan sulfate units bearing side chains of galactose, xylose, and uronic acid residues. Based in Kumar, et al. [38].
Figure 1.
Fucoidan is a polymer of fucan sulfate composed primarily of 1,2-linked L-fucose-4-sulfate units (as depicted). In some instances, it also contains 1,3- or 1,4-linked fucan sulfate units bearing side chains of galactose, xylose, and uronic acid residues. Based in Kumar, et al. [38].
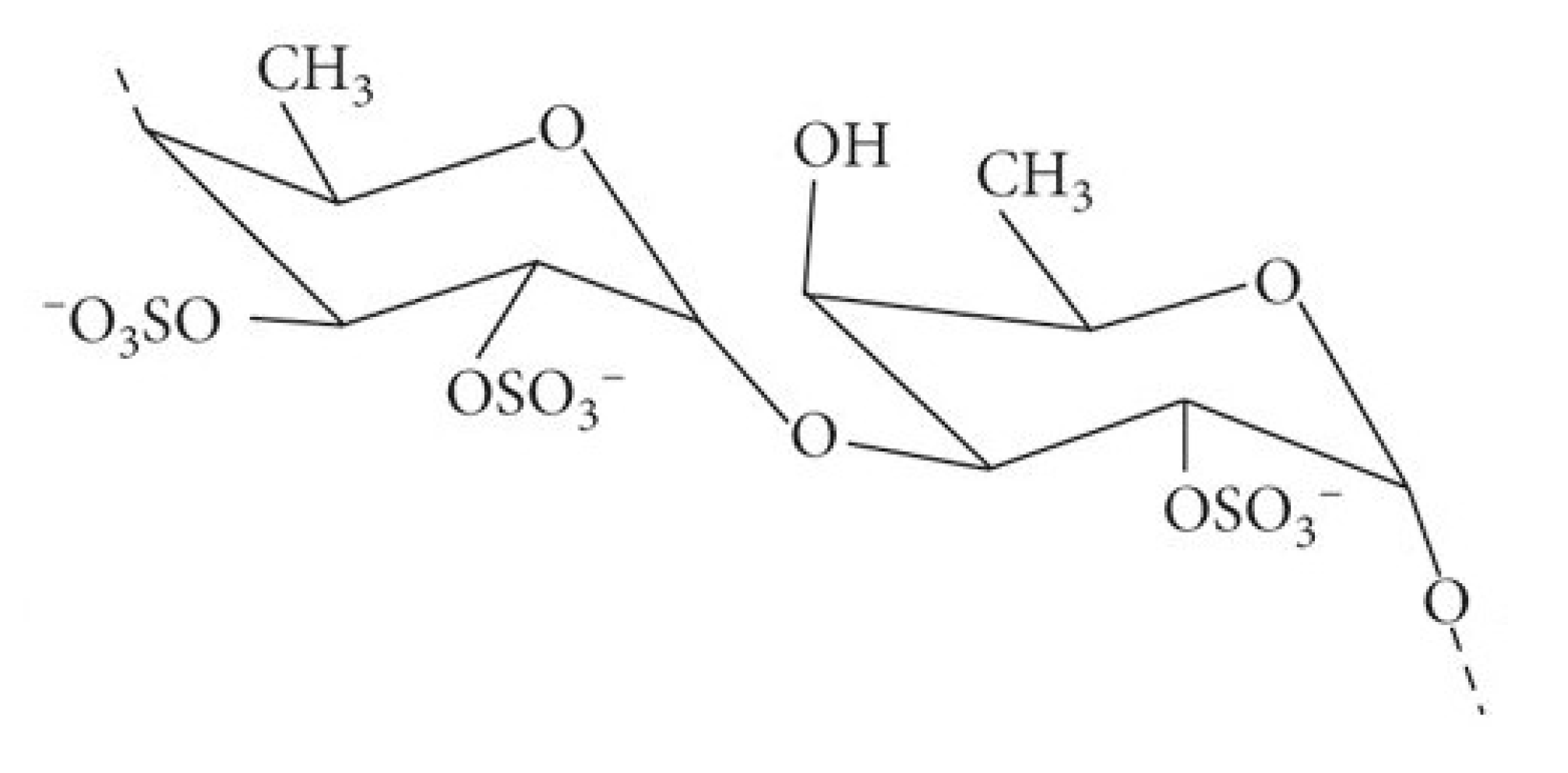
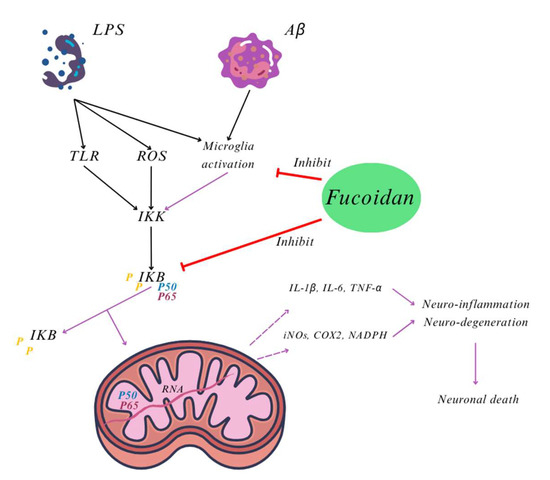
Figure 2.
Inhibitor of microglia activation and NF-kβ reaction mechanisms of fucoidan. Based in Khairinisa, et al. [39].
Figure 2.
Inhibitor of microglia activation and NF-kβ reaction mechanisms of fucoidan. Based in Khairinisa, et al. [39].
2.1.2. Alginates (Figure 3)
Another polysaccharide that forms the structural components of brown seaweed [40] is alginates. They include salts and derivatives of alginic acid and have a high content of dietary fibre [41]. Such fibres can increase the feeling of satiety, which regulates food intake and, therefore, has great significance in controlling obesity, in addition to increasing faecal volume, which helps digestive transit. For that, alginates are among the seaweed fibres best known for their anti-obesity effects. The alginate has also been shown to reduce the permeability of intestinal mucus, reducing problems associated with high lipid absorption, such as hyperlipidemia [42]. In addition, administration of calcium alginate has been shown to lower blood cholesterol levels in mice on a high cholesterol diet [43].
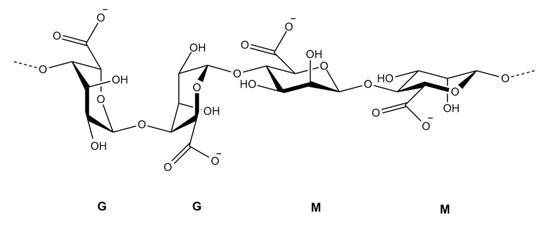
Figure 3.
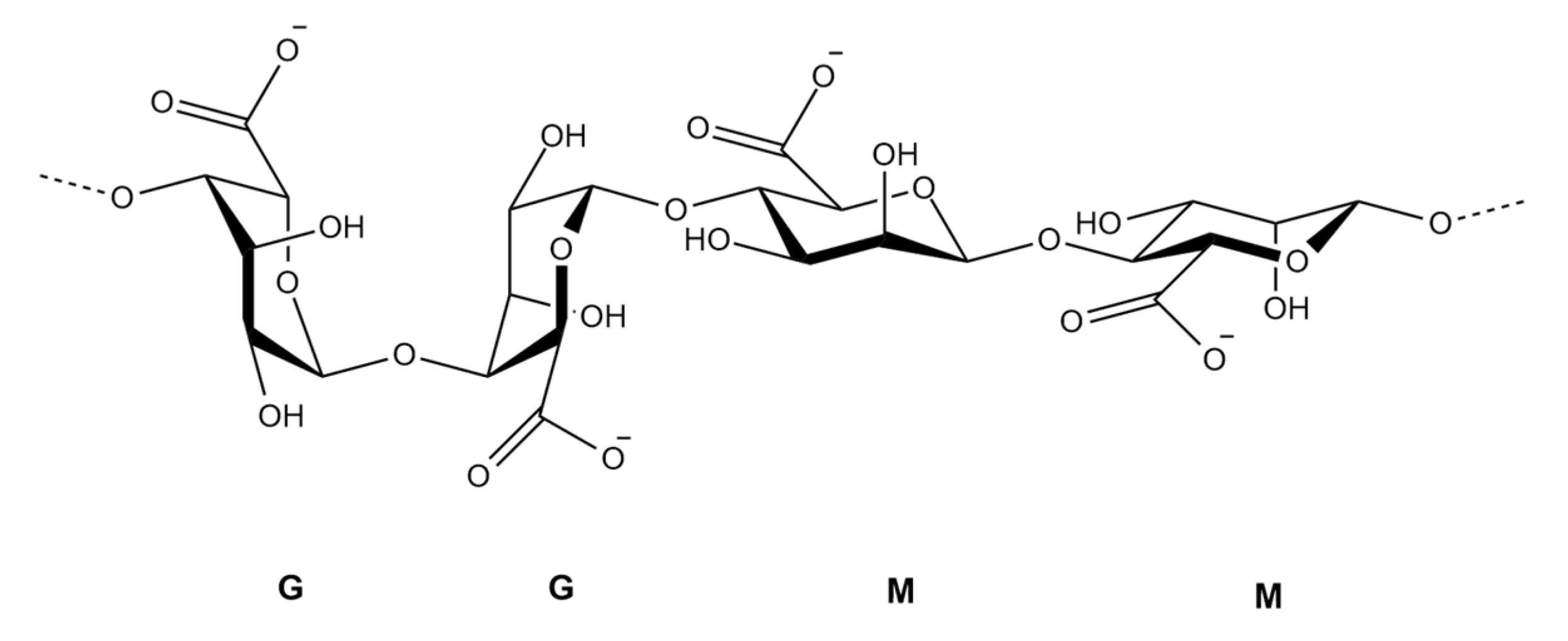
Chemical structure of alginate illustrating its block copolymer composition according to Choukaife, et al. [44]. The polymer consists of homopolymeric regions of α-L-guluronic acid (G blocks) and β-D-mannuronic acid (M blocks), connected via 1→4 glycosidic linkages. Sodium salts (NaOOC) represent the carboxylate groups present on each uronic acid unit [45].
Figure 3.
Chemical structure of alginate illustrating its block copolymer composition according to Choukaife, et al. [44]. The polymer consists of homopolymeric regions of α-L-guluronic acid (G blocks) and β-D-mannuronic acid (M blocks), connected via 1→4 glycosidic linkages. Sodium salts (NaOOC) represent the carboxylate groups present on each uronic acid unit [45].
2.2. Pharmacological Activities of U. pinnatifida Polysaccharides
2.2.1. Anti-Angiogenic Activity
Angiogenesis is a complex process characterized by several key events, namely the degradation of the extracellular matrix, proliferation, migration, and differentiation of endothelial cells, followed by tube formation and the sprouting of new capillary branches. This process is tightly regulated by a diverse array of proangiogenic and antiangiogenic factors [3,46]. Furthermore, angiogenesis is an important component of tumour growth and development, and there is no doubt that blocking angiogenesis is one of the effective strategies to inhibit tumour growth [3,47]. Using a human umbilical vein endothelial cell (HUVEC)-based culture model, Liu, et al. [48] demonstrated that treatment with U. pinnatifida fucoidan (UPF) significantly inhibited HUVEC proliferation, migration, tube formation, and vascular network formation in a dose-dependent manner. Specifically, 400 μg/mL UPF inhibited approximately 40% of cell proliferation and migration, and 61% of tube formation in HUVECs Beyond the cellular model, UPF also exhibited anti-angiogenic effects in an in vitro rat aortic ring assay. Mechanistically, UPF was found to significantly reduce both the mRNA and protein expression of the proangiogenic factor VEGF-A (Vascular Endothelial Growth Factor-A) [48]. However, further detailed in vitro and in vivo investigations are still required to fully elucidate these mechanisms.
2.2.2. Anti-Tumor Activity
Malignant tumors pose significant global public health challenges due to their high morbidity and mortality [3,49], underscoring the urgent need for effective, low-toxicity anti-tumor drugs. Numerous studies indicate that UPPs exhibit promising anti-tumor activity, both alone and in combination with other bioactive compounds. Furthermore, UPPs have demonstrated considerable anti-cancer potential in both in vitro and in vivo investigations.
For instance, Maruyama, et al. [50] reported that intraperitoneal injection of 50 mg/kg fucoidan significantly extended the survival of P-388 lymphoma cell tumor-bearing mice. This effect was accompanied by a notable increase in the cytolytic activity of NK cells and elevated interferon-gamma (IFN-γ) production by T cells. Subsequent research showed that a diet supplemented with 1% UPF (0.034 g/mouse/day) for 40 days in A20 leukemia cell tumor-bearing mice significantly reduced tumor size and weight, while concurrently enhancing IFN-γ production and NK cell activity. This anti-tumor effect of UPF appears to be mediated by tumor destruction via interferon-activated NK cells [3,50,51].
