The Nrf2-Related Pathways and the Antiandrogenic Effects Are Enhanced In Vitro and In Silico by the Combination of Graminex®G96® Pollen and Teupol 25P in Cell Models of Benign Prostate Hyperplasia
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Cultures
2.2. Establishment of the Inflamed Environment and Cell Treatments
2.3. Cell Metabolic Activity (MTT)
2.4. LDH Released (Cytotoxicity Assay)
2.5. Catalase Activity Assay
2.6. Protein Extraction
2.7. Immunoblotting (Western Blot)
2.8. Measurement of Epiandrosterone Concentration
2.9. Determination of HO-1 and IL-6 Levels in BPH-1 Cells
2.10. RNA Extraction and Quantitative Real-Time PCR Analysis
2.11. Cell-Free Evaluation of Antioxidant Capacity
2.12. ROS Measurement
2.13. Homology Modeling of Human 5α-Reductase 1
2.14. Molecular Docking Study
2.15. Statistical Analysis
3. Results
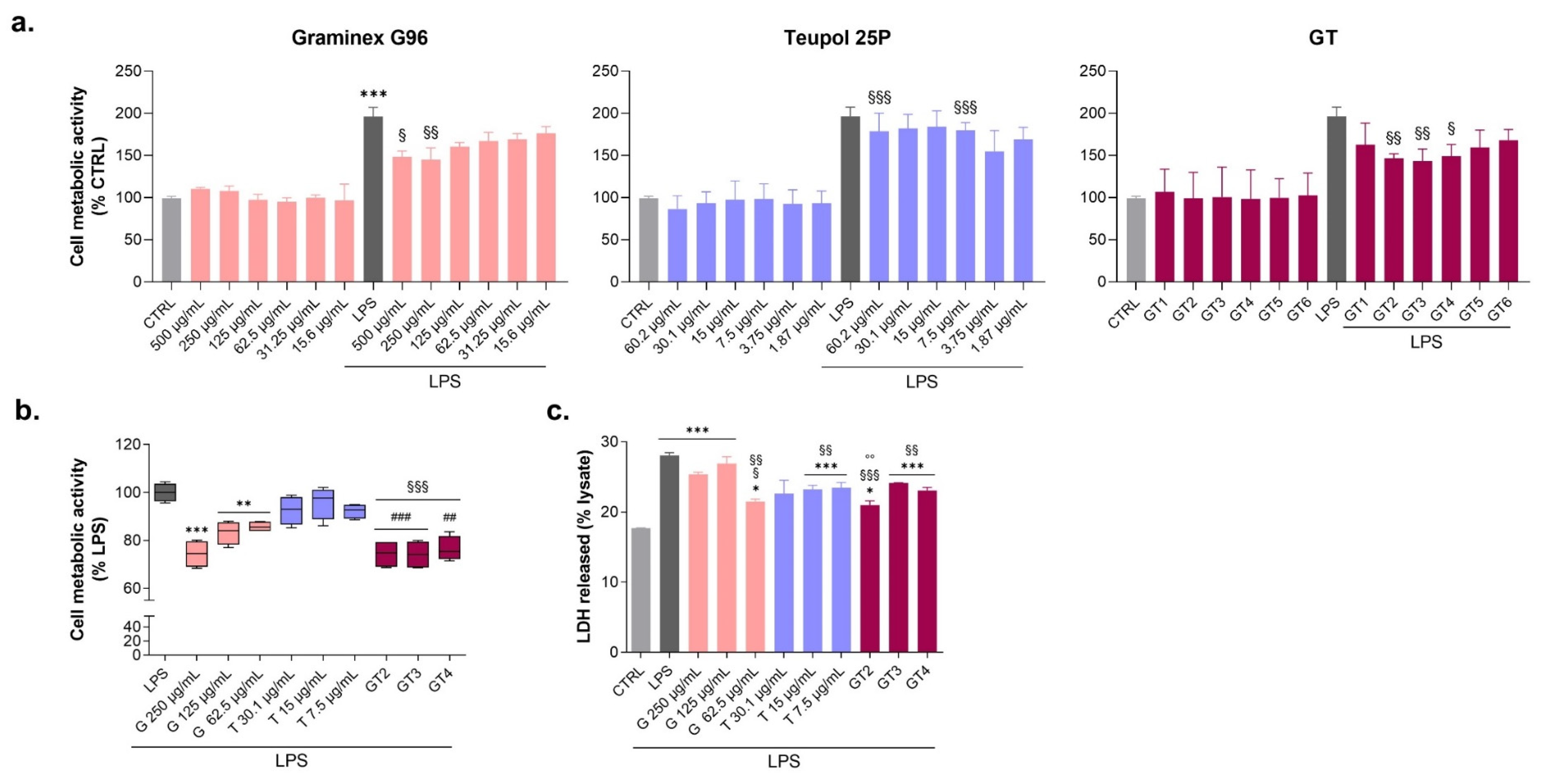
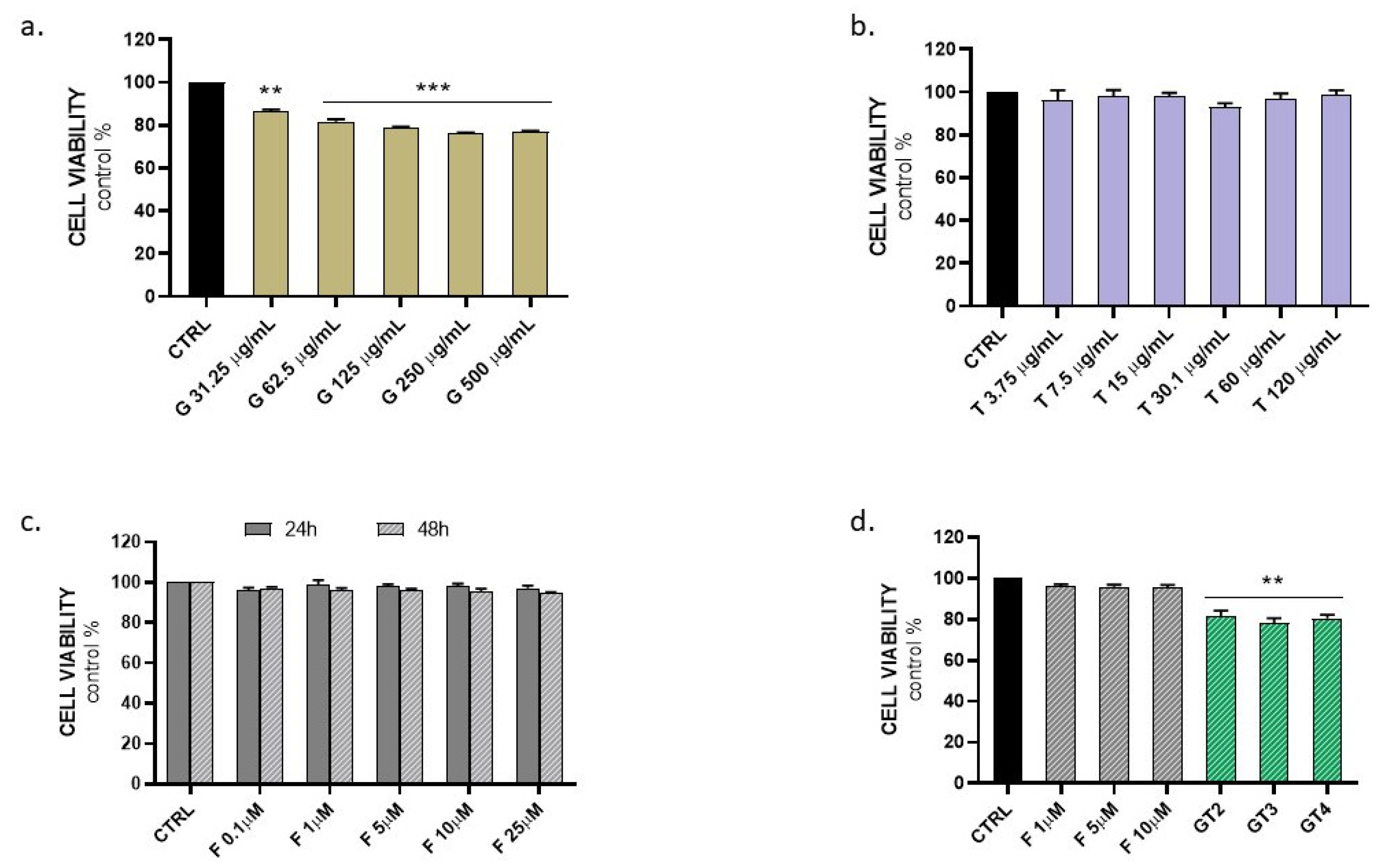
3.1. Evaluation of Graminex®G96® and Teupol 25P Cytotoxicity and Their Effect on the Cell Metabolic Activity of LPS-Stimulated Macrophages
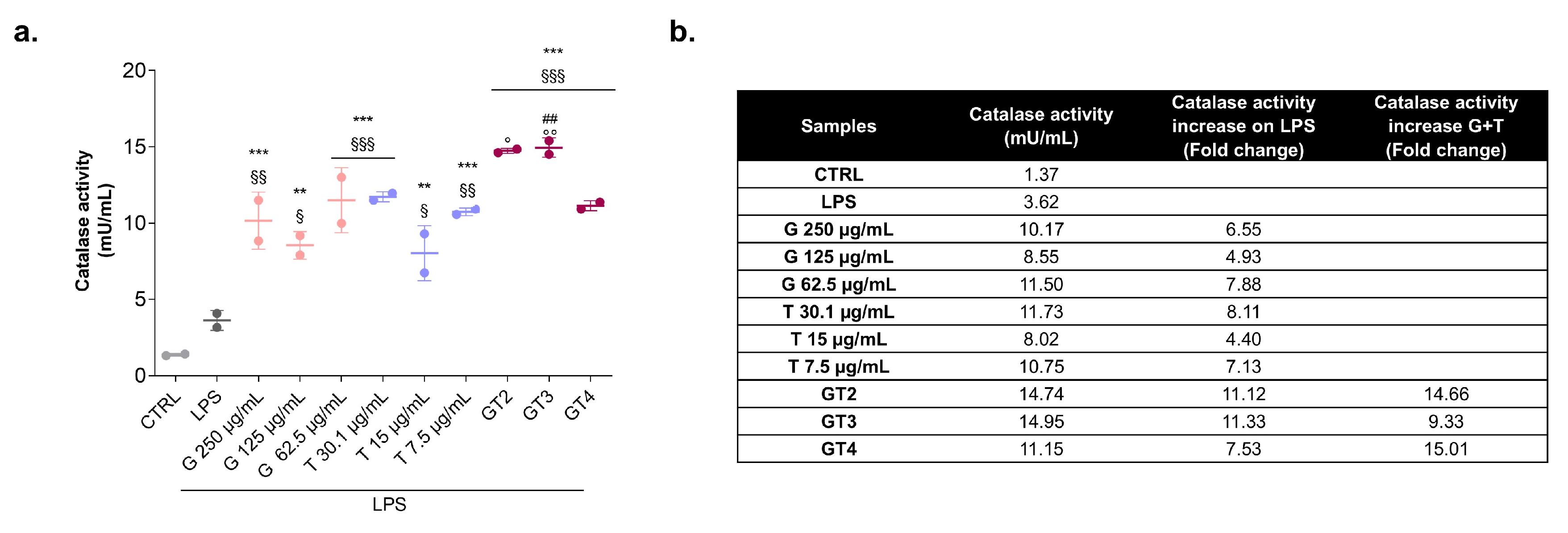
3.2. Catalase Activity in the Presence of Graminex®G96® and Teupol 25P in LPS-Stimulated Macrophages
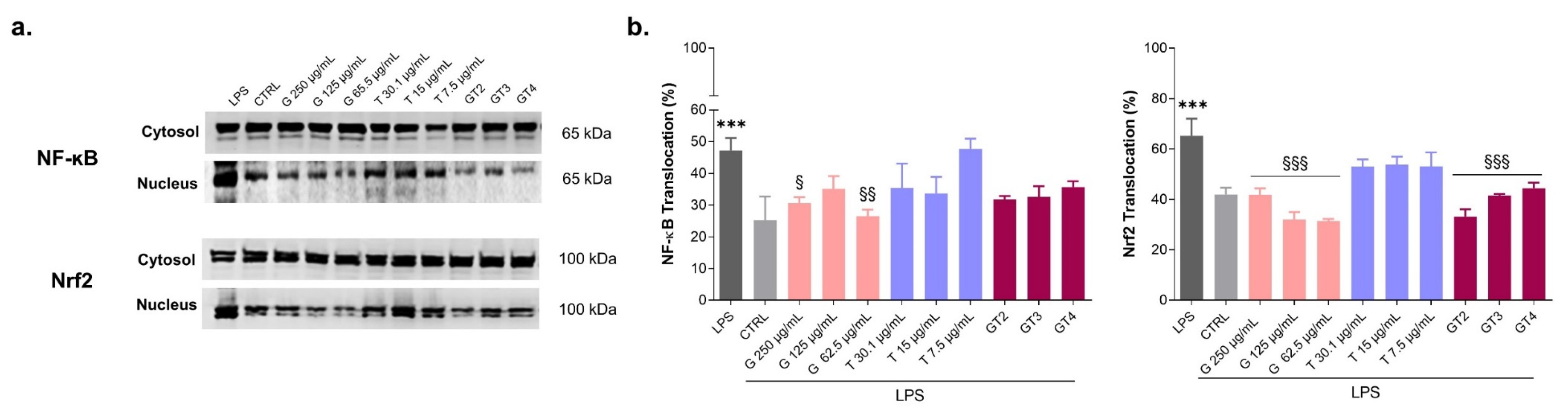
3.3. Levels of Expression of NF-ĸB and Nrf2 in LPS-Stimulated Macrophages in the Presence of Graminex®G96® and Teupol 25P
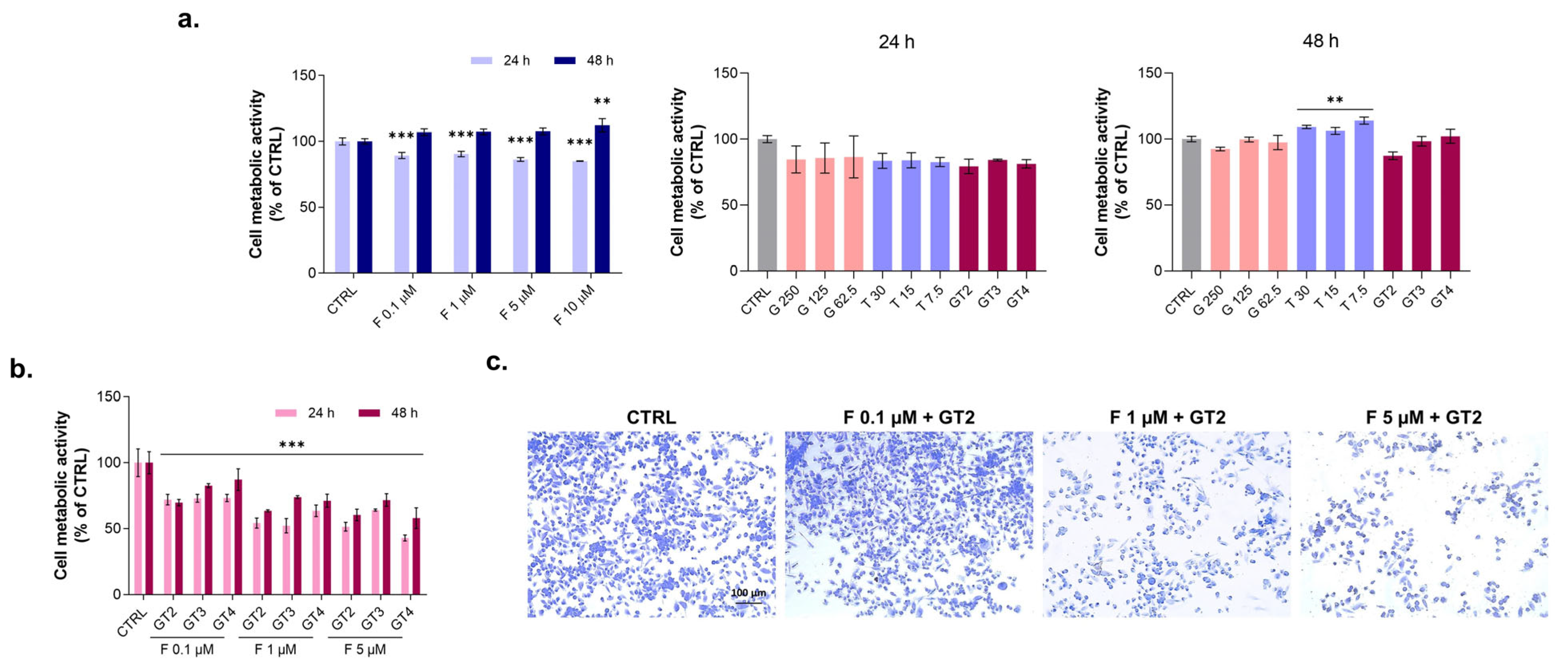
3.4. Evaluation of Graminex®G96® and Teupol 25P Effect on Cell Metabolic Activity of PC-3 Cells in the Presence of Finasteride
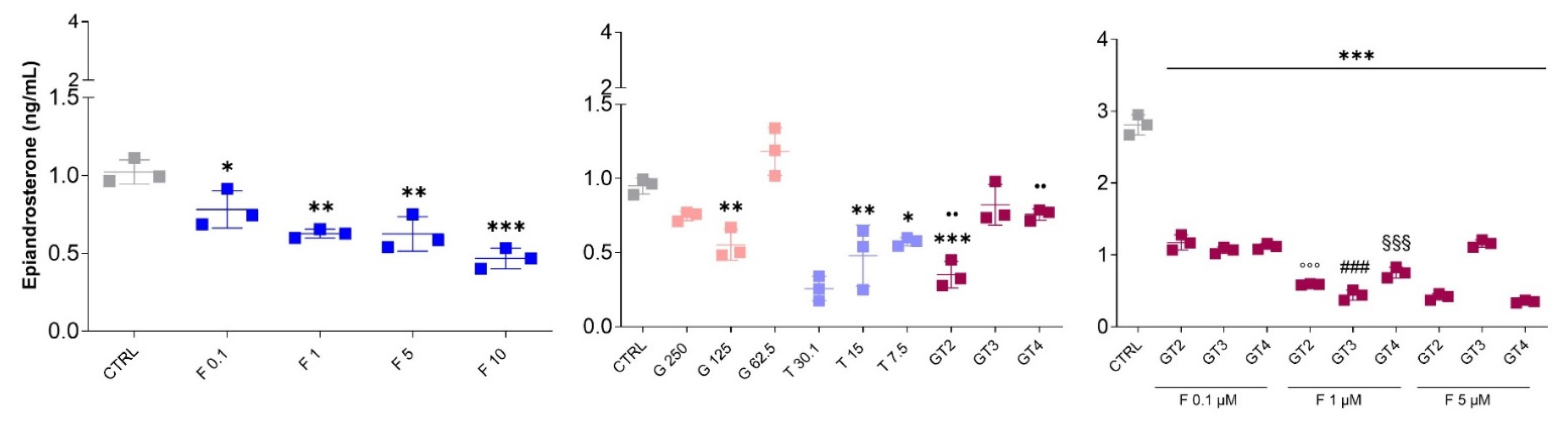
3.5. Epiandrosterone Detection in PC-3 Cells in the Presence of Graminex®G96®, Teupol 25P, or Finasteride
3.6. Evaluation of Graminex®G96® and Teupol 25P Cytotoxicity in Benign Prostatic Hyperplasia Cells
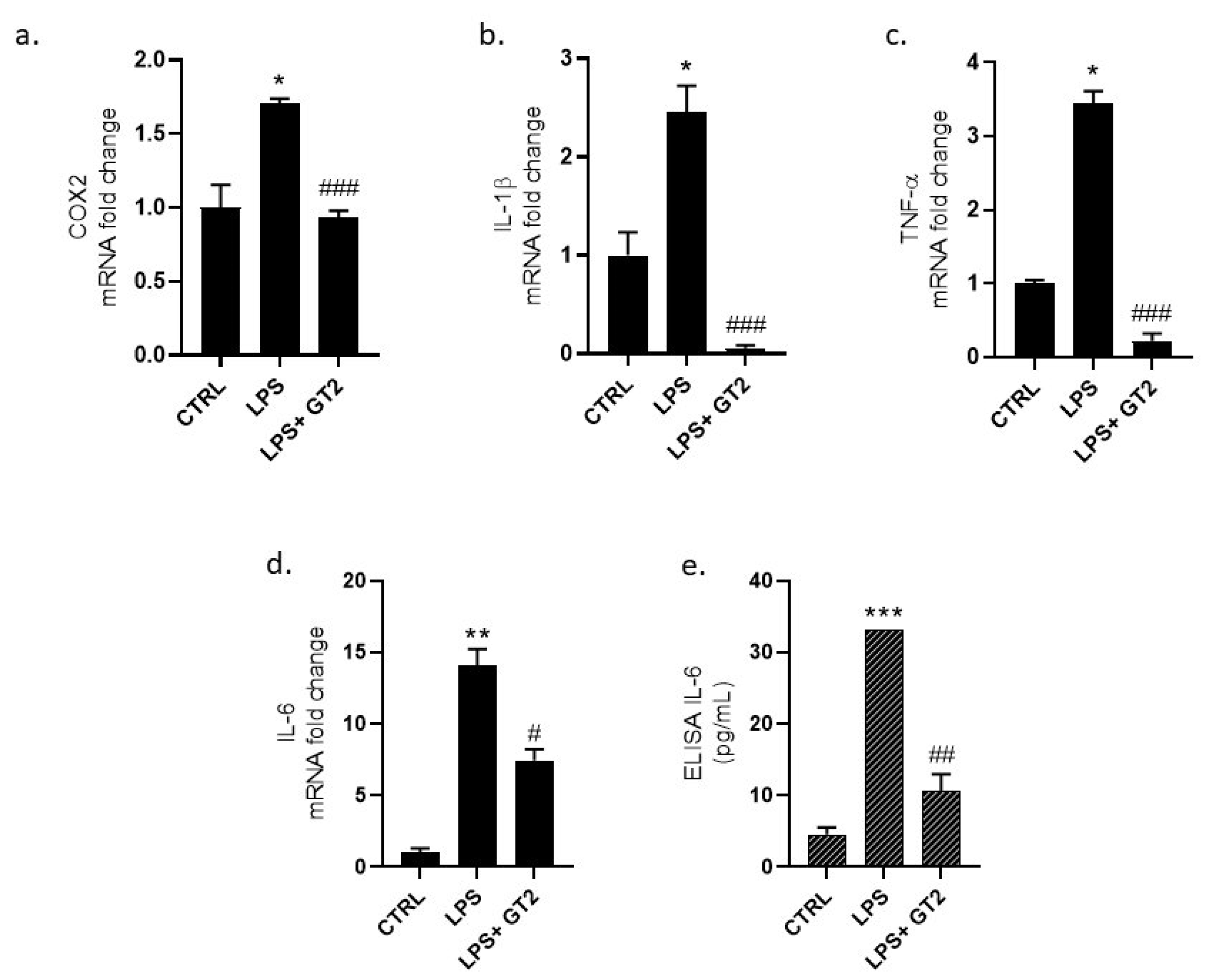
3.7. Evaluation of GT Anti-Inflammatory Activity
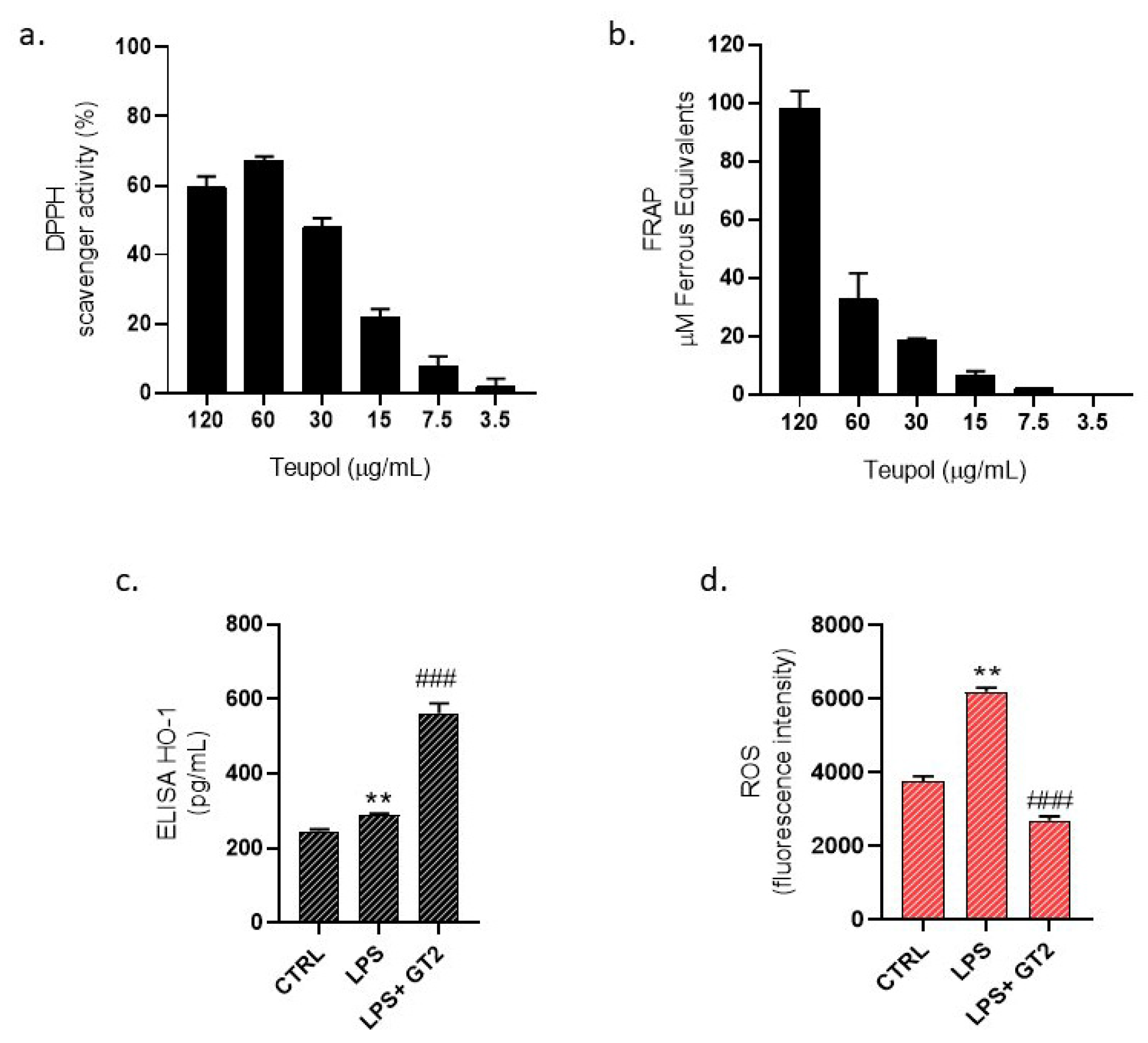
3.8. Evaluation of GT Antioxidant Activity
3.9. The Homology Modeling for Target Human 5α-Reductase 1
3.10. Evaluation of Binding Interactions of Teupolioside Assessed by Molecular Docking Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vuichoud, C.; Loughlin, K.R. Benign prostatic hyperplasia: Epidemiology, economics and evaluation. Can. J. Urol. 2015, 22 (Suppl. S1), 1–6. [Google Scholar] [PubMed]
- Welén, K.; Damber, J.E. Androgens, aging, and prostate health. Rev. Endocr. Metab. Disord. 2022, 23, 1221–1231. [Google Scholar] [CrossRef]
