Adjunctive Therapies in Rheumatoid Arthritis
Abstract
1. Introduction
2. Current Treatment of Rheumatoid Arthritis
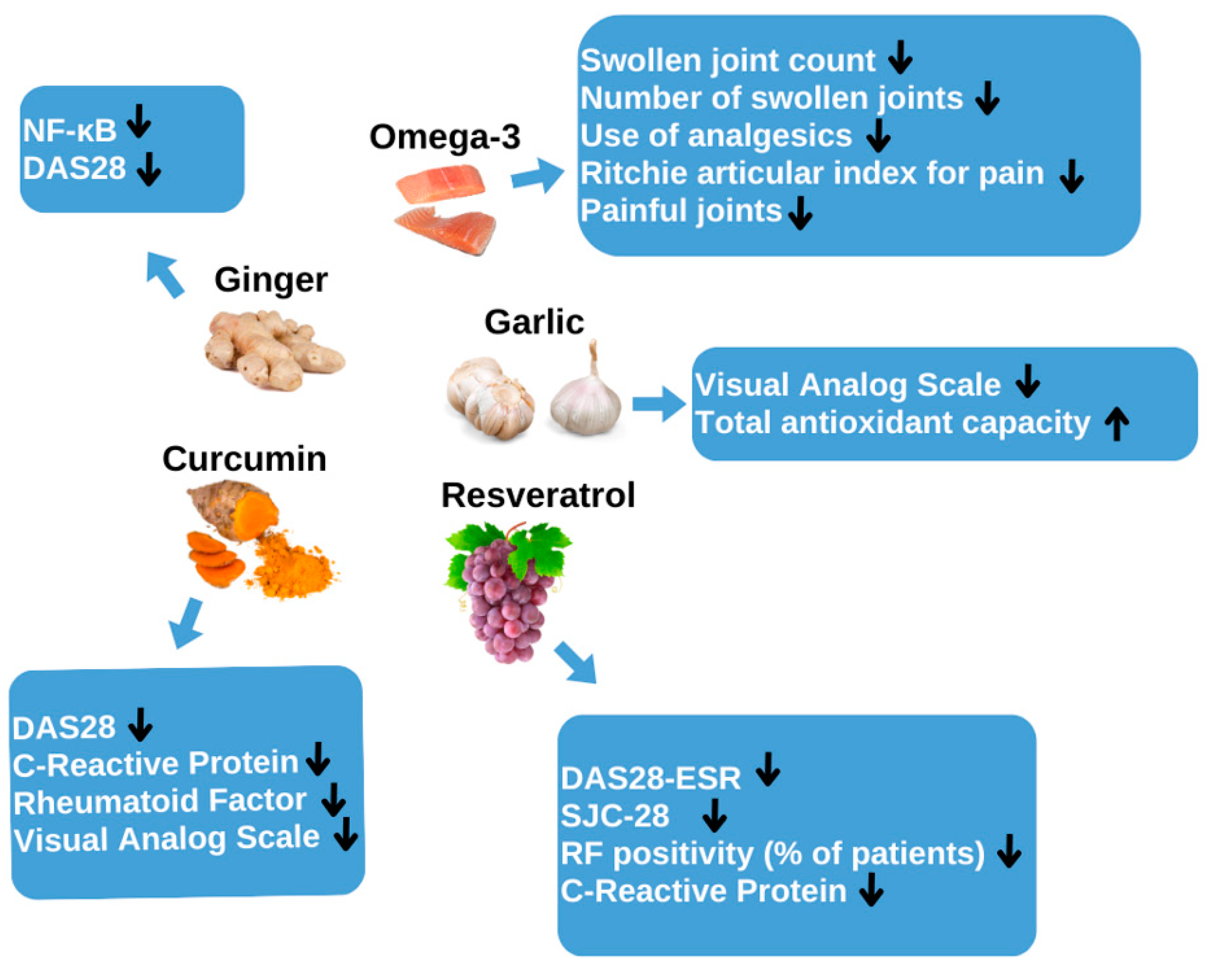
3. Garlic as an Adjuvant Treatment for Rheumatoid Arthritis
3.1. Mechanism of Action of Garlic
3.2. Preclinical Studies
3.3. Clinical Studies
| Author/Year | Study Population | Intervention | Base/Final | Changes in the Mean | References |
|---|---|---|---|---|---|
| Moosavian S, Paknahad Z, Habibagahi Z | 70 patients with active RA | Tablets containing 500 mg of dry garlic powder 8 weeks | TAC: (26.58 ± 77.30 nmol of Trolox equivalent/mL) | TAC ↑ | [8] |
| MDA: (−0.82 ± 1.99 nmol/mL vs. 0.36 ± 2.57 nmol/mL | MDA ↓ | ||||
| HAQ: −11.96 ± 13.43 mm frente a −0.06 ± 13.41 mm | HAQ ↓ | ||||
| Zare, 2019 | 42 peritoneal dialysis patients | 400 mg twice a day for 8 weeks | IL-6: 2.2 vs. 0.7 | IL-6 ↓ | [14] |
| CRP: 13 vs. 2 | CRP ↓ | ||||
| ESR: 45.3 vs. 35.4 | ESR ↓ |
4. Ginger as an Adjunctive Treatment for Rheumatoid Arthritis
- Apoptosis;
- Cell growth and DNA repair;
- Gene expression and regulation;
- Cell structure and movement;
- Immune function and inflammation;
- Nervous system function [15].
- Respiratory issues: asthma, cough;
- Digestive problems: diarrhea, loss of appetite;
- Heart conditions;
- Infections: fever;
- Metabolic disorders: diabetes;
- Nervous system disorders: difficulty urinating;