Beyond these findings, other studies suggest that UPF induces apoptosis in SMMC-7721 cells through a reactive oxygen species (ROS)-mediated mitochondrial pathway [3,52]. Additionally, UPPs have been shown to attenuate the growth of human prostate cancer cells in vitro and in vivo [3,53] indicating their potential as a complementary agent for prostate cancer prevention or treatment. Consistent with these in vivo findings, Wu, et al. [54] extracted a sulphated polysaccharide from U. pinnatifida (SPUP) and observed that SPUP decreased the viability and colony formation efficiency of MCF-7 breast cancer cells in a dose-dependent manner. These mechanisms are summarized in Figure 4, which illustrates how UPPs mediate tumor suppression through immune activation, apoptosis induction, and cell cycle regulation.
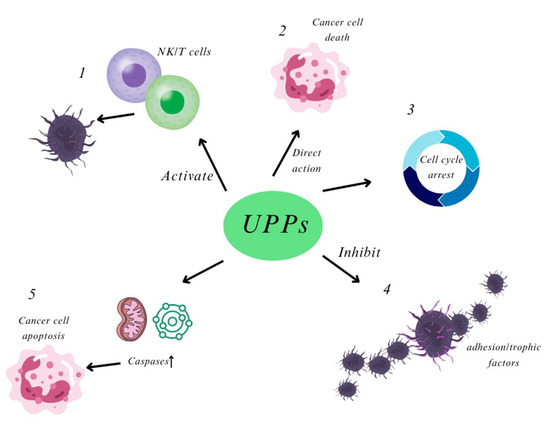
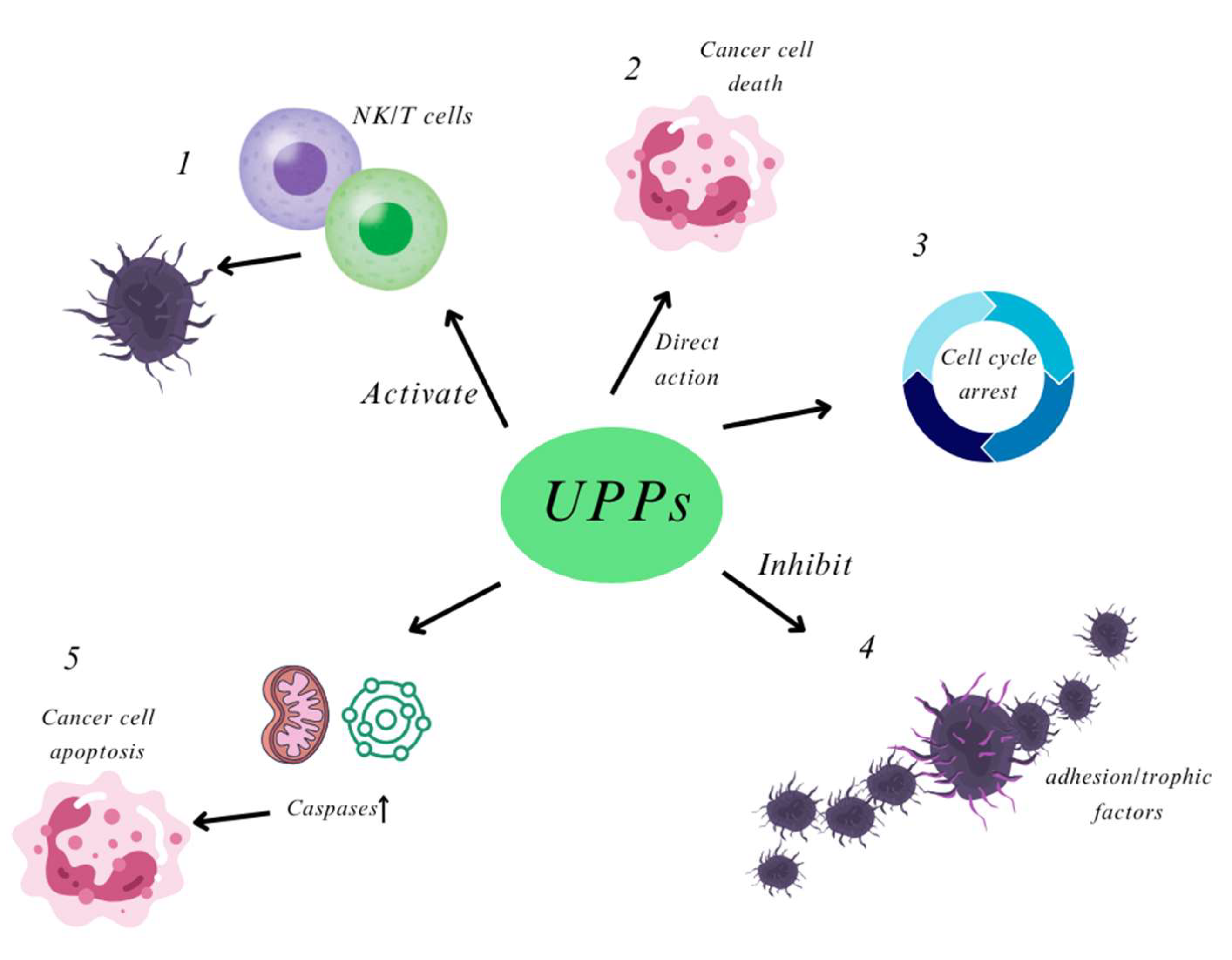
Figure 4.
UPPs appear to exert their anti-cancer effects through several key mechanisms: (1) Immunomodulation: UPPs can activate the immune function of T cells and Natural Killer (NK) cells, thereby inhibiting tumor growth; (2) Direct Cytotoxicity: They may directly reduce the viability of tumor cells or exert cytotoxic effects against them; (3) Cell Cycle Regulation: UPPs can inhibit the progression of the tumor cell cycle by modulating cell cycle-related proteins and genes, thus impacting cell division and proliferation; (4) Inhibition of Invasion and Metastasis: They can hinder tumor invasion and metastasis by inhibiting the secretion of adhesion factors or trophic factors by tumor cells; (5) Apoptosis Induction: UPPs may induce imbalances in the antioxidant system and disrupt mitochondrial function, leading to enhanced caspase-mediated apoptosis in tumor cells.
In summary, fucoidans derived from U. pinnatifida demonstrate the ability to inhibit proliferation and colony formation in both breast cancer and melanoma cell lines in a dose-dependent manner. This suggests a promising potential as a therapeutic approach for cancer treatment [55,56]. Overall, UPPs consistently exhibit beneficial anti-cancer effects in both in vitro and in vivo models across a range of malignancies, including lung, liver, prostate, breast, melanoma, pancreatic, and colon cancers [3,57,58,59].
2.2.3. Antihypertension
Angiotensin I-Converting Enzyme (ACE) inhibitory peptides play an important part in the renin-angiotensin system, which can decrease blood pressure (BP). Studies suggest that ACE inhibitory peptides have an antihypertensive effect after a single oral administration in Spontaneously Hypertensive Rats (SHR) [60]. The authors also found that these peptides are resistant to gastrointestinal proteases in vitro.
Beyond effectively and consistently reducing high blood pressure, oral administration of UPF offers other potential benefits. These include protection against structural vascular damage, enhancement of endothelium-dependent vascular function, and inhibition of abnormal smooth muscle cell proliferation via the Akt-eNOS signaling pathway. These effects were found to be superior to those achieved with nifedipine [3,61]. Collectively, these findings suggest that UPF holds advantages over traditional therapeutics in the prevention of hypertension. Such biological activities could therefore support the use of UPF as a functional food in novel therapeutic strategies for hypertension management [3].
2.2.4. Anti-Inflammatory Activity
The inflammatory response is a complex pathological process that can arise in various tissues and organs. The anti-inflammatory properties of numerous compounds within U. pinnatifida have been extensively researched [3,62].
Murayama, et al. [28] investigated the anti-allergic inflammatory capacity of UPF. Their findings indicated that UPF can significantly modulate Th2 responses, suggesting its utility in treating allergic inflammation. They further assessed its anti-rheumatoid activity in mice with collagen-induced arthritis (CIA). Additionally, UPF was observed to downregulate COX-2 expression in rabbit articular chondrocytes in a dose- and time-dependent manner.
Simultaneously, UPF ameliorated paw edema and effectively regulated platelet and circulatory cell (WBC, RBC) counts, hemoglobin levels, and organ index in carrageenan-induced arthritic rats. It also restored the levels of antioxidant enzymes such as catalase (CAT), peroxidase (POD), and superoxide dismutase (SOD) [3,63]. More recently, Herath, et al. [64] discovered that oral administration of UPF (400 mg/kg for 7 days) significantly attenuated particulate matter (PM)-induced asthma symptoms, including mucus hypersecretion and goblet cell hyperplasia.
On the other hand, Khan, et al. [65] successfully isolated Stearidonic Acid (SA), Eicosapentaenoic Acid (EPA), and Arachidonic Acid (AA) from U. pinnatifida. Their research showed that SA was effective against mouse ear inflammation induced by phorbol myristate acetate, reducing edema, erythema, and blood flow. EPA also demonstrated activity against edema, erythema, and blood flow. Interestingly, AA displayed anti-inflammatory effects at low concentrations when measured 10 h post-application. However, AA doses exceeding 243 µg per ear surprisingly induced inflammatory symptoms after just one hour.
2.2.5. Antioxidant Activity
Oxidative stress occurs when there’s an imbalance between the body’s oxidative and antioxidant systems. This state, caused by an excess of free radicals, is a significant contributor to various diseases [3,66]. Antioxidants play a crucial role in delaying or inhibiting the oxidation of cellular substrates, thereby slowing the progression of many chronic illnesses.
SPUP have demonstrated notable antioxidant activities. For instance, Hu, et al. [26] conducted an in vitro investigation of sulfated polysaccharide fractions (S1 and S2) from U. pinnatifida. They found that both S1 and S2 exhibited strong scavenging abilities against superoxide radicals, hydroxyl radicals, and DPPH (2,2-diphenyl-1-picrylhydrazyl) free radicals, as well as metal-chelating properties. These sulfated fractions displayed superior antioxidant properties compared to their de-sulfated counterparts (DS-1 and DS-2). Furthermore, Mak, et al. [67] confirmed the in vitro antioxidant activity of crude fucoidan (F0) and its three fractions (F1, F2, F3). All three fucoidan fractions primarily contained fucose, with lesser amounts of galactose, xylose, glucose, and mannose.
More recently, extensive research has corroborated the antioxidant activity of UPPs [3]. Specifically, Phull, et al. [63] observed that UPPs demonstrated potent in vitro antioxidant capacity at a dose of 0.5 mg/mL. Additionally, a significant reduction in liver tissue necrosis and cirrhosis was noted in rats treated with U. pinnatifida extract (FE-treated rats) [3,68].
2.2.6. Immunomodulatory Activity
The pivotal role of immune system regulation in disease pathology and development highlights the significance of the immunomodulatory activity exhibited by UPPs, as confirmed by diverse in vitro and in vivo investigations [3].
For instance, fucoidan, isolated from the sporophyll of U. pinnatifida, was observed to induce muscle contraction in silkworms, an effect indicative of innate immune system activation [69].
In previous studies [70,71], it was shown that a fraction UPP-2 significantly promoted the proliferation and pinocytic activity of RAW264.7 macrophage cells and upregulated mRNA expression levels of NO, iNOS, TNF-α, IL-6, and IL-1β in a concentration-dependent manner. Other recent studies have shown that UPP supplementation can modulate several pathways of immunological mechanisms [3,71,72]. In addition, it was proved that UPF significantly increase the levels of secreted immunoglobulins IgG, IgA, and IgM in mouse spleen lymphocytes in vitro [3,73]. Moreover, Injected fucoidans also elicited a range of immune responses in healthy C57BL/6 mice, including NK cell activation, DC cell maturation, T cell immune responses, antigen-specific antibody production, and memory T cell generation. As well, there was a significant increase in the in vitro release of IL-6, IL-8, and TNF-α from peripheral blood neutrophils [3,74].
Collectively, the immunomodulatory effects of UPPs involve regulating the balance of immune responses, inducing the release of immunoreactive substances, slowing down the apoptosis of immune cells, and enhancing immune cell function. These effects are primarily achieved through the activation of the PI3K/Akt and MAPK/NF-κB signalling pathways [3].
2.2.7. Antiviral and Antibacterial Activity
Viral infections currently pose a significant threat to human health due to their severe detrimental effects [3,75]. Galactofucan sulfate (GFS) extracted from U. pinnatifida has demonstrated in vitro inhibitory effects against several viruses, including HSV-1, HSV-2, and HCMV, primarily by blocking viral attachment and entry into host cells [3,25,56,76]. Another study further revealed that GFS also inhibited acyclovir-resistant (ACV-R) strains of HSV-1 and HSV-2 [3,77], as well as HCMV and influenza viruses [3,78]. More recent investigations have consistently confirmed that UPPs exhibit excellent antiviral effects, mainly by interfering with the early stages of viral replication, such as viral adsorption and penetration [3,76,78].
In summary, UPPs act as natural antiviral macromolecules that can both interfere with the initial steps of viral infection (adsorption and penetration) and stimulate the host’s immune system to help reduce viral loads.