- O’Quin, C.; White, K.L.; Campbell, J.R.; Myers, S.H.; Patil, S.; Chandler, D.; Ahmadzadeh, S.; Varrassi, G.; Shekoohi, S.; Kaye, A.D. Pharmacological Approaches in Managing Symptomatic Relief of Benign Prostatic Hyperplasia: A Comprehensive Review. Cureus 2023, 15, e51314. [Google Scholar] [CrossRef] [PubMed]
- Traish, A.M. Health Risks Associated with Long-Term Finasteride and Dutasteride Use: It’s Time to Sound the Alarm. World J. Men’s Health 2020, 38, 323–337. [Google Scholar] [CrossRef] [PubMed]
- Jahan, N.; Chowdhury, A.; Li, T.; Xu, K.; Wei, F.; Wang, S. Neferine improves oxidative stress and apoptosis in benign prostate hyperplasia via Nrf2-ARE pathway. Redox Rep. 2021, 26, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Song, G.; Tong, J.; Wang, Y.; Li, Y.; Liao, Z.; Fan, D.; Fan, X. Nrf2-mediated macrophage function in benign prostatic hyperplasia: Novel molecular insights and implications. Biomed. Pharmacother. 2023, 167, 115566. [Google Scholar] [CrossRef]
- Miernik, A.; Gratzke, C. Current Treatment for Benign Prostatic Hyperplasia. Dtsch. Arztebl. Int. 2020, 117, 843–854. [Google Scholar] [CrossRef]
- Cicero, A.F.G.; Allkanjari, O.; Busetto, G.M.; Cai, T.; Larganà, G.; Magri, V.; Perletti, G.; Robustelli Della Cuna, F.S.; Russo, G.I.; Stamatiou, K.; et al. Nutraceutical treatment and prevention of benign prostatic hyperplasia and prostate cancer. Arch. Ital. Urol. Androl. 2019, 91, 139–152. [Google Scholar] [CrossRef]
- Chiavaroli, A.; Recinella, L.; Ferrante, C.; Locatelli, M.; Carradori, S.; Macchione, N.; Zengin, G.; Leporini, L.; Leone, S.; Martinotti, S.; et al. Crocus sativus, Serenoa repens and Pinus massoniana extracts modulate inflammatory response in isolated rat prostate challenged with LPS. J. Biol. Regul. Homeost. Agents 2017, 31, 531–541. [Google Scholar]
- De Monte, C.; Carradori, S.; Granese, A.; Di Pierro, G.B.; Leonardo, C.; De Nunzio, C. Modern extraction techniques and their impact on the pharmacological profile of Serenoa repens extracts for the treatment of lower urinary tract symptoms. BMC Urol. 2014, 14, 63. [Google Scholar] [CrossRef]
- Locatelli, M.; Macchione, N.; Ferrante, C.; Chiavaroli, A.; Recinella, L.; Carradori, S.; Zengin, G.; Cesa, S.; Leporini, L.; Leone, S.; et al. Graminex Pollen: Phenolic Pattern, Colorimetric Analysis and Protective Effects in Immortalized Prostate Cells (PC3) and Rat Prostate Challenged with LPS. Molecules 2018, 23, 1145. [Google Scholar] [CrossRef] [PubMed]