- Immune system disorders: rheumatoid arthritis, inflammation, rheumatism [16].
4.1. Mechanism of Action of Ginger
4.2. Preclinical Studies
4.3. Clinical Studies
5. Omega-3 as an Adjunctive Treatment for Rheumatoid Arthritis
5.1. Mechanism of Action of Omega-3
5.2. Preclinical Studies
5.3. Clinical Studies
6. Resveratrol as an Adjunctive Treatment for Rheumatoid Arthritis
6.1. Mechanism of Action of Resveratrol
- Adenosine monophosphate-activated protein kinase (AMPK);
- Cyclooxygenase type 2 (COX-2);
- Mitogen-activated protein kinase (MAPK);
- Nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB);
- Nuclear erythroid 2-related factor 2 (Nrf2);
- Phosphatidylinositol-3-kinase (PI3K)/protein kinase B (Akt)/mammalian target of rapamycin (mTOR) pathway;
- Silent information regulator 1 (SIRT1);
- Signal transducer and activator of transcription (STAT3);
- Aryl hydrocarbon receptor (AhR);
- The Wnt/β-catenin pathway;
- JNK inhibition mitigates bone erosion.
- p38 MAPK inhibition lessens synovial inflammation, cartilage erosion, bone damage, and angiogenesis, which is crucial for chronic synovial inflammation.
6.2. Preclinical Studies
6.3. Clinical Studies
7. Curcumin as an Adjunctive Treatment for Rheumatoid Arthritis
- Reduces inflammation;
- Lowers blood cholesterol and triglycerides;
- Improves blood sugar control.