Moreover, the anti-adhesive bacterial effects of UPFs should be mentioned. While UPFs may not directly reduce bacterial virulence, they can significantly decrease the adhesion of H. pylori to human gastric epithelial (AGS) cells and of C. parvum to human intestinal cells [3,79]. This suggests a potential role in preventing bacterial colonization and subsequent infection.
2.2.8. Anticoagulating and Antithrombotic Activities
Algal polysaccharides are well-recognized for their medicinal attributes, particularly their prominent anticoagulating activity. Specific fractions of UPFs containing low-molecular-weight fuco-oligosaccharides (LMFOs) have been shown to significantly prolong both activated partial thromboplastin time (APTT) and thrombin time (TT) in normal human platelet-poor plasma in a dose-dependent manner, exhibiting greater efficacy than intact UPF [3,80]. Furthermore, Min, et al. [81] demonstrated that UPF possesses strong antithrombotic effects without the potential for bleeding, a notable advantage over heparin in an arterial thrombus model. Subsequent research by Song, et al. [82] unveiled the thrombolytic potential of SPUPs. Their findings indicated a dose-dependent reduction in atherosclerotic plaque area induced by a high-fat diet following SPUP intervention. Importantly, SPUP was also found to significantly reduce the content of matrix metalloproteinase-9 (MMP-9) within atherosclerotic plaques, suggesting a promising application in achieving atherosclerotic plaque stabilization.
2.2.9. Renoprotective Activities
The degradation of SPUPs with H2O2 produces degraded polysaccharides (DUP) [83] which exhibit a reparative effect. Mechanistic studies have elucidated DUP’s protective function through its ability to repair subcellular structures, decrease lactate dehydrogenase leakage, improve cell membrane and lysosome integrity, and block cell cycle progression to lessen apoptosis and necrosis [84]. This evidence provides a compelling basis for inhibiting nephrolithiasis and for the future development of an innovative anti-stone polysaccharide therapeutic.
2.3. U. pinnatifida Non-Polysaccharide Compounds and Their Pharmacological Applications
In addition to polysaccharides, U. pinnatifida contains several other bioactive metabolites, such as fucoxanthin, fucosterol, glycoproteins, and phlorotannins. This section outlines their pharmacological roles and highlights their potential for application in disease prevention and treatment. By delving into the research surrounding these natural compounds, mainly those extracted from U. pinnatifida, scientists and researchers can uncover valuable insights into their mechanisms of action, therapeutic potential, and their application in various health conditions, which are syntetized in Table 1.

Table 1.
Main activities of bioactive compounds found in U. pinnatifida and the mechanisms involved.
This exploration contributes to a deeper understanding of the potential benefits of U. pinnatifida-based treatments and their metabolites, paving the way for the development of new therapeutic strategies [3,16,17,105]. According to them, bioactive metabolites and extracts have served the biomedical field with their therapeutically significant properties against microbial infections, cancer, cardiovascular, neurodegenerative diseases, inflammatory diseases, and other medical conditions. As illustrated in Figure 5, several bioactive metabolites from marine algae contribute to neuroprotection through antioxidative, anti-inflammatory, and enzyme inhibitory actions.
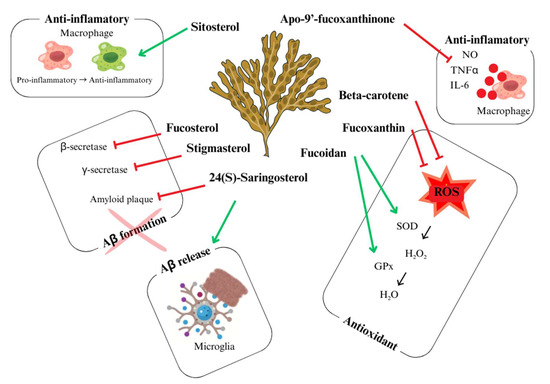
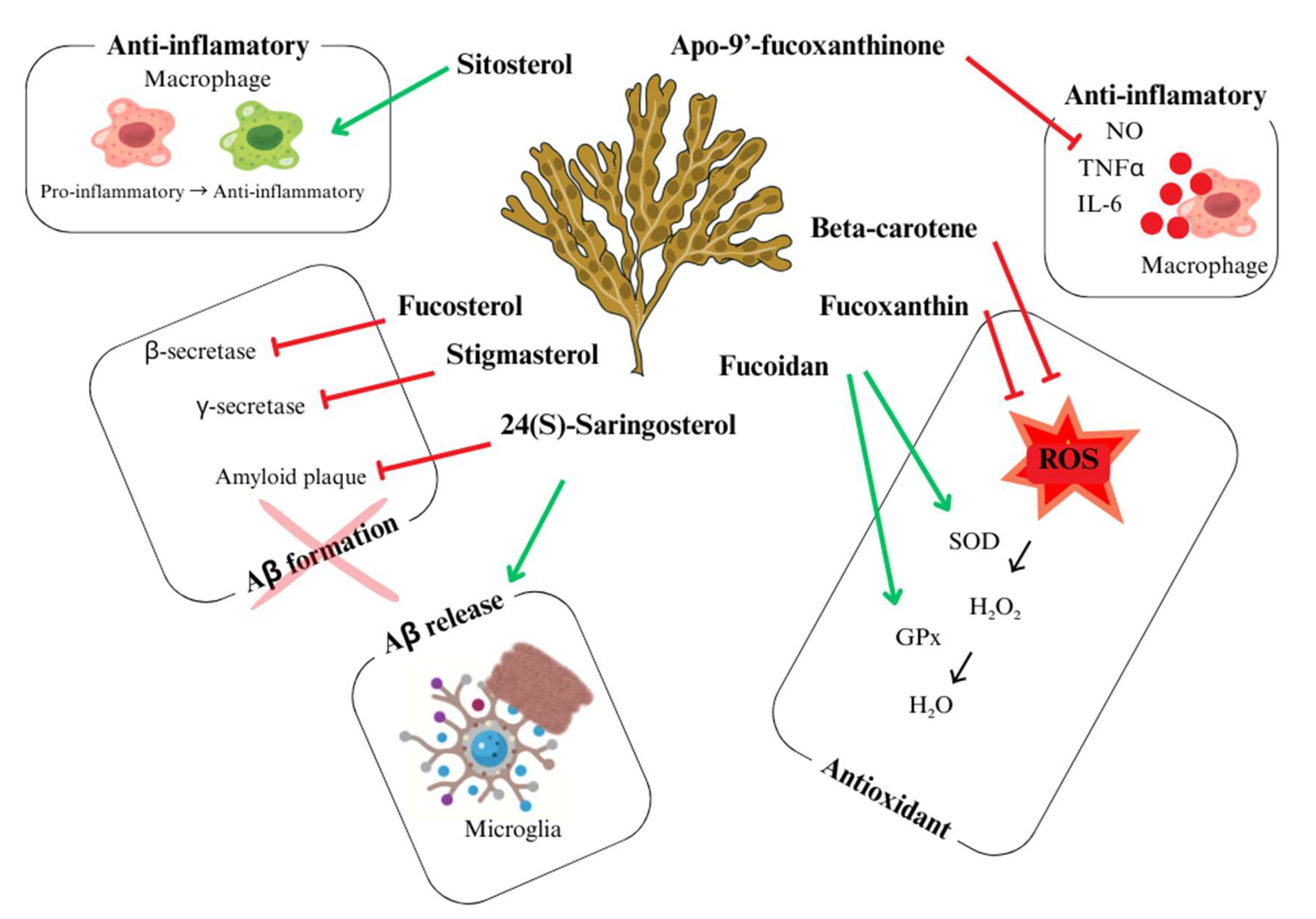
Figure 5.
Bioactive components with neuroprotector activity present in marine algae and their corresponding action. Adapted from Schepers, et al. [106].
2.3.1. Fucoxanthin
Fucoxanthin is a marine carotenoid present in brown seaweed, found abundantly in edible algae, such as U. pinnatifida [107]. Due to the variety of bioactive compounds that U. pinnatifida presents, the possibility of its antioxidant capacity has been studied [9,96,98]. There is strong evidence that its potent antioxidant action is due to the instability and unusual allelic bond, in which it is possible to remove ROS such as the radical O2- and the non-radical species H2O2. It is this antioxidant property that defines its anti-obesity and anti-diabetic activity. Additionally, fucoxanthin has shown anti-inflammatory, anti-photoaging properties, anti-carcinogenic, hepatoprotective, and cardiovascular and cerebrovascular protective effects [9,56,96,98].
So, fucoxanthin has been reported to have anti-obesity effects in diet-induced obesity mice fed a high-fat diet (20% fat wt/wt), significantly inhibiting various lipogenic enzyme activities in epididymal adipose tissue and decreasing the fatty acid β-oxidation activity [56]. In addition, it decreases the mass of white adipose tissue, the serum level of triacylglycerols and increases the serum level of HDL cholesterol (high-density lipoprotein) [94]. Fucoxanthin also exerts its effect on hepatic lipid content by regulating the activity of hepatic metabolic enzymes and stimulating the oxidation of fatty acids [95].
In addition to the previously mentioned activities, fucoxanthin also showed an inhibitory activity on MAO enzymes that are linked to the degradation of neurotransmitters such as dopamine and serotonin. There is also evidence that fucoxanthin has cholinesterase (AChE) inhibitory activity, which is favourable for the treatment of AD [97].
2.3.2. Fucoesterols
Fucosterol is a sterol from the phytosterol family, present in higher levels in brown algae, that can represent around 4 to 95% of their entire phytosterol content. Concerning U. pinnatifida, the fucosterol content varies between 83% and 97% of the total sterols present in the algae [11,100].
Clinical studies have demonstrated that dietary intake of plant sterols might help to lower blood cholesterol levels [99]. Besides this action, like fucoxanthin and fucoidan, fucosterol can also have an antioxidant action responsible for inhibiting oxidative stress that can lead to the onset of AD, PD and depression (ability to inhibit the enzyme β-secretase and MAO enzymes). On the other hand, it seems to have several other bioactive properties, namely anti-inflammatory activity [11,100].
2.3.3. Phenolic Compounds—Flavonoids and Phlorotannins
Numerous studies have elucidated the reaction mechanisms of phenols as effective antioxidants [39]. Phenolic compounds isolated from seaweed have been identified as having a neuroprotective effect [39,103] largely due to their potent antioxidant properties [39,102]. These phenolic antioxidants show significant promise in effectively neutralizing free radicals, which are major contributors to neuronal damage. Consequently, they can exert substantial neuroprotective effects and play a crucial role in managing neurodegenerative diseases [39,102]. Beyond their antioxidant capacity, phenolic compounds can also inhibit AChE and BChE, as well as amyloid-beta (Aβ) aggregation [39,103]. This makes them particularly relevant in AD management, as AD progression is linked to cholinergic pathway disruption caused by the upregulation of AChE and BChE [39,108].
Polyphenols represent a diverse group of compounds, constituting the largest class of phytochemicals in the human diet, found abundantly in fruits, vegetables, seeds, essential oils, and various other foods and beverages. Based on the chemical structure of their aglycones, polyphenols are categorized into phenolic acids, flavonoids, stilbenes, lignans, and other phenolic compounds. Among these, flavonoids are the most widespread, further divided into six subclasses: flavonols, flavanols, flavanones, flavones, isoflavones, and anthocyanins [109].
Seaweeds are a rich source of polyphenolic compounds. The major proportion of phenolic compounds found in green and red algae are bromophenols, phenolic acids and flavonoids. On the other hand, phlorotannins are the dominant polyphenolic group of secondary metabolites found only in brown algae such as U. pinnatifida [110,111,112].
Many pharmacological effects are related to flavonoid antioxidants; their biological function is maintaining oxidative stress levels below the critical point [39,101]. The activity of flavonoids as antioxidants combines several pathways for reducing oxidase enzymes, such as cyclooxygenase, lipoxygenase, xanthine oxidase, myeloperoxidase, and NADPH oxidase [101].
Flavonoids are the largest group of phenolic compounds which can be found in U. pinnatifida. In this macroalgae, flavonoids such as catechin, epigallocatechin, epicatechin gallate, catechin gallate, epigallocatechin gallate, and epicatechin gallate have been identified. Phlorotannins are also abundant compounds in the brown seaweed class [110,112].
Phlorotannins have attracted considerable research interest due to their high antioxidant activity. They can be classified into six subclasses based on the type of bond between the phloroglucinol subunits, as well as the additional number of hydroxyl groups in the aromatic skeleton: phloroecol, ecol, dichlorohydroxycarmalol, diecol, eckstolonol, and phloroglucinol. Phlorotannins have several biological activities, such as antioxidant and prevention of skin carcinogenesis [113]. Different studies have shown that phlorotannins can also improve obesity disorders through several mechanisms, for example, inhibition of pancreatic lipase and obstruction of adipocyte differentiation [104].