- Chiavaroli, A.; Di Simone, S.C.; Acquaviva, A.; Libero, M.L.; Campana, C.; Recinella, L.; Leone, S.; Brunetti, L.; Orlando, G.; Nilofar; et al. Protective Effects of PollenAid Plus Soft Gel Capsules’ Hydroalcoholic Extract in Isolated Prostates and Ovaries Exposed to Lipopolysaccharide. Molecules 2022, 27, 6279. [Google Scholar] [CrossRef] [PubMed]
- Di Pasquale, A.B.; Cassani, A.; Masciovecchio, S.; Zasa, G.; Ranieri, G.; Romano, G.; Di Clemente, L. Postoperative treatment with phytotheraphy Graminex G63 (CERNILEN-Flogo®) after greenlight laser XPS (180W) photovaporization of the prostate (PVP), can affect patient’s quality of life? Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 9116–9120. [Google Scholar] [CrossRef]
- Cai, T.; Verze, P.; La Rocca, R.; Palmieri, A.; Tiscione, D.; Luciani, L.G.; Mazzoli, S.; Mirone, V.; Malossini, G. The Clinical Efficacy of Pollen Extract and Vitamins on Chronic Prostatitis/Chronic Pelvic Pain Syndrome Is Linked to a Decrease in the Pro-Inflammatory Cytokine Interleukin-8. World J. Men’s Health 2017, 35, 120–128. [Google Scholar] [CrossRef]
- Di Paola, R.; Esposito, E.; Mazzon, E.; Riccardi, L.; Caminiti, R.; Dal Toso, R.; Pressi, G.; Cuzzocrea, S. Teupolioside, a phenylpropanoid glycosides of Ajuga reptans, biotechnologically produced by IRBN22 plant cell line, exerts beneficial effects on a rodent model of colitis. Biochem. Pharmacol. 2009, 77, 845–857. [Google Scholar] [CrossRef]
- Korkina, L.G.; Mikhal’chik, E.; Suprun, M.V.; Pastore, S.; Dal Toso, R. Molecular mechanisms underlying wound healing and anti-inflammatory properties of naturally occurring biotechnologically produced phenylpropanoid glycosides. Cell. Mol. Biol. 2007, 53, 84–91. [Google Scholar] [PubMed]
- Pastore, S.; Potapovich, A.; Kostyuk, V.; Mariani, V.; Lulli, D.; De Luca, C.; Korkina, L. Plant polyphenols effectively protect HaCaT cells from ultraviolet C-triggered necrosis and suppress inflammatory chemokine expression. Ann. N. Y. Acad. Sci. 2009, 1171, 305–313. [Google Scholar] [CrossRef]
- Muraca, L.; Scuteri, A.; Burdino, E.; Marcianò, G.; Rania, V.; Catarisano, L.; Casarella, A.; Cione, E.; Palleria, C.; Colosimo, M.; et al. Effectiveness and Safety of a New Nutrient Fixed Combination Containing Pollen Extract Plus Teupolioside, in the Management of LUTS in Patients with Benign Prostatic Hypertrophy: A Pilot Study. Life 2022, 12, 965. [Google Scholar] [CrossRef]
- Gallorini, M.; Carradori, S.; Resende, D.I.S.P.; Saso, L.; Ricci, A.; Palmeira, A.; Cataldi, A.; Pinto, M.; Sousa, E. Natural and Synthetic Xanthone Derivatives Counteract Oxidative Stress via Nrf2 Modulation in Inflamed Human Macrophages. Int. J. Mol. Sci. 2022, 23, 13319. [Google Scholar] [CrossRef]