7.1. Mechanism of Action of Curcumin
7.2. Preclinical Studies
8. Discussion
9. Conclusions
10. Future Directions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Pope, J.E. Management of Fatigue in Rheumatoid Arthritis. RMD Open 2020, 6, e001084. [Google Scholar] [CrossRef] [PubMed]
- Smolen, J.S.; Aletaha, D.; McInnes, I.B. Rheumatoid arthritis. Lancet 2016, 388, 2023–2038. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Yu, L.; Li, W.; Ge, G.; Ma, Y.; Xiao, L.; Qiao, Y.; Huang, W.; Huang, W.; Wei, M.; et al. Prevention and treatment of inflammatory arthritis with traditional Chinese medicine: Underlying mechanisms based on cell and molecular targets. Ageing Res. Rev. 2023, 89, 101981. [Google Scholar] [CrossRef]
- Itaya, T.; Torii, M.; Hashimoto, M.; Tanigawa, K.; Urai, Y.; Kinoshita, A.; Nin, K.; Jindai, K.; Watanabe, R.; Murata, K.; et al. Prevalence of anxiety and depression in patients with rheumatoid arthritis before and during the COVID-19 pandemic. Rheumatology 2021, 60, 2023–2024. [Google Scholar] [CrossRef]
- Miguel-Lavariega, D.; Elizararrás-Rivas, J.; Villarreal-Ríos, E.; Baltiérrez-Hoyos, R.; Velasco-Tobón, U.; Vargas-Daza, E.R.; Galicia-Rodríguez, L. Epidemiological profile of rheumatoid arthritis. Rev. Med. Inst. Mex. Seguro Soc. 2023, 61, 574–582. [Google Scholar] [CrossRef]
- Nygaard, G.; Firestein, G.S. Restoring synovial homeostasis in rheumatoid arthritis by targeting fibroblast-like synoviocytes. Nat. Rev. Rheumatol. 2020, 16, 316–333. [Google Scholar] [CrossRef] [PubMed]
- Shang, A.; Cao, S.-Y.; Xu, X.-Y.; Gan, R.-Y.; Tang, G.-Y.; Corke, H.; Mavumengwana, V.; Li, H.-B. Bioactive compounds and biological functions of garlic (Allium sativum L.). Foods 2019, 8, 246. [Google Scholar] [CrossRef]
- Moosavian, S.P.; Paknahad, Z.; Habibagahi, Z. A randomized, double-blind, placebo-controlled clinical trial, evaluating the garlic supplement effects on some serum biomarkers of oxidative stress, and quality of life in women with rheumatoid arthritis. Int. J. Clin. Pract. 2020, 74, e13498. [Google Scholar] [CrossRef]
- Enayatjazi, M.; Esfarjani, F.; Reisi, J.; Moshtaghian, S. Studying the effect of garlic consumption and endurance training on serum levels of some pro-and anti-inflammatory cytokines in female mice with breast cancer—A randomized trial. Int. J. Prev. Med. 2022, 13, 38. [Google Scholar] [CrossRef]
- Percival, S.S. Aged garlic extract modifies human immunity. J. Nutr. 2016, 146, 433S–436S. [Google Scholar] [CrossRef]
- Liang, J.J.; Li, H.R.; Chen, Y.; Zhang, C.; Chen, D.G.; Liang, Z.C.; Shi, Y.Q.; Zhang, L.L.; Xin, L.; Zhao, D.B. Diallyl Trisulfide can induce fibroblast-like synovial apoptosis and has a therapeutic effect on collagen-induced arthritis in mice via blocking NF-κB and Wnt pathways. Int. Immunopharmacol. 2019, 71, 132–138. [Google Scholar] [CrossRef] [PubMed]
- Letarouilly, J.-G.; Sanchez, P.; Nguyen, Y.; Sigaux, J.; Czernichow, S.; Flipo, R.-M.; Sellam, J.; Daïen, C. Efficacy of spice supplementation in rheumatoid arthritis: A systematic literature review. Nutrients 2020, 12, 3800. [Google Scholar] [CrossRef]