3. Focus on the Neuroprotective Actions of U. pinnatifida: Mechanisms and Key Compounds
The imbalance in the release of neurotransmitters is identified as one of the main causes for the appearance of neurodegenerative diseases, because it is through the release of neurotransmitters that the transmission of nerve impulses occurs. The main neurotransmitters that influence the onset of neurodegenerative diseases are glutamate, gamma-aminobutyric acid (GABA), serotonin (5-HT), acetylcholine (ACh) and dopamine [114,115,116].
Glutamate is one of the main excitatory neurotransmitters in the central nervous system (CNS), associated with learning and memory at normal levels. At high levels, glutamate causes its receptors (for example, the ionotropic N-methyl D-aspartate (NMDA) and alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic (AMPA) receptors to be active for longer, causing a calcium-dependent intracellular signaling cascade, leading to excitotoxicity. Memantine, widely used in the treatment of Alzheimer’s disease (AD), is an NMDA receptor antagonist which binds to it, enabling the regulation of glutamate levels and consequently reducing its excitotoxicity [115,116].
Gamma-aminobutyric acid (GABA) is the main inhibitory neurotransmitter of the CNS, synthesised from glutamate through the enzyme glutamate decarboxylase. However, the function of GABA opposes the function of glutamate, since this neurotransmitter has the function of reducing neuronal excitability through the inhibition of nervous transmission. In other words, GABA, when found at normal levels, has the ability to inhibit excitatory neurotransmitters such as glutamate, in order to maintain stability in neurological functions. Therefore, reduced levels of this neurotransmitter cause anxiety attacks that may also be associated with symptoms of depression [115,117].
Serotonin (5-hydroxytryptamine or 5-HT) is a monoamine-type neurotransmitter produced from the hydroxylation of tryptophan through the enzyme tryptophan hydroxylase, resulting in 5-hydroxytryptophan, which is subsequently decarboxylated to form serotonin. Therefore, serotonin levels are controlled by the ability to capture tryptophan. This neurotransmitter plays a fundamental role in several behavioral functions as it regulates sleep, satiety, aggression and mood changes or depression. Furthermore, the enzyme monoamine oxidase A (MAO-A) also has effects on serotonin levels, as it promotes the oxidative deamination of monoamines after transmission, and therefore, its action causes a decrease in serotonin levels [115,116,118,119].
Acetylcholine (ACh) is the main neurotransmitter responsible for cognitive and memory processes. The regulation of the neurotransmitter’s time of action on the ACh receptor, that is, the nerve impulse, is carried out by Acetylcholinesterase (AChE), which removes ACh from the receptor, hydrolysing it to give rise to choline and acetate. ACh levels are regulated by choline acetyltransferase, which promotes choline uptake for ACh production. Low levels of the neurotransmitter ACh, or intense AChE activity, favor the onset of neurodegenerative diseases such as AD [115,116].
Cholinesterases are divided into two types: the aforementioned AChE, more selective and present in skeletal muscle and the CNS, and butyrylcholinesterase (BuChE), nonspecific and present in blood plasma. These enzymes are evolutionarily similar. However, their distribution in tissues, their kinetic properties and their substrate specificities are factors that allow their differentiation [56,120].
Dopamine is an inhibitory neurotransmitter that is involved in motor control, endocrine functions, cognition and emotions. Dopamine is produced from the hydrolysis of the amino acid tyrosine through the enzyme tyrosine hydroxylase, which results in levodopa (L-DOPA), which in turn is decarboxylated and converted into dopamine in dopaminergic neurons. The lack of dopamine in the brain’s basal ganglia leads to a loss of control over performing smooth, controlled movements, which is the main cause of Parkinson’s disease (PD). Dopamine levels are regulated by L-DOPA production and the uptake of dopamine by receptors. Furthermore, as dopamine is also a monoamine, the action of MAO-B and tyrosinase enzymes also has effects on reducing dopamine levels [115,116,119,121].
Tyrosine can also be oxidised by tyrosinase, producing dopaquinone, which is an extremely unstable molecule that can bind to other molecules and produce dangerous compounds capable of deteriorating and destroying nerve cells. One of the steps in the neuromelanin production process involves the spontaneous auto-oxidation of dopaquinone, however it is a difficult step to regulate and therefore, high levels of this molecule can be reached, consequently causing not only oxidative stress, but also an undesirable inhibition of the tyrosine hydroxylase responsible for the production of dopamine [122,123,124,125].
Neurodegenerative disorders, such as AD and PD, are the leading cause of mortality and morbidity among the elderly globally [87,126]. According to the World Health Organization [127], the population suffering from dementia worldwide are deeply increasing rapidly to more than 35.6 million people, with AD accounting for around 21.5 million and PD around 8.5 million. This number will double by 2030 and more than triple by 2050. Dementia is overwhelming not only for the people who have it, but also for their caregivers and families. The predominant risk factors for neurodegenerative disorders are multifaceted, involving oxidative stress, protein aggregation and misfolding, tau phosphorylation, neuroinflammation, and neuronal apoptosis [87,128]. Furthermore, other potential etiological factors, such as environmental risks, chemical exposure, immune and metabolic dysfunction, and genetic mutations (e.g., amyloid polymorphisms, mitochondrial mutations, and epigenetic changes), are also considered. Importantly, these diverse factors collectively represent promising targets for neuroprotective interventions [87,129,130].
Therefore, as neuroinflammation and oxidative stress play a relevant role in the emergence of neurodegenerative diseases, it is urgent to develop effective strategies for treating them. Currently, natural compounds with anti-inflammatory and antioxidant action have been sought through scientific research as a means of protection against these diseases [131,132,133,134].
Natural compounds have been reported to possess different pharmacological and biological activities, including antioxidant, anti-inflammatory, and antiapoptotic effects [87,135,136,137], being considered promising alternatives for the treatment or prevention of neurodegeneration [87,138,139,140,141].
Many of the bioactive compounds in macroalgae are derived from Phaeophyceae, brown algae (57.6%), followed by Rhodophyta, red algae (28.3%) and Chlorophyta, green algae (14.1%) [91,142]. Among the various components, polysaccharides are generally the main component of brown, green, and red algae [91,143], and monosaccharides and oligosaccharides are also present.
Brown algae primarily store laminarin, red algae store floridean starch (which is more highly branched than amylopectin), and green algae store starch as their reserve polysaccharides. Laminarin, a polysaccharide composed of (1,3)-β-D-glucan with some β(1,6) branching, has demonstrated antibacterial and chemopreventive activities. Furthermore, it exhibits prebiotic properties crucial for the modulation of the intestinal microbiota, which, in turn, can regulate neuro-inflammation [91,131]. These findings collectively suggest that polysaccharide extracts from seaweeds possess significant neuroprotective and reparative activities. Consequently, these polysaccharides hold promise as a potential major advancement in the treatment of neurodegenerative diseases [91].
In fact, Brown algae have been shown to have useful therapeutic properties in the prevention and treatment of neurodegenerative diseases: PD, AD, Multiple Sclerosis, Depression and other chronic diseases which are greatly induced by the reactive oxygen species (ROS—collectively, H2O2, OH, and O2−) or free radicals produced by the body [39,86,87,91,92]. Figure 6 graphically represents the potential neuroprotective mechanisms of seaweed.
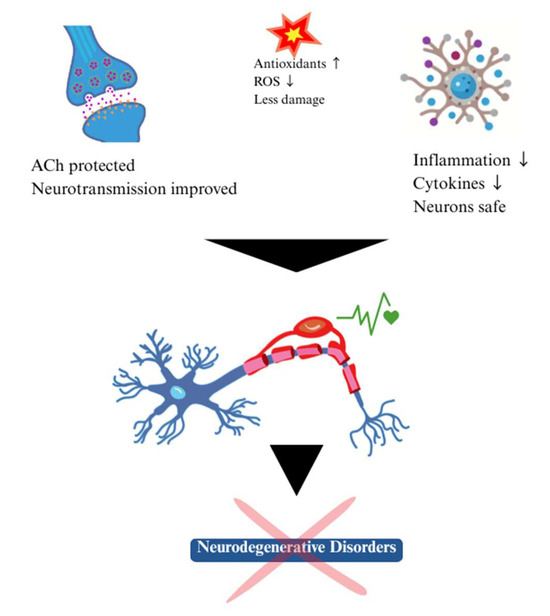
Figure 6.
Neurotherapeutic potential effect of marine-algae compounds.
Despite the high variety of seaweed and their different compositions, the brown seaweed U. pinnatifida properties are important for the treatment or prevention of diseases such as AD, PD and depression [9,11].
AD accounts for 60–70% of dementia cases [86,144]. Histopathological studies of the AD-affected brains demonstrate the presence of amyloid beta (Aβ) plaques and neurofibrillary tangles (NFT) [86,145]. Aβ plaque accumulation and its hyperphosphorylation are thought to play a role in the neuronal damage found in AD. Since ACh is the main neurotransmitter responsible for the aforementioned cognitive and memory processes, with the structural progression of AD, the levels of this neurotransmitter decrease, as it is enzymatically degraded by the AChE enzyme [11,146]. According to these authors, the relief of AD symptoms may also be associated with the action of AChE enzyme inhibitors, which can, in turn, lead to more positive control of ACh levels in the transmission of nerve impulses. Currently, there is no available disease-modifying drug for AD, and the five approved AD therapy drugs only alleviate some symptoms of the disease [86,147]. Thus, drugs that act on the pathognomonic mechanism(s) of AD are needed. Therefore, currently the prevailing hypotheses are the cholinergic and the amyloid cascade mechanisms [92,148]. As a result, anti-AD drug development has focused on drugs acting on ACh levels, mainly AChE and BChE inhibitors, or those that reduce the formation of toxic amyloid β peptides, mainly noncompetitive β-secretase (BACE-1) and γ-secretase inhibitors [92,149].
Several studies on the pharmacological properties of U. pinnatifida, described that bioactive compounds such as fucodian, fucoxanthine, phlorotanin, PUFA, peptides and glycoproteins showed various bio-functional activities, including antioxidant, anticancer, antiviral, antimicrobial, antidiabetic and anti-inflammatory properties [56,88,92,150,151]
In particular, fucoidan exhibits broad neuroprotective effects. These include inhibition of oxidative stress and neuroinflammation, suppression of cholinesterases (AChE and BuChE), and modulation of monoamine oxidases (MAO-A and MAO-B), which are involved in neurotransmitter metabolism. fucoidan has also been shown to reduce amyloid beta (Aβ1–42)- and hydrogen peroxide (H2O2)-induced cytotoxicity in neuronal cells, attenuate neurotoxic damage from MPTP and 6-OHDA in experimental models, and promote brain-derived neurotrophic factor (BDNF) release [34,85,86,90,93,152,153]. Together, these mechanisms support its promise as a multi-target agent for managing AD and PD.
On the other hand, according to Rafiquzzaman, Kim, Lee, Mohibbullah, Alam, Soo Moon, Kim and Kong [92], glycoproteins from U. pinnatifida (UPGP) showed predominantly AChE, BChE, and BACE1 inhibitory activities at dose-dependent concentrations. The addition of UPGP (5 μg/mL) to the culture medium showed that it was not cytotoxic to cultured hippocampal cells, but rather, protected neurons from natural death. The antioxidant ability of UPGP was demonstrated by inhibition of cyclooxygenase enzymes (COX-1 and COX-2) and of nitric oxide (NO) production. Moreover, UPGP improved antioxidant activities of superoxide dismutase (SOD) and inhibited xanthine oxidase (Xox) activity. Thus, these results suggest that UPGP is a bioactive compound with the potential to control AD and inflammatory and oxidative stress-related diseases.
PD is a degenerative, slowly progressive neurological disorder affecting specific regions of the central nervous system (brain and spinal cord). Clinically, it is characterized by a distinctive constellation of motor symptoms: resting tremor, increased muscle tone (rigidity), bradykinesia (slowness of movement), and postural instability (difficulty maintaining balance). Cognitive impairment also affects many patients.
At a neuropathological level, PD involves the degeneration of nerve cells, particularly within the substantia nigra, a component of the basal ganglia. The basal ganglia are critical structures intimately involved in movement, although they do not directly project to the spinal cord or cranial nerves. Their fundamental functions are primarily associated with the cognitive aspects of motor control, such as planning and executing complex motor acts. Dysfunction of these basal ganglia circuits leads to altered reciprocal muscle control, manifesting as the characteristic rigidity, tremors, and akinesia observed in PD. [16,91].
The inhibition of MAO-B and tyrosinase enzymes prevents dopamine from being degraded and keeps the levels controlled, thus pointing out a possible treatment for the relief of PD symptoms.
PD is generally characterised, as we have seen previously, by the loss of dopaminergic neurons, and the presence of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) can induce PD [91,154]. The administration of this substance may result in motor dysfunction, such as occurs in PD, which makes it a suitable experimental model for this disease [91,155]. Fucoidan has been found to attenuate the neurotoxicity of MPTP activity. This sulphated polysaccharide derived from Phaeophyceae brown algae, has been demonstrated in mice models to be effective protecting the cells from MPTP-induced neurotoxicity through the reduction of the behavioral deficits and cell death and the level of dopamine increase [90,91]. Furthermore, this natural compound has excellent inhibitory activity on MAO enzymes, thus being able to better control dopamine levels that are relevant to preventing the worsening of PD.