- Sorrenti, V.; D’Amico, A.G.; Barbagallo, I.; Consoli, V.; Grosso, S.; Vanella, L. Tin Mesoporphyrin Selectively Reduces Non-Small-Cell Lung Cancer Cell Line A549 Proliferation by Interfering with Heme Oxygenase and Glutathione Systems. Biomolecules 2021, 11, 917. [Google Scholar] [CrossRef]
- Krieger, E.; Vriend, G. YASARA view-molecular graphics for all devices-from smartphones to workstations. Bioinformatics 2014, 30, 2981–2982. [Google Scholar] [CrossRef] [PubMed]
- Krieger, E.; Vriend, G. New ways to boost molecular dynamics simulations. J. Comput. Chem. 2015, 36, 996–1007. [Google Scholar] [CrossRef] [PubMed]
- Morris, G.M.; Huey, R.; Lindstrom, W.; Sanner, M.F.; Belew, R.K.; Goodsell, D.S.; Olson, A.J. AutoDock4 and AutoDockTools4: Automated docking with selective receptor flexibility. J. Comput. Chem. 2009, 30, 2785–2791. [Google Scholar] [CrossRef]
- Xiao, Q.; Wang, L.; Supekar, S.; Shen, T.; Liu, H.; Ye, F.; Huang, J.; Fan, H.; Wei, Z.; Zhang, C. Structure of human steroid 5α-reductase 2 with the anti-androgen drug finasteride. Nat. Commun. 2020, 11, 5430. [Google Scholar] [CrossRef]
- Maier, J.A.; Martinez, C.; Kasavajhala, K.; Wickstrom, L.; Hauser, K.E.; Simmerling, C. ff14SB: Improving the accuracy of protein side chain and backbone parameters from ff99SB. J. Chem. Theory Comput. 2015, 11, 3696–3713. [Google Scholar] [CrossRef]
- Dassault Systèmes BIOVIA. Discovery Studio Modeling Environment; Release 24.1.0.23298; Dassault Systèmes: San Diego, CA, USA, 2024. [Google Scholar]
- Mencarelli, N.; Arena, D.; Salamone, M.; Pietrangelo, L.; Berardi, A.C.; Cataldi, A.; Carradori, S.; Gallorini, M. The combination of hyaluronic acids and collagen boosts human Achilles tendon-derived cell escape from inflammation and matrix remodeling in vitro. Inflamm. Res. 2025, 74, 4. [Google Scholar] [CrossRef]
- Ricci, A.; Zara, S.; Carta, F.; Di Valerio, V.; Sancilio, S.; Cataldi, A.; Selleri, S.; Supuran, C.T.; Carradori, S.; Gallorini, M. 2-Substituted-4,7-dihydro-4-ethylpyrazolo [1,5-a]pyrimidin-7-ones alleviate LPS-induced inflammation by modulating cell metabolism via CD73 upon macrophage polarization. Mol. Immunol. 2024, 170, 99–109. [Google Scholar] [CrossRef] [PubMed]
- Jones, D.T. Protein secondary structure prediction based on position-specific scoring matrices. J. Mol. Biol. 1999, 292, 195–202. [Google Scholar] [CrossRef]
- Mondaini, N.; Gontero, P.; Giubilei, G.; Lombardi, G.; Cai, T.; Gavazzi, A.; Bartoletti, R. Finasteride 5 mg and sexual side effects: How many of these are related to a nocebo phenomenon? J. Sex. Med. 2007, 4, 1708–1712. [Google Scholar] [CrossRef]