- Bayan, L.; Koulivand, P.H.; Gorji, A. Garlic: A Review of Potential Therapeutic Effects. Avicenna J. Phytomed. 2014, 4, 1. [Google Scholar]
- Zare, E.; Alirezaei, A.; Bakhtiyari, M.; Mansouri, A. Evaluating the effect of garlic extract on serum inflammatory markers of peritoneal dialysis patients: A randomized double-blind clinical trial study. BMC Nephrol. 2019, 20, 26. [Google Scholar] [CrossRef]
- Shaukat, M.N.; Nazir, A.; Fallico, B. Ginger Bioactives: A Comprehensive Review of Health Benefits and Potential Food Applications. Antioxidants 2023, 12, 2015. [Google Scholar] [CrossRef] [PubMed]
- Semwal, R.B.; Semwal, D.K.; Combrinck, S.; Viljoen, A.M. Gingerols and shogaols: Important nutraceutical principles from ginger. Phytochemistry 2015, 117, 554–568. [Google Scholar] [CrossRef] [PubMed]
- Ballester, P.; Cerdá, B.; Arcusa, R.; Marhuenda, J.; Yamedjeu, K.; Zafrilla, P. Effect of Ginger on Inflammatory Diseases. Molecules 2022, 27, 7223. [Google Scholar] [CrossRef]
- Long, Z.; Xiang, W.; He, Q.; Xiao, W.; Wei, H.; Li, H.; Guo, H.; Chen, Y.; Yuan, M.; Yuan, X.; et al. Efficacy and safety of dietary polyphenols in rheumatoid arthritis: A systematic review and meta-analysis of 47 randomized controlled trials. Front. Immunol. 2023, 14, 1024120. [Google Scholar] [CrossRef]
- Zhang, Y.-M.; Shen, J.; Zhao, J.-M.; Guan, J.; Wei, X.-R.; Miao, D.-Y.; Li, W.; Xie, Y.-C.; Zhao, Y.-Q. Cedrol from Ginger Ameliorates Rheumatoid Arthritis via Reducing Inflammation and Selectively Inhibiting JAK3 Phosphorylation. J. Agric. Food Chem. 2021, 69, 5332–5343. [Google Scholar] [CrossRef]
- Aryaeian, N.; Shahram, F.; Mahmoudi, M.; Tavakoli, H.; Yousefi, B.; Arablou, T. The effect of ginger supplementation on some immunity and inflammation intermediate genes expression in patients with active Rheumatoid Arthritis. Gene 2019, 698, 179–185. [Google Scholar] [CrossRef]
- Ansari, U.A.M.A.; Uddin, Q.; Husain, N.; Ahmad, T.; Fatima, S.H.; Minhajuddin, A. Evaluation of the efficacy and safety of a herbal formulation for rheumatoid arthritis—A non-inferiority randomized controlled trial. J. Ethnopharmacol. 2024, 325, 117833. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Guo, M.; Hu, Y.; Liu, T.; Yan, J.; Luo, Y.; Yun, M.; Yang, M.; Zhang, J.; Guo, L. Effect of Sanhuangwuji powder, anti-rheumatic drugs, and ginger-partitioned acupoint stimulation on the treatment of rheumatoid arthritis with peptic ulcer: A randomized controlled study. J. Tradit. Chin. Med. 2015, 35, 273–280. [Google Scholar] [PubMed]
- Kim, E.Y.; Moudgil, K.D. Regulation of autoimmune inflammation by pro-inflammatory cytokines. Immunol. Lett. 2008, 120, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Sweeney, S.E.; Firestein, G.S. Rheumatoid arthritis: Regulation of synovial inflammation. Int. J. Biochem. Cell Biol. 2004, 36, 372–378. [Google Scholar] [CrossRef]
- Burmester, G.R.; Pope, J.E. Novel treatment strategies in rheumatoid arthritis. Lancet 2017, 389, 2338–2348. [Google Scholar] [CrossRef]
- van Delft, M.A.M.; Huizinga, T.W.J. An overview of autoantibodies in rheumatoid arthritis. J. Autoimmun. 2020, 110, 102392. [Google Scholar] [CrossRef]