Deep depression is considered a disease responsible for the appearance of serious and persistent symptoms of mood disorders, such as feelings of guilt, insomnia, mental irritability, low self-esteem, fatigue, and anhedonia, among others. Therefore, due to the vast list of symptoms it presents, it is considered one of the most common neuropsychiatric diseases that affect cognitive functions, and consequently, quality of life. Furthermore, depression can occur simultaneously with diseases such as obesity, diabetes, anxiety, schizophrenia, AD and PD. The characteristics of major depression, such as its severity and pathogenesis, are influenced by factors such as genetics, but also by psychological, environmental and biological factors [156,157].
Globally, according to the World Health Organization [158], it is estimated that 3.8% of the population suffers from depression, of which 5.0% are adults and 5.7% are adults over 60 years of age, that is, about 280 million people worldwide suffer from depression. Recent scientific evidence demonstrates that depression is also associated with changes in brain function, neuronal plasticity, and a reduction in the volume of the frontal cortex and hippocampus. It is also known that the main cause for these disorders is oxidative/nitrosative stress caused by the uncontrolled release of ROS and RNS during neuroinflammation processes, but also due to the dysregulation of neurotransmitters such as glutamate, serotonin, and GABA mentioned above.
4. Conclusions
U. pinnatifida, a widely consumed brown macroalga, presents an abundant source of structurally diverse bioactive compounds with compelling pharmacological relevance. Among these, fucoidan and other polysaccharides demonstrate strong potential in modulating oxidative stress, inflammation, and enzymatic dysfunction implicated in chronic and neurodegenerative diseases. These properties suggest practical applications in the development of multi-target therapeutics for conditions such as AD and PD.
However, significant challenges remain in translating these findings to clinical practice. Critical issues include poor oral bioavailability, lack of standardisation across extracts, and limited data on long-term safety and tolerability in humans. While numerous in vitro and in vivo studies show promise, robust evidence from well-designed human clinical trials is still lacking.
Addressing these gaps will require interdisciplinary collaboration to optimise extraction methods, enhance compound stability and delivery, and conduct mechanistic and clinical investigations. With such efforts, U. pinnatifida could become a valuable component of integrative strategies aimed at treating not only neurodegenerative disorders but also other inflammation-driven and metabolic diseases.
Author Contributions
Conceptualization, H.M. and J.P.M.; methodology, J.P.M.; software, J.M.R.; validation, J.P.M., C.S., C.G., J.M.R. and M.B.C.; formal analysis, H.M. and J.P.M.; investigation, H.M., J.P.M. and M.B.C.; resources, J.M.R.; data curation, C.A., C.S., C.G. and J.M.R.; writing—original draft preparation, H.M. and J.P.M.; writing—review and editing, J.P.M., C.A., C.S., C.G., J.M.R. and M.B.C.; visualization, H.M., C.S., C.G., J.M.R. and M.B.C.; supervision, J.P.M. and M.B.C.; project administration, J.P.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Acknowledgments
We are thankful to the reviewers for their insightful comments, suggestions, and contributions to this article.
Conflicts of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- European Commission. Undaria pinnatifida. Available online: https://fish-commercial-names.ec.europa.eu/fish-names/species/undaria-pinnatifida_pt (accessed on 29 May 2025).
- Rubal, M.; Fernandez-Gutierrez, J.; Carreira-Flores, D.; Gomes, P.T.; Veiga, P. Current Distribution of the Invasive Kelp Undaria pinnatifida (Harvey) Suringar, 1873 Along Artificial and Natural Habitats in North Portugal-Impacts and Mitigation Initiatives. Plants 2025, 14, 658. [Google Scholar] [CrossRef]
- Zeng, J.; Luan, F.; Hu, J.; Liu, Y.; Zhang, X.; Qin, T.; Zhang, X.; Liu, R.; Zeng, N. Recent research advances in polysaccharides from Undaria pinnatifida: Isolation, structures, bioactivities, and applications. Int. J. Biol. Macromol. 2022, 206, 325–354. [Google Scholar] [CrossRef] [PubMed]
- Martinez, M.A.; Becherucci, M.E. Study of the potential use of the invasive marine algae Undaria pinnatifida in the preliminary development of a functional textile. J. Ind. Text. 2020, 51, 8127S–8141S. [Google Scholar] [CrossRef]
- Mizuno, M.; Nishitani, Y.; Tanoue, T.; Matoba, Y.; Ojima, T.; Hashimoto, T.; Kanazawa, K. Quantification and localization of fucoidan in Laminaria japonica using a novel antibody. Biosci. Biotechnol. Biochem. 2009, 73, 335–338. [Google Scholar] [CrossRef]
- Zhao, Y.; Zheng, Y.; Wang, J.; Ma, S.; Yu, Y.; White, W.L.; Yang, S.; Yang, F.; Lu, J. Fucoidan Extracted from Undaria pinnatifida: Source for Nutraceuticals/Functional Foods. Mar. Drugs 2018, 16, 321. [Google Scholar] [CrossRef]
- Martinez-Villaluenga, C.; Penas, E.; Rico, D.; Martin-Diana, A.B.; Portillo, M.P.; Macarulla, M.T.; de Luis, D.A.; Miranda, J. Potential Usefulness of a Wakame/Carob Functional Snack for the Treatment of Several Aspects of Metabolic Syndrome: From In Vitro to In Vivo Studies. Mar. Drugs 2018, 16, 512. [Google Scholar] [CrossRef]
- Taboada, M.C.; Millán, R.; Miguez, M.I. Nutritional value of the marine algae wakame (Undaria pinnatifida) and nori (Porphyra purpurea) as food supplements. J. Appl. Phycol. 2012, 25, 1271–1276. [Google Scholar] [CrossRef]
- Wang, L.; Park, Y.-J.; Jeon, Y.-J.; Ryu, B. Bioactivities of the edible brown seaweed, Undaria pinnatifida: A review. Aquaculture 2018, 495, 873–880. [Google Scholar] [CrossRef]
- Khan, M.N.; Yoon, S.J.; Choi, J.S.; Park, N.G.; Lee, H.H.; Cho, J.Y.; Hong, Y.K. Anti-edema effects of brown seaweed (Undaria pinnatifida) extract on phorbol 12-myristate 13-acetate-induced mouse ear inflammation. Am. J. Chin. Med. 2009, 37, 373–381. [Google Scholar] [CrossRef]
- Hannan, M.A.; Dash, R.; Haque, M.N.; Mohibbullah, M.; Sohag, A.A.M.; Rahman, M.A.; Uddin, M.J.; Alam, M.; Moon, I.S. Neuroprotective potentials of marine algae and their bioactive metabolites: Pharmacological insights and therapeutic advances. Mar. Drugs 2020, 18, 347. [Google Scholar] [CrossRef]
- Li, S.; Hu, M.; Tong, Y.; Xia, Z.; Tong, Y.; Sun, Y.; Cao, J.; Zhang, J.; Liu, J.; Zhao, S.; et al. A review of volatile compounds in edible macroalgae. Food Res. Int. 2023, 165, 112559. [Google Scholar] [CrossRef]
- Pohnert, G.; Boland, W. The oxylipin chemistry of attraction and defense in brown algae and diatoms. Nat. Prod. Rep. 2002, 19, 108–122. [Google Scholar] [CrossRef]
- Pu, D.; Xu, Z.; Sun, B.; Wang, Y.; Xu, J.; Zhang, Y. Advances in Food Aroma Analysis: Extraction, Separation, and Quantification Techniques. Foods 2025, 14, 1302. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.F.; Liu, J.H.; Xu, N.G.; Liang, Z.H.; Xu, Z.H.; Xu, S.J.; Fu, W.B. Effects of acupuncture treatment on depression insomnia: A study protocol of a multicenter randomized controlled trial. Trials 2013, 14, 2. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Hou, Z.; Chen, X.; Zhao, L.; Zhu, D.; Wang, N.; Zhao, Y.; Zhang, B. Analysis of the medication rules of traditional Chinese medicines (TCMs) in treating liver cancer and potential TCMs exploration. Pharmacol. Res.-Mod. Chin. Med. 2022, 3, 100086. [Google Scholar] [CrossRef]
- Wu, D.-Y.; Hao, R.-W.; Lan, Q.-Y.; Liang, J.-Q.; Du, Z.-C.; Lu, B.-D.; Deng, J.-G.; Hou, X.-T. Analysis of varieties and characteristics of marine Chinese medicines recorded in Compendium of Materia Medica. Zhongcaoyao 2020, 51, 4338–4347. [Google Scholar]
- Nemoto, M.; Kuda, T.; Eda, M.; Yamakawa, H.; Takahashi, H.; Kimura, B. Protective Effects of Mekabu Aqueous Solution Fermented by Lactobacillus plantarum Sanriku-SU7 on Human Enterocyte-Like HT-29-luc Cells and DSS-Induced Murine IBD Model. Probiotics Antimicrob. Proteins 2017, 9, 48–55. [Google Scholar] [CrossRef]
- Hemmingson, J.A.; Falshaw, R.; Furneaux, R.; Thompson, K. Structure and antiviral activity of the galactofucan sulfates extracted from Undaria pinnatifida (Phaeophyta). J. Appl. Phycol. 2006, 18, 185–193. [Google Scholar] [CrossRef]
- Vishchuk, O.S.; Ermakova, S.P.; Zvyagintseva, T.N. The fucoidans from brown algae of Far-Eastern seas: Anti-tumor activity and structure-function relationship. Food Chem. 2013, 141, 1211–1217. [Google Scholar] [CrossRef]
- Chen, N.; Zhang, H.; Zong, X.; Li, S.; Wang, J.; Wang, Y.; Jin, M. Polysaccharides from Auricularia auricula: Preparation, structural features and biological activities. Carbohydr. Polym. 2020, 247, 116750. [Google Scholar] [CrossRef]
- Maruyama, H.; Suzuki, K.; Miyai, S.; Ohtsuki, K. Characterization of meFucoidan as a selective inhibitor for secretory phospholipase A2-IIA and the phosphorylation of meFucoidan-binding proteins by A-kinase in vitro. Biol. Pharm. Bull. 2008, 31, 714–718. [Google Scholar] [CrossRef]
- Michel, C.; Lahaye, M.; Bonnet, C.; Mabeau, S.; Barry, J.L. In vitro fermentation by human faecal bacteria of total and purified dietary fibres from brown seaweeds. Br. J. Nutr. 1996, 75, 263–280. [Google Scholar] [CrossRef] [PubMed]
- Togari, N.; Ogawa, N.; Sakata, T. Poor fermentability of “mekabu” (sporophyll of Undaria pinnatifida) alginic acid in batch culture using pig cecal bacteria. J. Nutr. Sci. Vitaminol. 1995, 41, 179–185. [Google Scholar] [CrossRef] [PubMed]
- Cooper, R.; Dragar, C.; Elliot, K.; Fitton, J.; Godwin, J.; Thompson, K. GFS, a preparation of Tasmanian Undaria pinnatifida is associated with healing and inhibition of reactivation of Herpes. BMC Complement. Altern. Med. 2002, 2, 11. [Google Scholar] [CrossRef]
- Hu, T.; Liu, D.; Chen, Y.; Wu, J.; Wang, S. Antioxidant activity of sulfated polysaccharide fractions extracted from Undaria pinnitafida in vitro. Int. J. Biol. Macromol. 2010, 46, 193–198. [Google Scholar] [CrossRef]
- Kim, K.J.; Yoon, K.Y.; Lee, B.Y. Low molecular weight fucoidan from the sporophyll of Undaria pinnatifida suppresses inflammation by promoting the inhibition of mitogen-activated protein kinases and oxidative stress in RAW264.7 cells. Fitoterapia 2012, 83, 1628–1635. [Google Scholar] [CrossRef]