- Edwards, J.E.; Moore, R.A. Finasteride in the treatment of clinical benign prostatic hyperplasia: A systematic review of randomised trials. BMC Urol. 2002, 2, 14. [Google Scholar] [CrossRef]
- Lowe, F.C.; McConnell, J.D.; Hudson, P.B.; Romas, N.A.; Boake, R.; Lieber, M.; Elhilali, M.; Geller, J.; Imperto-McGinely, J.; Andriole, G.L.; et al. Long-term 6-year experience with finasteride in patients with benign prostatic hyperplasia. Urology 2003, 61, 791–796. [Google Scholar] [CrossRef] [PubMed]
- Sens-Albert, C.; Weisenburger, S.; König, B.C.; Melcher, S.F.; Scheyhing, U.A.M.; Rollet, K.; Lluel, P.; Koch, E.; Lehner, M.D.; Michel, M.C. Effects of a proprietary mixture of extracts from. Front. Pharmacol. 2024, 15, 1379456. [Google Scholar] [CrossRef]
- Murtola, T.J.; Gurel, B.; Umbehr, M.; Lucia, M.S.; Thompson, I.M.; Goodman, P.J.; Kristal, A.R.; Parnes, H.L.; Lippman, S.M.; Sutcliffe, S.; et al. Inflammation in Benign Prostate Tissue and Prostate Cancer in the Finasteride Arm of the Prostate Cancer Prevention Trial. Cancer Epidemiol. Biomark. Prev. 2016, 25, 463–469. [Google Scholar] [CrossRef] [PubMed]
- Stewart, K.L.; Lephart, E.D. Overview of BPH: Symptom Relief with Dietary Polyphenols, Vitamins and Phytochemicals by Nutraceutical Supplements with Implications to the Prostate Microbiome. Int. J. Mol. Sci. 2023, 24, 5486. [Google Scholar] [CrossRef]
- Tusubira, D.; Aja, P.M.; Munezero, J.; Ssedyabane, F.; Namale, N.; Ifie, J.E.; Agu, P.C.; Ajayi, C.O.; Okoboi, J. Safety profile of colocasia esculenta tuber extracts in benign prostate hyperplasia. BMC Complement. Med. Ther. 2023, 23, 187. [Google Scholar] [CrossRef] [PubMed]
- Crocerossa, F.; Cantiello, F.; Bagalá, L.; Sicoli, F.; Carbonara, U.; Manfredi, C.; Falagario, U.; Veccia, A.; Pandolfo, S.D.; Napolitano, L.; et al. Clinical Effects of Oral Supplementation of Gamma-Cyclodextrin Curcumin Complex in Male Patients with Moderate-To-Severe Benign Prostatic Hyperplasia-Related Lower Urinary Tract Symptoms. Urol. Int. 2023, 107, 924–934. [Google Scholar] [CrossRef]
- Norouzi, M.; Mahboobi, S.; Eftekhari, M.H.; Salehipour, M.; Ghaem, H.; Mirzakhanlouei, A.; Mohsenpour, M.A. Effects of L-Carnitine and Coenzyme Q10 Supplementation on Lower Urinary Tract Symptoms in Men with Benign Prostatic Hyperplasia: A Randomized, Controlled, Clinical Trial. Nutr. Cancer 2024, 76, 207–214. [Google Scholar] [CrossRef]
- Consoli, V.; Burò, I.; Gulisano, M.; Castellano, A.; D’Amico, A.G.; D’Agata, V.; Vanella, L.; Sorrenti, V. Evaluation of the Antioxidant and Antiangiogenic Activity of a Pomegranate Extract in BPH-1 Prostate Epithelial Cells. Int. J. Mol. Sci. 2023, 24, 10719. [Google Scholar] [CrossRef]
- Huynh, H.; Seyam, R.M.; Brock, G.B. Reduction of ventral prostate weight by finasteride is associated with suppression of insulin-like growth factor I (IGF-I) and IGF-I receptor genes and with an increase in IGF binding protein 3. Cancer Res. 1998, 58, 215–218. [Google Scholar]
- Wang, K.; Jin, S.; Fan, D.; Wang, M.; Xing, N.; Niu, Y. Anti-proliferative activities of finasteride in benign prostate epithelial cells require stromal fibroblasts and c-Jun gene. PLoS ONE 2017, 12, e0172233. [Google Scholar] [CrossRef]
- Nair, V.S.; Doman, C.E.; Morrison, M.S.; Miller, G.D.; Husk, J.; van Eenoo, P.; Crouch, A.K.; Eichner, D. Evaluation of epiandrosterone as a long-term marker of testosterone use. Drug Test. Anal. 2020, 12, 1554–1560. [Google Scholar] [CrossRef] [PubMed]
- Dušková, M.; Hill, M.; Stárka, L. The influence of low dose finasteride, a type II 5α-reductase inhibitor, on circulating neuroactive steroids. Horm. Mol. Biol. Clin. Investig. 2010, 1, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Fibbi, B.; Penna, G.; Morelli, A.; Adorini, L.; Maggi, M. Chronic inflammation in the pathogenesis of benign prostatic hyperplasia. Int. J. Androl. 2010, 33, 475–488. [Google Scholar] [CrossRef]
- Jin, R.; Forbes, C.M.; Miller, N.L.; Lafin, J.; Strand, D.W.; Case, T.; Cates, J.M.; Liu, Q.; Ramirez-Solano, M.; Mohler, J.L.; et al. Transcriptomic analysis of benign prostatic hyperplasia identifies critical pathways in prostatic overgrowth and 5-alpha reductase inhibitor resistance. Prostate 2024, 84, 441–459. [Google Scholar] [CrossRef]
- Ene, C.V.; Nicolae, I.; Geavlete, B.; Geavlete, P.; Ene, C.D. IL-6 Signaling Link between Inflammatory Tumor Microenvironment and Prostatic Tumorigenesis. Anal. Cell. Pathol. 2022, 2022, 5980387. [Google Scholar] [CrossRef]
- Russo, G.I.; Vanella, L.; Castelli, T.; Cimino, S.; Reale, G.; Urzì, D.; Li Volti, G.; Gacci, M.; Carini, M.; Motta, F.; et al. Heme oxygenase levels and metaflammation in benign prostatic hyperplasia patients. World J. Urol. 2016, 34, 1183–1192. [Google Scholar] [CrossRef]
- Vanella, L.; Russo, G.I.; Cimino, S.; Fragalà, E.; Favilla, V.; Li Volti, G.; Barbagallo, I.; Sorrenti, V.; Morgia, G. Correlation between lipid profile and heme oxygenase system in patients with benign prostatic hyperplasia. Urology 2014, 83, 1444.e7–1444.e13. [Google Scholar] [CrossRef] [PubMed]