- Maruyama, H.; Tamauchi, H.; Hashimoto, M.; Nakano, T. Suppression of Th2 immune responses by mekabu fucoidan from Undaria pinnatifida sporophylls. Int. Arch. Allergy Immunol. 2005, 137, 289–294. [Google Scholar] [CrossRef]
- Teng, H.; Yang, Y.; Wei, H.; Liu, Z.; Liu, Z.; Ma, Y.; Gao, Z.; Hou, L.; Zou, X. Fucoidan Suppresses Hypoxia-Induced Lymphangiogenesis and Lymphatic Metastasis in Mouse Hepatocarcinoma. Mar. Drugs 2015, 13, 3514–3530. [Google Scholar] [CrossRef]
- Chung, H.J.; Jeun, J.; Houng, S.J.; Jun, H.J.; Kweon, D.K.; Lee, S.J. Toxicological evaluation of fucoidan from Undaria pinnatifida in vitro and in vivo. Phytother. Res. PTR 2010, 24, 1078–1083. [Google Scholar] [CrossRef]
- Kadekaru, T.; Toyama, H.; Yasumoto, T. Safety Evaluation of Fucoxanthin purified from Undaria pinnatifida. Nippon Shokuhin Kagaku Kogaku Kaishi 2008, 55, 304–308. [Google Scholar] [CrossRef]
- Berteau, O.; Mulloy, B. Sulfated fucans, fresh perspectives: Structures, functions, and biological properties of sulfated fucans and an overview of enzymes active toward this class of polysaccharide. Glycobiology 2003, 13, 29R–40R. [Google Scholar] [CrossRef] [PubMed]
- Ho, T.T.; Bremmell, K.E.; Krasowska, M.; Stringer, D.N.; Thierry, B.; Beattie, D.A. Tuning polyelectrolyte multilayer structure by exploiting natural variation in fucoidan chemistry. Soft Matter 2015, 11, 2110–2124. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.S.; Jin, G.H.; Yeo, M.G.; Jang, C.H.; Lee, H.; Kim, G.H. Fabrication of electrospun biocomposites comprising polycaprolactone/fucoidan for tissue regeneration. Carbohydr. Polym. 2012, 90, 181–188. [Google Scholar] [CrossRef] [PubMed]
- Sanjeewa, K.K.A.; Herath, K.; Yang, H.W.; Choi, C.S.; Jeon, Y.J. Anti-Inflammatory Mechanisms of Fucoidans to Treat Inflammatory Diseases: A Review. Mar. Drugs 2021, 19, 678. [Google Scholar] [CrossRef]
- Subaraja, M.; Anantha Krishnan, D.; Edwin Hillary, V.; William Raja, T.R.; Mathew, P.; Ravikumar, S.; Gabriel Paulraj, M.; Ignacimuthu, S. Fucoidan serves a neuroprotective effect in an Alzheimer’s disease model. Front. Biosci. (Elite Ed.) 2020, 12, 1–34. [Google Scholar] [CrossRef]
- Koh, H.S.A.; Lu, J.; Zhou, W. Structure characterization and antioxidant activity of fucoidan isolated from Undaria pinnatifida grown in New Zealand. Carbohydr. Polym. 2019, 212, 178–185. [Google Scholar] [CrossRef]
- Kumar, Y.; Tarafdar, A.; Badgujar, P.C.; El-Sohaimy, S. Seaweed as a Source of Natural Antioxidants: Therapeutic Activity and Food Applications. J. Food Qual. 2021, 2021, 5753391. [Google Scholar] [CrossRef]
- Khairinisa, M.A.; Latarissa, I.R.; Athaya, N.S.; Charlie, V.; Musyaffa, H.A.; Prasedya, E.S.; Puspitasari, I.M. Potential Application of Marine Algae and Their Bioactive Metabolites in Brain Disease Treatment: Pharmacognosy and Pharmacology Insights for Therapeutic Advances. Brain Sci. 2023, 13, 1686. [Google Scholar] [CrossRef]
- Draget, K.I.; Taylor, C. Chemical, physical and biological properties of alginates and their biomedical implications. Food Hydrocoll. 2011, 25, 251–256. [Google Scholar] [CrossRef]
- MacArtain, P.; Gill, C.I.; Brooks, M.; Campbell, R.; Rowland, I.R. Nutritional value of edible seaweeds. Nutr. Rev. 2007, 65, 535–543. [Google Scholar] [CrossRef]
- Mackie, A.R.; Macierzanka, A.; Aarak, K.; Rigby, N.M.; Parker, R.; Channell, G.A.; Harding, S.E.; Bajka, B.H. Sodium alginate decreases the permeability of intestinal mucus. Food Hydrocoll. 2016, 52, 749–755. [Google Scholar] [CrossRef]
- Idota, Y.; Kogure, Y.; Kato, T.; Ogawa, M.; Kobayashi, S.; Kakinuma, C.; Yano, K.; Arakawa, H.; Miyajima, C.; Kasahara, F.; et al. Cholesterol-Lowering Effect of Calcium Alginate in Rats. Biol. Pharm. Bull. 2016, 39, 62–67. [Google Scholar] [CrossRef]
- Choukaife, H.; Doolaanea, A.A.; Alfatama, M. Alginate Nanoformulation: Influence of Process and Selected Variables. Pharmaceuticals 2020, 13, 335. [Google Scholar] [CrossRef]
- Feng, Y.; Quinnell, S.P.; Lanzi, A.M.; Vegas, A.J. Alginate-Based Amphiphilic Block Copolymers as a Drug Codelivery Platform. Nano Lett. 2021, 21, 7495–7504. [Google Scholar] [CrossRef]
- Haibe, Y.; Kreidieh, M.; El Hajj, H.; Khalifeh, I.; Mukherji, D.; Temraz, S.; Shamseddine, A. Resistance Mechanisms to Anti-angiogenic Therapies in Cancer. Front. Oncol. 2020, 10, 221. [Google Scholar] [CrossRef]
- Fogli, S.; Porta, C.; Del Re, M.; Crucitta, S.; Gianfilippo, G.; Danesi, R.; Rini, B.I.; Schmidinger, M. Optimizing treatment of renal cell carcinoma with VEGFR-TKIs: A comparison of clinical pharmacology and drug-drug interactions of anti-angiogenic drugs. Cancer Treat. Rev. 2020, 84, 101966. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Wang, J.; Chang, A.K.; Liu, B.; Yang, L.; Li, Q.; Wang, P.; Zou, X. Fucoidan extract derived from Undaria pinnatifida inhibits angiogenesis by human umbilical vein endothelial cells. Phytomed. Int. J. Phytother. Phytopharm. 2012, 19, 797–803. [Google Scholar] [CrossRef] [PubMed]
- Kohler, L.N.; Garcia, D.O.; Harris, R.B.; Oren, E.; Roe, D.J.; Jacobs, E.T. Adherence to Diet and Physical Activity Cancer Prevention Guidelines and Cancer Outcomes: A Systematic Review. Cancer Epidemiol. Biomark. Prev. 2016, 25, 1018–1028. [Google Scholar] [CrossRef] [PubMed]
- Maruyama, H.; Tamauchi, H.; Iizuka, M.; Nakano, T. The role of NK cells in antitumor activity of dietary fucoidan from Undaria pinnatifida sporophylls (Mekabu). Planta Med. 2006, 72, 1415–1417. [Google Scholar] [CrossRef]
- Maruyama, H.; Tamauchi, H.; Hashimoto, M.; Nakano, T. Antitumor activity and immune response of Mekabu fucoidan extracted from Sporophyll of Undaria pinnatifida. In Vivo 2003, 17, 245–249. [Google Scholar]
- Yang, L.; Wang, P.; Wang, H.; Li, Q.; Teng, H.; Liu, Z.; Yang, W.; Hou, L.; Zou, X. Fucoidan derived from Undaria pinnatifida induces apoptosis in human hepatocellular carcinoma SMMC-7721 cells via the ROS-mediated mitochondrial pathway. Mar. Drugs 2013, 11, 1961–1976. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Wang, S.; Trangle, S.S.; Li, Y.; White, W.L.; Li, J.; Ying, T.; Kong, Q.; Zhao, Y.; Lu, J. Investigation of Different Molecular Weight Fucoidan Fractions Derived from New Zealand Undaria pinnatifida in Combination with GroA Therapy in Prostate Cancer Cell Lines. Mar. Drugs 2018, 16, 454. [Google Scholar] [CrossRef]
- Wu, J.; Li, H.; Wang, X.; Zhang, X.; Liu, W.; Wang, Y.; Zhang, Y.; Pan, H.; Wang, Q.; Han, Y. Effect of polysaccharide from Undaria pinnatifida on proliferation, migration and apoptosis of breast cancer cell MCF7. Int. J. Biol. Macromol. 2019, 121, 734–742. [Google Scholar] [CrossRef]
- Vishchuk, O.S.; Ermakova, S.P.; Zvyagintseva, T.N. Sulfated polysaccharides from brown seaweeds Saccharina japonica and Undaria pinnatifida: Isolation, structural characteristics, and antitumor activity. Carbohydr. Res. 2011, 346, 2769–2776. [Google Scholar] [CrossRef]
- Zhang Hui, Z.H.; Pang ZunTing, P.Z.; Han ChunChao, H.C. Undaria pinnatifida (Wakame): A seaweed with pharmacological properties. Sci. Int. 2014, 2, 32–36. [Google Scholar] [CrossRef]
- Bobiński, M.; Okła, K.; Bednarek, W.; Wawruszak, A.; Dmoszyńska-Graniczka, M.; García-Sanz, P.; Wertel, I.; Kotarski, J. The effect of fucoidan, a potential new, natural, anti-neoplastic agent on uterine sarcomas and carcinosarcoma cell lines: ENITEC collaborative study. Arch. Immunol. Ther. Exp. 2019, 67, 125–131. [Google Scholar] [CrossRef]
- Bovet, L.; Samer, C.; Daali, Y. Preclinical Evaluation of Safety of Fucoidan Extracts from Undaria pinnatifida and Fucus vesiculosus for Use in Cancer Treatment. Integr. Cancer Ther. 2019, 18, 1534735419876325. [Google Scholar] [CrossRef]
- Corban, M.; Ambrose, M.; Pagnon, J.; Stringer, D.; Karpiniec, S.; Park, A.; Eri, R.; Fitton, J.H.; Gueven, N. Pathway Analysis of Fucoidan Activity Using a Yeast Gene Deletion Library Screen. Mar. Drugs 2019, 17, 54. [Google Scholar] [CrossRef]
- Sato, M.; Hosokawa, T.; Yamaguchi, T.; Nakano, T.; Muramoto, K.; Kahara, T.; Funayama, K.; Kobayashi, A.; Nakano, T. Angiotensin I-converting enzyme inhibitory peptides derived from wakame (Undaria pinnatifida) and their antihypertensive effect in spontaneously hypertensive rats. J. Agric. Food Chem. 2002, 50, 6245–6252. [Google Scholar] [CrossRef]
- Li, X.; Li, J.; Li, Z.; Sang, Y.; Niu, Y.; Zhang, Q.; Ding, H.; Yin, S. Fucoidan from Undaria pinnatifida prevents vascular dysfunction through PI3K/Akt/eNOS-dependent mechanisms in the l-NAME-induced hypertensive rat model. Food Funct. 2016, 7, 2398–2408. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Jeong, S.M.; Lee, J.E.; Kang, W.S.; Ryu, S.H.; Kim, K.; Byun, E.H.; Cho, Y.J.; Ahn, D.H. Characterization of Undaria pinnatifida Root Enzymatic Extracts Using Crude Enzyme from Shewanella oneidensis PKA 1008 and Its Anti-Inflammatory Effect. J. Microbiol. Biotechnol. 2020, 30, 79–84. [Google Scholar] [CrossRef] [PubMed]
- Phull, A.R.; Majid, M.; Haq, I.U.; Khan, M.R.; Kim, S.J. In vitro and in vivo evaluation of anti-arthritic, antioxidant efficacy of fucoidan from Undaria pinnatifida (Harvey) Suringar. Int. J. Biol. Macromol. 2017, 97, 468–480. [Google Scholar] [CrossRef] [PubMed]
- Herath, K.; Kim, H.J.; Kim, A.; Sook, C.E.; Lee, B.Y.; Jee, Y. The Role of Fucoidans Isolated from the Sporophylls of Undaria pinnatifida against Particulate-Matter-Induced Allergic Airway Inflammation: Evidence of the Attenuation of Oxidative Stress and Inflammatory Responses. Molecules 2020, 25, 2869. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.N.; Cho, J.Y.; Lee, M.C.; Kang, J.Y.; Park, N.G.; Fujii, H.; Hong, Y.K. Isolation of two anti-inflammatory and one pro-inflammatory polyunsaturated fatty acids from the brown seaweed Undaria pinnatifida. J. Agric. Food Chem. 2007, 55, 6984–6988. [Google Scholar] [CrossRef]
- Chen, X.; Wang, Y.; Shen, M.; Yu, Q.; Chen, Y.; Huang, L.; Xie, J. The water-soluble non-starch polysaccharides from natural resources against excessive oxidative stress: A potential health-promoting effect and its mechanisms. Int. J. Biol. Macromol. 2021, 171, 320–330. [Google Scholar] [CrossRef]
- Mak, W.; Hamid, N.; Liu, T.; Lu, J.; White, W.L. Fucoidan from New Zealand Undaria pinnatifida: Monthly variations and determination of antioxidant activities. Carbohydr. Polym. 2013, 95, 606–614. [Google Scholar] [CrossRef]