- Schweikl, H.; Gallorini, M.; Pöschl, G.; Urmann, V.; Petzel, C.; Bolay, C.; Hiller, K.A.; Cataldi, A.; Buchalla, W. Functions of transcription factors NF-κB and Nrf2 in the inhibition of LPS-stimulated cytokine release by the resin monomer HEMA. Dent. Mater. 2018, 34, 1661–1678. [Google Scholar] [CrossRef]
- Gallorini, M.; Carradori, S.; Panieri, E.; Sova, M.; Saso, L. Modulation of NRF2: Biological Dualism in Cancer, Targets and Possible Therapeutic Applications. Antioxid. Redox Signal. 2024, 40, 636–662. [Google Scholar] [CrossRef]
- Langlois, V.S.; Zhang, D.; Cooke, G.M.; Trudeau, V.L. Evolution of steroid-5α-reductases and comparison of their function with 5β-reductase. Gen. Comp. Endocrinol. 2010, 166, 489–497. [Google Scholar] [CrossRef]
- Russell, D.W.; Wilson, J.D. Steroid 5α-reductase: Two genes/two enzymes. Annu. Rev. Biochem. 1994, 63, 25–61. [Google Scholar] [CrossRef] [PubMed]
- Azzouni, F.; Godoy, A.; Li, Y.; Mohler, J. The 5 alpha-reductase isozyme family: A review of basic biology and their role in human diseases. Adv. Urol. 2012, 2012, 530121. [Google Scholar] [CrossRef] [PubMed]
- Tomkins, G.M. The enzymatic reduction of Δ4-3-ketosteroids. J. Biol. Chem. 1957, 225, 13–24. [Google Scholar] [CrossRef]
- Aggarwal, S.; Thareja, S.; Verma, A.; Bhardwaj, T.R.; Kumar, M. An overview on 5α-reductase inhibitors. Steroids 2010, 75, 109–153. [Google Scholar] [CrossRef]
- Occhiato, E.G.; Guarna, A.; Danza, G.; Serio, M. Selective non-steroidal inhibitors of 5α-reductase type 1. J. Steroid Biochem. Mol. Biol. 2004, 88, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Liao, S.S.; Hiipakka, R.A. Selective-Inhibition of steroid 5 α-reductase isozymes by tea epicatechin-3-gallate and epigallocatechin-3-gallate. Biochem. Biophys. Res. Commun. 1995, 214, 833–838. [Google Scholar] [CrossRef]
- Lo Re, M.; Pezzoli, M.; Cadenar, A.; Fuligni, E.; Gajo, L.; Minervini, A.; Cocci, A. Discovering a new nutraceutical based on pollen extract and teupolioside: A prospective monocentric study evaluating its role in alleviating lower urinary tract symptoms in benign prostatic hyperplasia patients. Arch. Ital. Urol. Androl. 2025, 97, 13412. [Google Scholar] [CrossRef]
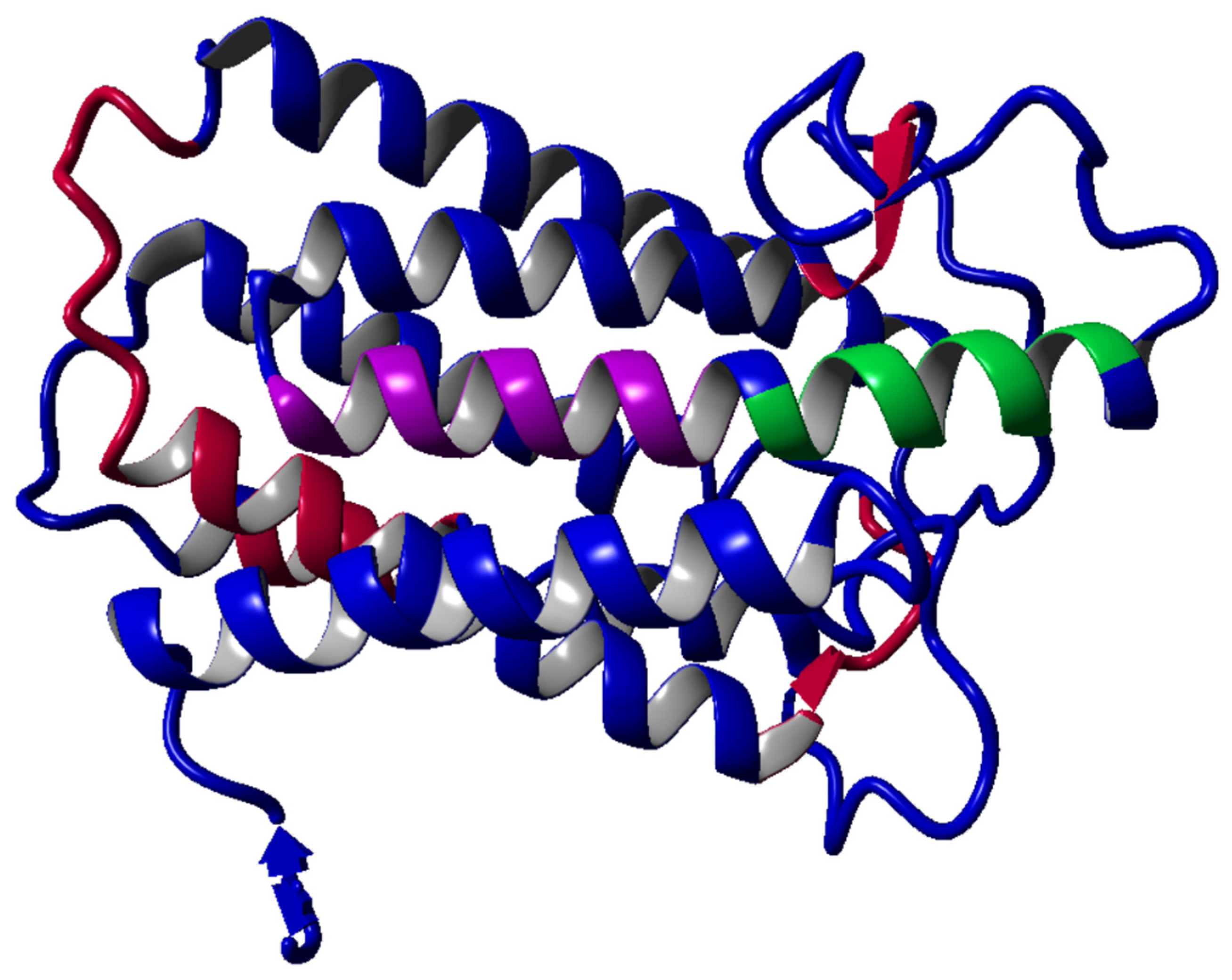
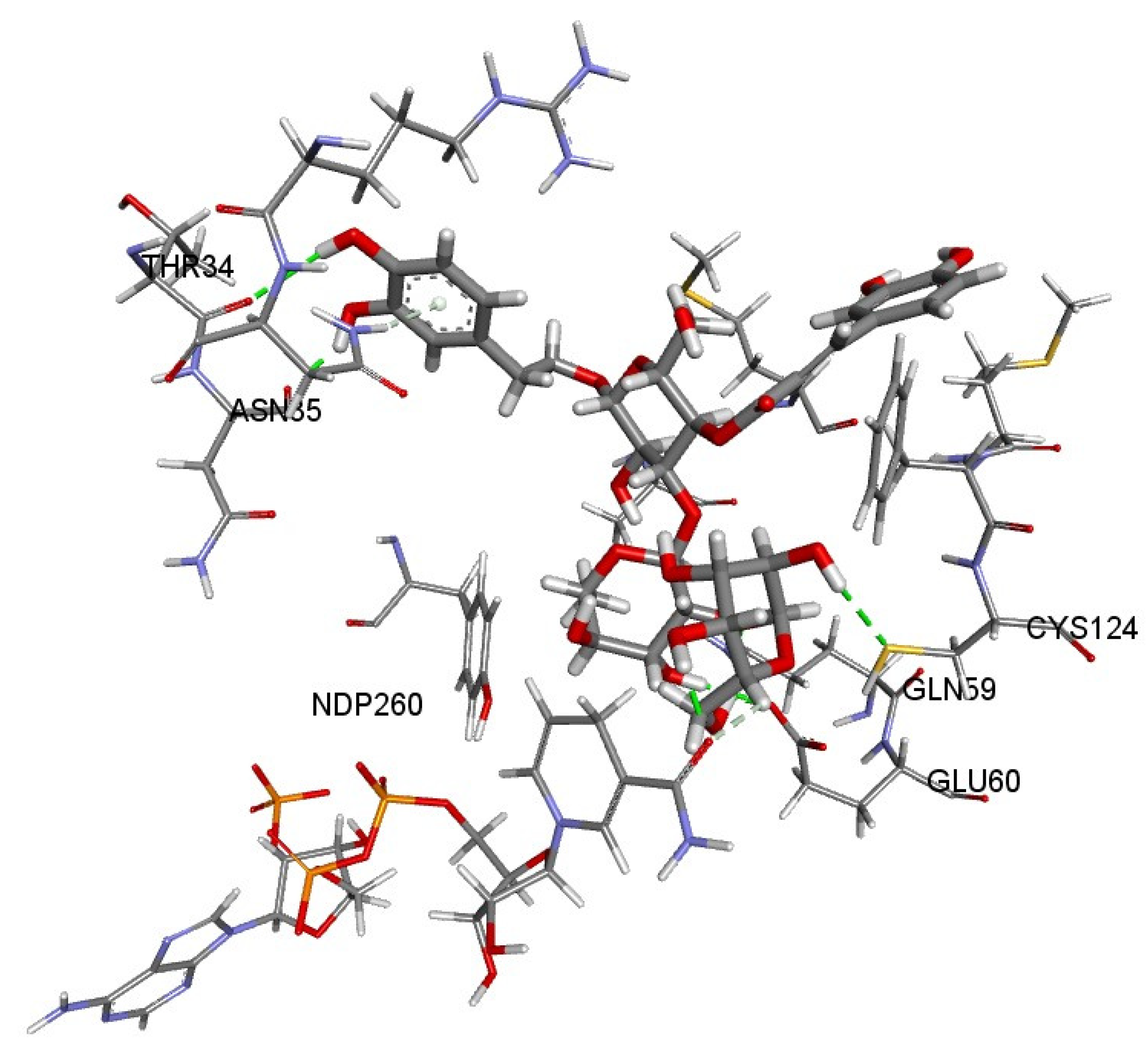

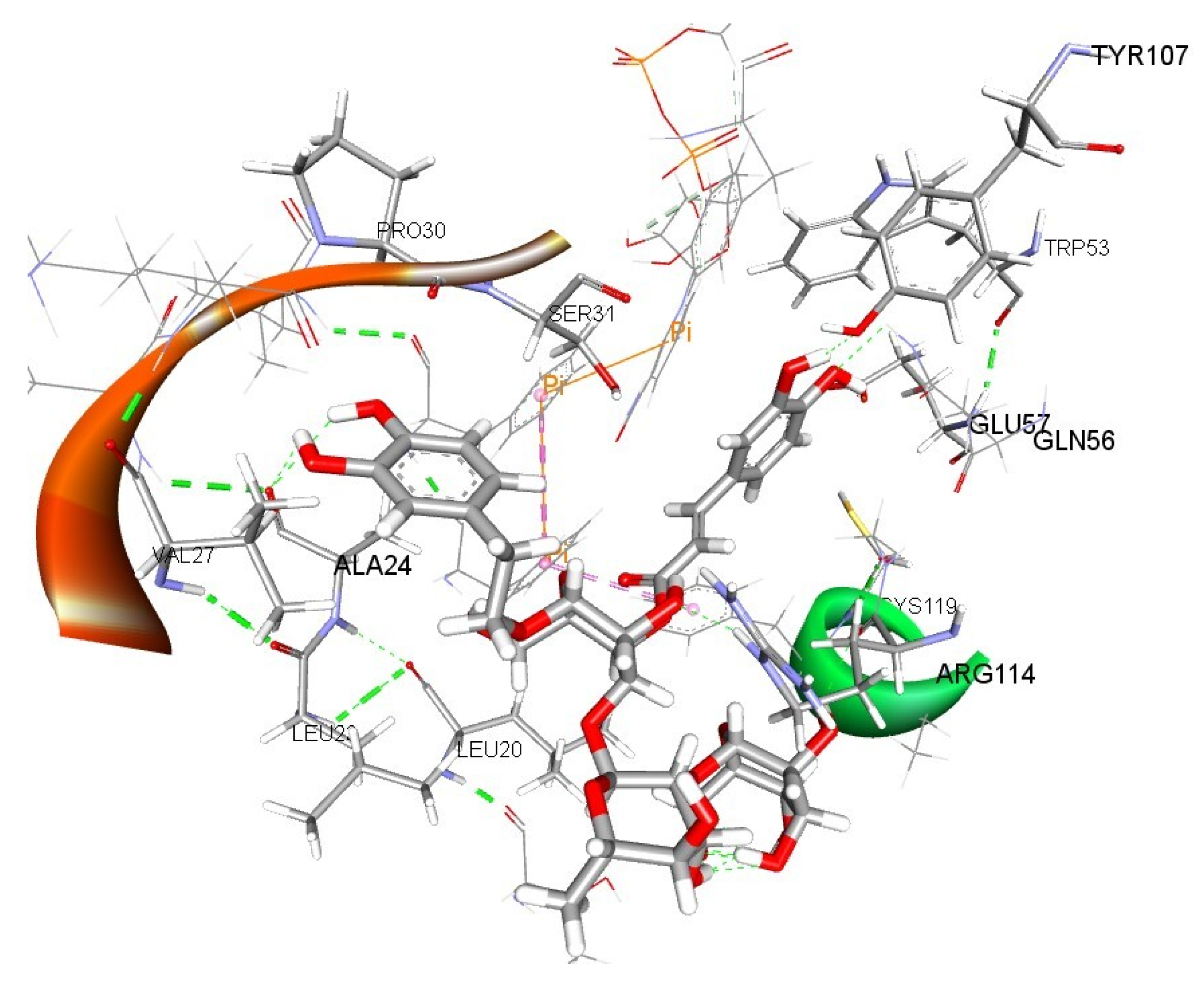
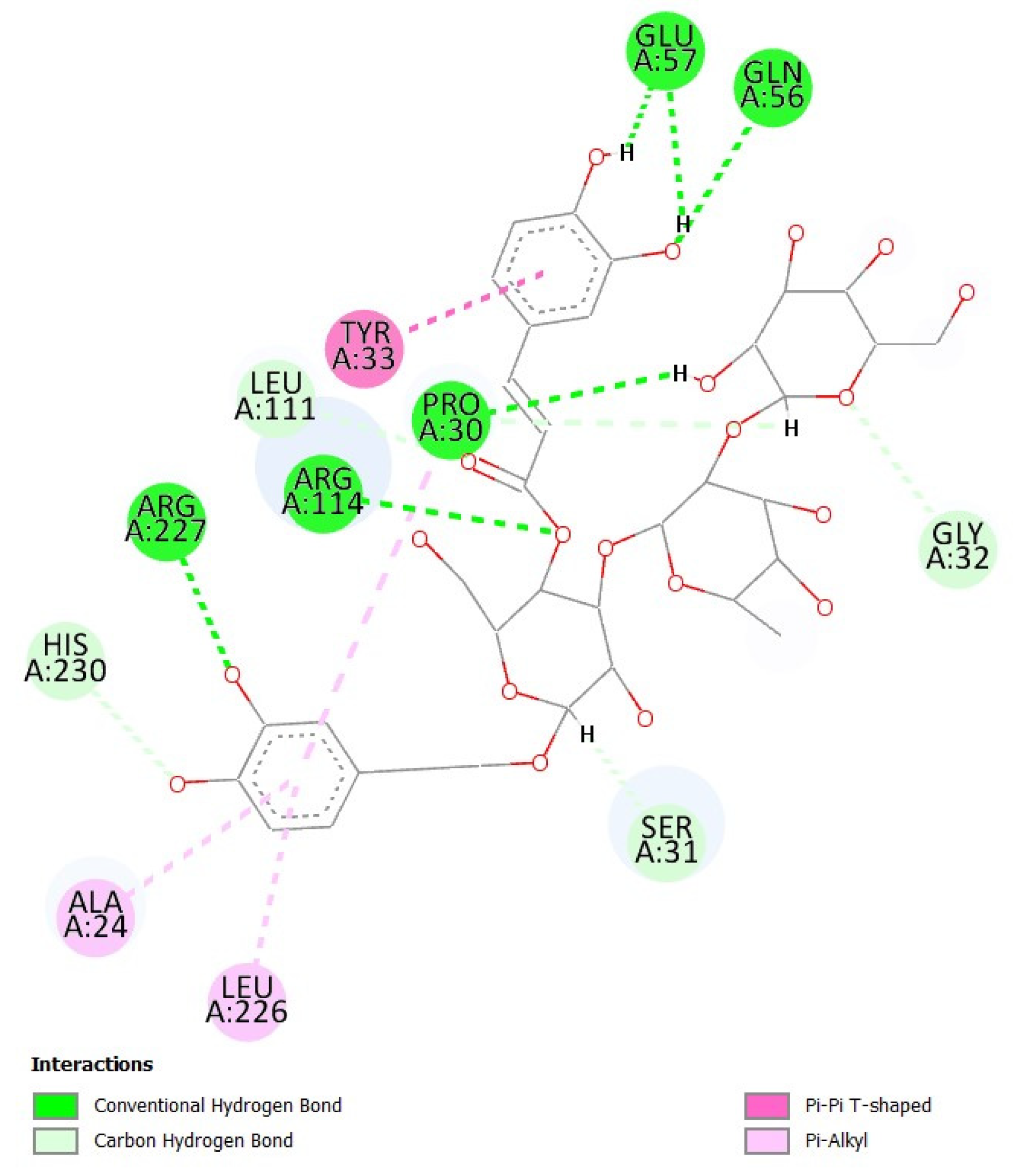
| Template | Total Score | BLAST E-Value | Align Score | Cover | ID | Resolution | Header |
|---|---|---|---|---|---|---|---|