- Kang, K.S.; Kim, I.D.; Kwon, R.H.; Lee, J.Y.; Kang, J.S.; Ha, B.J. The effects of fucoidan extracts on CCl(4)-induced liver injury. Arch. Pharm. Res. 2008, 31, 622–627. [Google Scholar] [CrossRef]
- Fujiyuki, T.; Hamamoto, H.; Ishii, K.; Urai, M.; Kataoka, K.; Takeda, T.; Shibata, S.; Sekimizu, K. Evaluation of innate immune stimulating activity of polysaccharides using a silkworm (Bombyx mori) muscle contraction assay. Drug Discov. Ther. 2012, 6, 88–93. [Google Scholar] [CrossRef]
- Yu, Y.; Zhang, Y.; Hu, C.; Zou, X.; Lin, Y.; Xia, Y.; You, L. Chemistry and immunostimulatory activity of a polysaccharide from Undaria pinnatifida. Food Chem. Toxicol. 2019, 128, 119–128. [Google Scholar] [CrossRef]
- Bi, D.; Yu, B.; Han, Q.; Lu, J.; White, W.L.; Lai, Q.; Cai, N.; Luo, W.; Gu, L.; Li, S.; et al. Immune Activation of RAW264.7 Macrophages by Low Molecular Weight Fucoidan Extracted from New Zealand Undaria pinnatifida. J. Agric. Food Chem. 2018, 66, 10721–10728. [Google Scholar] [CrossRef]
- Jin, J.O.; Yu, Q. Fucoidan delays apoptosis and induces pro-inflammatory cytokine production in human neutrophils. Int. J. Biol. Macromol. 2015, 73, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Takai, M.; Miyazaki, Y.; Tachibana, H.; Yamada, K. The enhancing effect of fucoidan derived from Undaria pinnatifida on immunoglobulin production by mouse spleen lymphocytes. Biosci. Biotechnol. Biochem. 2014, 78, 1743–1747. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Oda, T.; Yu, Q.; Jin, J.O. Fucoidan from Macrocystis pyrifera has powerful immune-modulatory effects compared to three other fucoidans. Mar. Drugs 2015, 13, 1084–1104. [Google Scholar] [CrossRef] [PubMed]
- Ti, H.; Zhuang, Z.; Yu, Q.; Wang, S. Progress of Plant Medicine Derived Extracts and Alkaloids on Modulating Viral Infections and Inflammation. Drug Des. Dev. Ther. 2021, 15, 1385–1408. [Google Scholar] [CrossRef]
- Harden, E.A.; Falshaw, R.; Carnachan, S.M.; Kern, E.R.; Prichard, M.N. Virucidal activity of polysaccharide extracts from four algal species against herpes simplex virus. Antivir. Res. 2009, 83, 282–289. [Google Scholar] [CrossRef]
- Thompson, K.D.; Dragar, C. Antiviral activity of Undaria pinnatifida against herpes simplex virus. Phytother. Res. PTR 2004, 18, 551–555. [Google Scholar] [CrossRef]
- Lee, J.B.; Hayashi, K.; Hashimoto, M.; Nakano, T.; Hayashi, T. Novel antiviral fucoidan from sporophyll of Undaria pinnatifida (Mekabu). Chem. Pharm. Bull. 2004, 52, 1091–1094. [Google Scholar] [CrossRef]
- Chua, E.G.; Verbrugghe, P.; Perkins, T.T.; Tay, C.Y. Fucoidans Disrupt Adherence of Helicobacter pylori to AGS Cells In Vitro. Evid.-Based Complement. Altern. Med. ECAM 2015, 2015, 120981. [Google Scholar] [CrossRef]
- Kim, W.J.; Koo, Y.K.; Jung, M.K.; Moon, H.R.; Kim, S.M.; Synytsya, A.; Yun-Choi, H.S.; Kim, Y.S.; Park, J.K.; Park, Y.I. Anticoagulating activities of low-molecular weight fuco-oligosaccharides prepared by enzymatic digestion of fucoidan from the sporophyll of Korean Undaria pinnatifida. Arch. Pharm. Res. 2010, 33, 125–131. [Google Scholar] [CrossRef]
- Min, S.K.; Kwon, O.C.; Lee, S.; Park, K.H.; Kim, J.K. An antithrombotic fucoidan, unlike heparin, does not prolong bleeding time in a murine arterial thrombosis model: A comparative study of Undaria pinnatifida sporophylls and Fucus vesiculosus. Phytother. Res. PTR 2012, 26, 752–757. [Google Scholar] [CrossRef]
- Song, Z.; Li, H.; Liang, J.; Xu, Y.; Zhu, L.; Ye, X.; Wu, J.; Li, W.; Xiong, Q.; Li, S. Sulfated polysaccharide from Undaria pinnatifida stabilizes the atherosclerotic plaque via enhancing the dominance of the stabilizing components. Int. J. Biol. Macromol. 2019, 140, 621–630. [Google Scholar] [CrossRef]
- Bhadja, P.; Tan, C.Y.; Ouyang, J.M.; Yu, K. Repair Effect of Seaweed Polysaccharides with Different Contents of Sulfate Group and Molecular Weights on Damaged HK-2 Cells. Polymers 2016, 8, 188. [Google Scholar] [CrossRef]
- Ma, X.T.; Sun, X.Y.; Yu, K.; Gui, B.S.; Gui, Q.; Ouyang, J.M. Effect of Content of Sulfate Groups in Seaweed Polysaccharides on Antioxidant Activity and Repair Effect of Subcellular Organelles in Injured HK-2 Cells. Oxid. Med. Cell. Longev. 2017, 2017, 2542950. [Google Scholar] [CrossRef]
- Zhang, F.L.; He, Y.; Zheng, Y.; Zhang, W.J.; Wang, Q.; Jia, Y.J.; Song, H.L.; An, H.T.; Zhang, H.B.; Qian, Y.J.; et al. Therapeutic effects of fucoidan in 6-hydroxydopamine-lesioned rat model of Parkinson’s disease: Role of NADPH oxidase-1. CNS Neurosci. Ther. 2014, 20, 1036–1044. [Google Scholar] [CrossRef] [PubMed]
- Alghazwi, M.; Smid, S.; Karpiniec, S.; Zhang, W. Comparative study on neuroprotective activities of fucoidans from Fucus vesiculosus and Undaria pinnatifida. Int. J. Biol. Macromol. 2019, 122, 255–264. [Google Scholar] [CrossRef] [PubMed]
- Jannat, K.; Balakrishnan, R.; Han, J.H.; Yu, Y.J.; Kim, G.W.; Choi, D.K. The Neuropharmacological Evaluation of Seaweed: A Potential Therapeutic Source. Cells 2023, 12, 2652. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.-Y.; Rafiquzzaman, S.M.; Lee, J.M.; Noh, G.; Jo, G.-a.; Lee, J.-H.; Kong, I.-S. Structural features of glycoprotein purified from Saccharina japonica and its effects on the selected probiotic properties of Lactobacillus plantarum in Caco-2 cell. J. Appl. Phycol. 2014, 27, 965–973. [Google Scholar] [CrossRef]
- Lee, B.; Sur, B.; Park, J.; Shin, H.; Kwon, S.; Yeom, M.; Kim, S.J.; Kim, K.; Shim, I.; Yin, C.S.; et al. Fucoidan ameliorates scopolamine-induced neuronal impairment and memory dysfunction in rats via activation of cholinergic system and regulation of cAMP-response element-binding protein and brain-derived neurotrophic factor expressions. J. Korean Soc. Appl. Biol. Chem. 2012, 55, 711–720. [Google Scholar] [CrossRef]
- Luo, D.; Zhang, Q.; Wang, H.; Cui, Y.; Sun, Z.; Yang, J.; Zheng, Y.; Jia, J.; Yu, F.; Wang, X.; et al. Fucoidan protects against dopaminergic neuron death in vivo and in vitro. Eur. J. Pharmacol. 2009, 617, 33–40. [Google Scholar] [CrossRef]
- Pereira, L.; Valado, A. The Seaweed Diet in Prevention and Treatment of the Neurodegenerative Diseases. Mar. Drugs 2021, 19, 128. [Google Scholar] [CrossRef]
- Rafiquzzaman, S.M.; Kim, E.Y.; Lee, J.M.; Mohibbullah, M.; Alam, M.B.; Soo Moon, I.; Kim, J.-M.; Kong, I.-S. Anti-Alzheimers and anti-inflammatory activities of a glycoprotein purified from the edible brown alga Undaria pinnatifida. Food Res. Int. 2015, 77, 118–124. [Google Scholar] [CrossRef]
- Rocha de Souza, M.C.; Marques, C.T.; Guerra Dore, C.M.; Ferreira da Silva, F.R.; Oliveira Rocha, H.A.; Leite, E.L. Antioxidant activities of sulfated polysaccharides from brown and red seaweeds. J. Appl. Phycol. 2007, 19, 153–160. [Google Scholar] [CrossRef]
- Grasa-López, A.; Miliar-García, Á.; Quevedo-Corona, L.; Paniagua-Castro, N.; Escalona-Cardoso, G.; Reyes-Maldonado, E.; Jaramillo-Flores, M.-E. Undaria pinnatifida and fucoxanthin ameliorate lipogenesis and markers of both inflammation and cardiovascular dysfunction in an animal model of diet-induced obesity. Mar. Drugs 2016, 14, 148. [Google Scholar] [CrossRef] [PubMed]
- Woo, M.N.; Jeon, S.M.; Kim, H.J.; Lee, M.K.; Shin, S.K.; Shin, Y.C.; Park, Y.B.; Choi, M.S. Fucoxanthin supplementation improves plasma and hepatic lipid metabolism and blood glucose concentration in high-fat fed C57BL/6N mice. Chem. Biol. Interact. 2010, 186, 316–322. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Tang, Y.; Zhang, Y.; Zhang, S.; Qu, J.; Wang, X.; Kong, R.; Han, C.; Liu, Z. Fucoxanthin: A Promising Medicinal and Nutritional Ingredient. Evid.-Based Complement. Altern. Med. ECAM 2015, 2015, 723515. [Google Scholar] [CrossRef]
- Aljanabi, R.; Alsous, L.; Sabbah, D.A.; Gul, H.I.; Gul, M.; Bardaweel, S.K. Monoamine oxidase (MAO) as a potential target for anticancer drug design and development. Molecules 2021, 26, 6019. [Google Scholar] [CrossRef] [PubMed]
- Fung, A.; Hamid, N.; Lu, J. Fucoxanthin content and antioxidant properties of Undaria pinnatifida. Food Chem. 2013, 136, 1055–1062. [Google Scholar] [CrossRef]
- Jones, P.J.; MacDougall, D.E.; Ntanios, F.; Vanstone, C.A. Dietary phytosterols as cholesterol-lowering agents in humans. Can. J. Physiol. Pharmacol. 1997, 75, 217–227. [Google Scholar] [CrossRef]
- Meinita, M.D.N.; Harwanto, D.; Tirtawijaya, G.; Negara, B.; Sohn, J.H.; Kim, J.S.; Choi, J.S. Fucosterol of Marine Macroalgae: Bioactivity, Safety and Toxicity on Organism. Mar. Drugs 2021, 19, 545. [Google Scholar] [CrossRef]
- Jabbari, M.; Jabbari, A. Antioxidant potential and DPPH radical scavenging kinetics of water-insoluble flavonoid naringenin in aqueous solution of micelles. Colloids Surf. A Physicochem. Eng. Asp. 2016, 489, 392–399. [Google Scholar] [CrossRef]
- Lomartire, S.; Goncalves, A.M.M. Marine Macroalgae Polyphenols as Potential Neuroprotective Antioxidants in Neurodegenerative Diseases. Mar. Drugs 2023, 21, 261. [Google Scholar] [CrossRef]
- Olasehinde, T.A.; Olaniran, A.O.; Okoh, A.I. Macroalgae as a Valuable Source of Naturally Occurring Bioactive Compounds for the Treatment of Alzheimer’s Disease. Mar. Drugs 2019, 17, 609. [Google Scholar] [CrossRef]
- Wan-Loy, C.; Siew-Moi, P. Marine Algae as a Potential Source for Anti-Obesity Agents. Mar. Drugs 2016, 14, 222. [Google Scholar] [CrossRef]
- Chen, Z. On the softening effect of salty drugs. Shanghai J. Tradit. Chin. Med. 2013, 47, 65. [Google Scholar]
- Schepers, M.; Martens, N.; Tiane, A.; Vanbrabant, K.; Liu, H.B.; Lutjohann, D.; Mulder, M.; Vanmierlo, T. Edible seaweed-derived constituents: An undisclosed source of neuroprotective compounds. Neural Regen. Res. 2020, 15, 790–795. [Google Scholar] [CrossRef] [PubMed]
- Maeda, H.; Tsukui, T.; Sashima, T.; Hosokawa, M.; Miyashita, K. Seaweed carotenoid, fucoxanthin, as a multi-functional nutrient. Asia Pac. J. Clin. Nutr. 2008, 17 (Suppl. S1), 196–199. [Google Scholar] [PubMed]
- Frozza, R.; Lourenco, M.; De Felice, F. Challenges for Alzheimer’s disease therapy: Insights from novel mechanisms beyond memory defects. Front. Neurosci. 2018, 12, 37. [Google Scholar] [CrossRef]
- Valdes, L.; Cuervo, A.; Salazar, N.; Ruas-Madiedo, P.; Gueimonde, M.; Gonzalez, S. The relationship between phenolic compounds from diet and microbiota: Impact on human health. Food Funct. 2015, 6, 2424–2439. [Google Scholar] [CrossRef]