| 1 | 450.33 | 3 × 10−55 | 608.0 | 95% | 7BW1 (chain-A) | 2.80 Å | 3-oxo-5-alpha-steroid 4-dehydrogenase 2 [Homo sapiens] (245 residues with quality score 0.783) |
| 2 | 401.82 | 4 × 10−58 | 498.0 | 94% | 7C83 (chain-A) | 2.00 Å | 3-oxo-5-alpha-steroid 4-dehydrogenase [Proteobacteria bacterium] (251 residues with quality score 0.860) |
| Check Type | Quality Z-Score | Comment |
|---|---|---|
| Dihedrals | 0.515 | Optimal |
| Packing 1D | 0.674 | Optimal |
| Packing 3D | −1.304 | Satisfactory |
| Overall | −0.269 | Good |
| Ligand | Binding Energy [kcal/mol] | Dissociation Constant [μM] |
|---|---|---|
| Teupolioside | −8.18 | 1.01 |
| Testosterone | −8.17 | 1.03 |
| Finasteride | −7.66 | 2.43 |
| Ligand | Binding Energy [kcal/mol] | Dissociation Constant [μM] |
|---|---|---|
| Teupolioside | −8.66 | 0.45 |
| Testosterone | −8.64 | 0.46 |
| Finasteride | −8.71 | 0.41 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mencarelli, N.; Consoli, V.; Gallorini, M.; Di Fazio, G.; Cataldi, A.; Gulisano, M.; Vanella, L.; Osmanović, A.; Carradori, S. The Nrf2-Related Pathways and the Antiandrogenic Effects Are Enhanced In Vitro and In Silico by the Combination of Graminex®G96® Pollen and Teupol 25P in Cell Models of Benign Prostate Hyperplasia. Nutraceuticals 2025, 5, 17. https://doi.org/10.3390/nutraceuticals5030017
Mencarelli N, Consoli V, Gallorini M, Di Fazio G, Cataldi A, Gulisano M, Vanella L, Osmanović A, Carradori S. The Nrf2-Related Pathways and the Antiandrogenic Effects Are Enhanced In Vitro and In Silico by the Combination of Graminex®G96® Pollen and Teupol 25P in Cell Models of Benign Prostate Hyperplasia. Nutraceuticals. 2025; 5(3):17. https://doi.org/10.3390/nutraceuticals5030017
Chicago/Turabian StyleMencarelli, Noemi, Valeria Consoli, Marialucia Gallorini, Gaetano Di Fazio, Amelia Cataldi, Maria Gulisano, Luca Vanella, Amar Osmanović, and Simone Carradori. 2025. "The Nrf2-Related Pathways and the Antiandrogenic Effects Are Enhanced In Vitro and In Silico by the Combination of Graminex®G96® Pollen and Teupol 25P in Cell Models of Benign Prostate Hyperplasia" Nutraceuticals 5, no. 3: 17. https://doi.org/10.3390/nutraceuticals5030017
APA StyleMencarelli, N., Consoli, V., Gallorini, M., Di Fazio, G., Cataldi, A., Gulisano, M., Vanella, L., Osmanović, A., & Carradori, S. (2025). The Nrf2-Related Pathways and the Antiandrogenic Effects Are Enhanced In Vitro and In Silico by the Combination of Graminex®G96® Pollen and Teupol 25P in Cell Models of Benign Prostate Hyperplasia. Nutraceuticals, 5(3), 17. https://doi.org/10.3390/nutraceuticals5030017