- Cruz, A.R.L.d. A Importância das Macroalgas Castanhas para o Desenvolvimento de Nutracêuticos. Bachelor’s Thesis, Universidade Fernando Pessoa, Porto, Portugal, 2018. [Google Scholar]
- Heo, S.J.; Park, E.J.; Lee, K.W.; Jeon, Y.J. Antioxidant activities of enzymatic extracts from brown seaweeds. Bioresour. Technol. 2005, 96, 1613–1623. [Google Scholar] [CrossRef]
- Oliveira, C.S.D. Prospeção de Compostos Bioativos nas Macroalgas Himanthalia elongata, Laminaria ochroleuca e Undaria pinnatifida. Ph.D. Thesis, Universidade de Aveiro, Aveiro, Portugal, 2015. [Google Scholar]
- Hwang, J.A.; Islam, M.M.; Ahmed, S.T.; Mun, H.S.; Kim, G.M.; Kim, Y.J.; Yang, C.J. Seamustard (Undaria pinnatifida) Improves Growth, Immunity, Fatty Acid Profile and Reduces Cholesterol in Hanwoo Steers. Asian-Australas J. Anim. Sci. 2014, 27, 1114–1123. [Google Scholar] [CrossRef]
- Lovinger, D.M. Communication networks in the brain: Neurons, receptors, neurotransmitters, and alcohol. Alcohol Res. Health 2008, 31, 196–214. [Google Scholar]
- Niyonambaza, S.D.; Kumar, P.; Xing, P.; Mathault, J.; De Koninck, P.; Boisselier, E.; Boukadoum, M.; Miled, A. A Review of Neurotransmitters Sensing Methods for Neuro-Engineering Research. Appl. Sci. 2019, 9, 4719. [Google Scholar] [CrossRef]
- Molina, E.M.B.; Mantilla, A.B.P.; Perera, O.H. Neurotransmitters, Their Effects on the Human Organism. Anat. Physiol. Biochem. Int. J. 2017, 2, 555–581. [Google Scholar] [CrossRef]
- Wong, C.G.; Bottiglieri, T.; Snead, O.C., 3rd. GABA, gamma-hydroxybutyric acid, and neurological disease. Ann. Neurol. 2003, 54 (Suppl. S6), S3–S12. [Google Scholar] [CrossRef] [PubMed]
- Berger, M.; Gray, J.A.; Roth, B.L. The expanded biology of serotonin. Annu. Rev. Med. 2009, 60, 355–366. [Google Scholar] [CrossRef] [PubMed]
- Canto, V.P.d. Estudo computacional das monoaminoxidases A e B com substratos e inibidores. Master’s Thesis, Universidade Federal do Rio Grande do Sul, Porto Alegre, Brazil, 2014. [Google Scholar]
- Petronilho, E.; Figueroa-Villar, J. Agents for defense against chemical warfare: Reactivators of acetylcholinesterase inhibited with neurotoxic organophosphorus compounds. Mil. Med. Sci. Lett. 2015, 84, 115–127. [Google Scholar] [CrossRef]
- Estevinho, M.F.; Soares-Fortunato, J. Dopamina e receptores. Rev. Port. Psicossomática 2003, 5, 21–31. [Google Scholar]
- Masuda, T.; Fujita, N.; Odaka, Y.; Takeda, Y.; Yonemori, S.; Nakamoto, K.; Kuninaga, H. Tyrosinase inhibitory activity of ethanol extracts from medicinal and edible plants cultivated in okinawa and identification of a water-soluble inhibitor from the leaves of Nandina domestica. Biosci. Biotechnol. Biochem. 2007, 71, 2316–2320. [Google Scholar] [CrossRef]
- Masuda, T.; Odaka, Y.; Ogawa, N.; Nakamoto, K.; Kuninaga, H. Identification of geranic acid, a tyrosinase inhibitor in lemongrass (Cymbopogon citratus). J. Agric. Food Chem. 2008, 56, 597–601. [Google Scholar] [CrossRef]
- Masuda, T.; Yamashita, D.; Takeda, Y.; Yonemori, S. Screening for tyrosinase inhibitors among extracts of seashore plants and identification of potent inhibitors from Garcinia subelliptica. Biosci. Biotechnol. Biochem. 2005, 69, 197–201. [Google Scholar] [CrossRef]
- Nagatsu, T.; Nakashima, A.; Watanabe, H.; Ito, S.; Wakamatsu, K. Neuromelanin in Parkinson’s Disease: Tyrosine Hydroxylase and Tyrosinase. Int. J. Mol. Sci. 2022, 23, 4176. [Google Scholar] [CrossRef]
- Wyss-Coray, T. Ageing, neurodegeneration and brain rejuvenation. Nature 2016, 539, 180–186. [Google Scholar] [CrossRef]
- World Health Organization. Dementia. Available online: https://www.who.int/news-room/fact-sheets/detail/dementia (accessed on 3 April 2025).
- Kovacs, G.G.; Budka, H. Current concepts of neuropathological diagnostics in practice: Neurodegenerative diseases. Clin. Neuropathol. 2010, 29, 271–288. [Google Scholar] [CrossRef]
- Peden, A.H.; Ironside, J.W. Molecular pathology in neurodegenerative diseases. Curr. Drug Targets 2012, 13, 1548–1559. [Google Scholar] [CrossRef] [PubMed]
- Tycko, R. Amyloid polymorphism: Structural basis and neurobiological relevance. Neuron 2015, 86, 632–645. [Google Scholar] [CrossRef] [PubMed]
- Barbalace, M.C.; Malaguti, M.; Giusti, L.; Lucacchini, A.; Hrelia, S.; Angeloni, C. Anti-Inflammatory Activities of Marine Algae in Neurodegenerative Diseases. Int. J. Mol. Sci. 2019, 20, 3061. [Google Scholar] [CrossRef]
- Khazdair, M.R.; Anaeigoudari, A.; Hashemzehi, M.; Mohebbati, R. Neuroprotective potency of some spice herbs, a literature review. J. Tradit. Complement. Med. 2019, 9, 98–105. [Google Scholar] [CrossRef]
- Milatovic, D.; Zaja-Milatovic, S.; Breyer, R.M.; Aschner, M.; Montine, T.J. Neuroinflammation and oxidative injury in developmental neurotoxicity. In Reproductive and Developmental Toxicology; Gupta, R.C., Ed.; Academic Press: San Diego, CA, USA, 2011; pp. 847–854. [Google Scholar]
- Salim, S. Oxidative Stress and the Central Nervous System. J. Pharmacol. Exp. Ther. 2017, 360, 201–205. [Google Scholar] [CrossRef]
- Hald, A.; Lotharius, J. Oxidative stress and inflammation in Parkinson’s disease: Is there a causal link? Exp. Neurol. 2005, 193, 279–290. [Google Scholar] [CrossRef]
- Reuter, S.; Gupta, S.C.; Chaturvedi, M.M.; Aggarwal, B.B. Oxidative stress, inflammation, and cancer: How are they linked? Free Radic. Biol. Med. 2010, 49, 1603–1616. [Google Scholar] [CrossRef]
- Sanchez-Lopez, F.; Tasset, I.; Aguera, E.; Feijoo, M.; Fernandez-Bolanos, R.; Sanchez, F.M.; Ruiz, M.C.; Cruz, A.H.; Gascon, F.; Tunez, I. Oxidative stress and inflammation biomarkers in the blood of patients with Huntington’s disease. Neurol Res. 2012, 34, 721–724. [Google Scholar] [CrossRef]
- Atanasov, A.G.; Zotchev, S.B.; Dirsch, V.M.; the International Natural Product Sciences; Supuran, C.T. Natural products in drug discovery: Advances and opportunities. Nat. Rev. Drug Discov. 2021, 20, 200–216. [Google Scholar] [CrossRef]
- Lamptey, R.N.L.; Chaulagain, B.; Trivedi, R.; Gothwal, A.; Layek, B.; Singh, J. A Review of the Common Neurodegenerative Disorders: Current Therapeutic Approaches and the Potential Role of Nanotherapeutics. Int. J. Mol. Sci. 2022, 23, 1851. [Google Scholar] [CrossRef]
- Oguntibeju, O.O. Type 2 diabetes mellitus, oxidative stress and inflammation: Examining the links. Int. J. Physiol. Pathophysiol. Pharmacol. 2019, 11, 45–63. [Google Scholar] [PubMed]
- Rojas-Gutierrez, E.; Munoz-Arenas, G.; Trevino, S.; Espinosa, B.; Chavez, R.; Rojas, K.; Flores, G.; Diaz, A.; Guevara, J. Alzheimer’s disease and metabolic syndrome: A link from oxidative stress and inflammation to neurodegeneration. Synapse 2017, 71, e21990. [Google Scholar] [CrossRef] [PubMed]
- Alghazwi, M.; Kan, Y.Q.; Zhang, W.; Gai, W.P.; Garson, M.J.; Smid, S. Neuroprotective activities of natural products from marine macroalgae during 1999–2015. J. Appl. Phycol. 2016, 28, 3599–3616. [Google Scholar] [CrossRef]
- Pereira, L. Nutritional Composition of the Main Edible Algae. In Therapeutic and Nutritional Uses of Algae, 1st ed.; CRC Press: Boca Raton, FL, USA, 2018. [Google Scholar]
- Anand, R.; Gill, K.D.; Mahdi, A.A. Therapeutics of Alzheimer’s disease: Past, present and future. Neuropharmacology 2014, 76 Pt A, 27–50. [Google Scholar] [CrossRef]
- Querfurth, H.W.; LaFerla, F.M. Alzheimer’s disease. N. Engl. J. Med. 2010, 362, 329–344. [Google Scholar] [CrossRef]
- Araujo, C.R.M.; Santos, V.d.A.; Gonsalves, A.A. Acetylcholinesterase-AChE: A pharmacological interesting enzyme. Rev. Virtual Química 2016, 8, 1818–1834. [Google Scholar] [CrossRef]
- Cummings, J.L.; Morstorf, T.; Zhong, K. Alzheimer’s disease drug-development pipeline: Few candidates, frequent failures. Alzheimer’s Res. Ther. 2014, 6, 37. [Google Scholar] [CrossRef]
- Parihar, M.S.; Hemnani, T. Alzheimer’s disease pathogenesis and therapeutic interventions. J. Clin. Neurosci. 2004, 11, 456–467. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, T.; Rao, P.P. Alzheimer’s disease: Emerging trends in small molecule therapies. Curr. Med. Chem. 2011, 18, 4299–4320. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.Y.; Kim, Y.R.; Nam, T.J.; Kong, I.S. Antioxidant and DNA protection activities of a glycoprotein isolated from a seaweed, Saccharina japonica. Int. J. Food Sci. Technol. 2012, 47, 1020–1027. [Google Scholar] [CrossRef]
- Zanella, L.; Vianello, F. Potential of microalgae as functional foods applied to mitochondria protection and healthy aging promotion. Nutraceuticals 2023, 3, 119–152. [Google Scholar] [CrossRef]
- Cui, Y.Q.; Jia, Y.J.; Zhang, T.; Zhang, Q.B.; Wang, X.M. Fucoidan protects against lipopolysaccharide-induced rat neuronal damage and inhibits the production of proinflammatory mediators in primary microglia. CNS Neurosci. Ther. 2012, 18, 827–833. [Google Scholar] [CrossRef]
- Cui, Y.Q.; Zhang, L.J.; Zhang, T.; Luo, D.Z.; Jia, Y.J.; Guo, Z.X.; Zhang, Q.B.; Wang, X.; Wang, X.M. Inhibitory effect of fucoidan on nitric oxide production in lipopolysaccharide-activated primary microglia. Clin. Exp. Pharmacol. Physiol. 2010, 37, 422–428. [Google Scholar] [CrossRef]
- Langston, J.W.; Forno, L.S.; Tetrud, J.; Reeves, A.G.; Kaplan, J.A.; Karluk, D. Evidence of active nerve cell degeneration in the substantia nigra of humans years after 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine exposure. Ann. Neurol. 1999, 46, 598–605. [Google Scholar] [CrossRef]
- Gerlach, M.; Maetzler, W.; Broich, K.; Hampel, H.; Rems, L.; Reum, T.; Riederer, P.; Stöffler, A.; Streffer, J.; Berg, D. Biomarker candidates of neurodegeneration in Parkinson’s disease for the evaluation of disease-modifying therapeutics. J. Neural Transm. 2012, 119, 39–52. [Google Scholar] [CrossRef]
- Bajpai, A.; Verma, A.K.; Srivastava, M.; Srivastava, R. Oxidative stress and major depression. J. Clin. Diagn. Res. 2014, 8, CC04–CC07. [Google Scholar] [CrossRef]
- Bhatt, S.; Nagappa, A.N.; Patil, C.R. Role of oxidative stress in depression. Drug Discov. Today 2020, 25, 1270–1276. [Google Scholar] [CrossRef]
- World Health Organization. Depressive Disorder (Depression). Available online: https://www.who.int/news-room/fact-sheets/detail/depression (accessed on 12 May 2025).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).