- Cush, J.J. Rheumatoid Arthritis: Early Diagnosis and Treatment. Med. Clin. N. Am. 2021, 105, 355–365. [Google Scholar] [CrossRef]
- Croia, C.; Bursi, R.; Sutera, D.; Petrelli, F.; Alunno, A.; Puxeddu, I. One year in review 2019: Pathogenesis of rheumatoid arthritis. Clin. Exp. Rheumatol. 2019, 37, 347–357. [Google Scholar]
- Ferreira, I.; Falcato, F.; Bandarra, N.; Rauter, A.P. Resolvins, Protectins, and Maresins: DHA-Derived Specialized Pro-Resolving Mediators, Biosynthetic Pathways, Synthetic Approaches, and Their Role in Inflammation. Molecules 2022, 27, 1677. [Google Scholar] [CrossRef]
- Serhan, C.N. Pro-resolving lipid mediators are leads for resolution physiology. Nature 2014, 510, 92–101. [Google Scholar] [CrossRef]
- Su, Y.; Han, Y.; Choi, H.S.; Lee, G.-Y.; Cho, H.W.; Choi, H.; Choi, J.H.; Jang, Y.-S.; Seo, J.-W. Lipid mediators obtained from docosahexaenoic acid by soybean lipoxygenase attenuate RANKL-induced osteoclast differentiation and rheumatoid arthritis. Biomed. Pharmacother. 2024, 171, 116153. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; Lim, K.; Kim, K.H.; Kim, J.H.; Choi, J.S.; Shim, S.C. N-3 polyunsaturated fatty acids restore Th17 and Treg balance in collagen antibody-induced arthritis. PLoS ONE 2018, 13, e0194331. [Google Scholar] [CrossRef]
- Nikiphorou, E.; Philippou, E. Nutrition and its role in prevention and management of rheumatoid arthritis. Autoimmun. Rev. 2023, 22, 103333. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Fu, X.; Chen, X.; Li, Z.; Huang, Y.; Liang, C. Promising Therapeutic Targets for Treatment of Rheumatoid Arthritis. Front. Immunol. 2021, 12, 686155. [Google Scholar] [CrossRef]
- Conforti, A.; Di Cola, I.; Pavlych, V.; Ruscitti, P.; Berardicurti, O.; Ursini, F.; Giacomelli, R.; Cipriani, P. Beyond the joints, the extra-articular manifestations in rheumatoid arthritis. Autoimmun. Rev. 2021, 20, 102735. [Google Scholar] [CrossRef]
- Laev, S.S.; Salakhutdinov, N.F. Anti-arthritic agents: Progress and potential. Bioorg. Med. Chem. 2015, 23, 3059–3080. [Google Scholar] [CrossRef]
- Smolen, J.S.; Aletaha, D. Rheumatoid arthritis therapy reappraisal: Strategies, opportunities, and challenges. Nat. Rev. Rheumatol. 2015, 11, 276–289. [Google Scholar] [CrossRef] [PubMed]
- Bahadori, B.; Uitz, E.; Thonhofer, R.; Trummer, M.; Pestemer-Lach, I.; McCarty, M.; Krejs, G.J. ω-3 fatty acids infusions as adjuvant therapy in rheumatoid arthritis. J. Parenter. Enter. Nutr. 2010, 34, 151–155. [Google Scholar] [CrossRef]
- Rajaei, E.; Mowla, K.; Ghorbani, A.; Bahadoram, S.; Bahadoram, M.; Dargahi-Malamir, M. The Effect of Omega-3 Fatty Acids in Patients with Active Rheumatoid Arthritis Receiving DMARDs Therapy: Double-Blind Randomized Controlled Trial. Glob. J. Health Sci. 2015, 8, 18–25. [Google Scholar] [CrossRef]
- Geusens, P.; Wouters, C.; Nijs, J.; Jiang, Y.; Dequeker, J. Long-Term Effect of OMEGA-3 Fatty Acid Supplementation in Active Rheumatoid Arthritis. Arthritis Rheum. 1994, 37, 824–829. [Google Scholar] [CrossRef]
- Moudgil, K.D.; Venkatesha, S.H. The Anti-Inflammatory and Immunomodulatory Activities of Natural Products to Control Autoimmune Inflammation. Int. J. Mol. Sci. 2023, 24, 95. [Google Scholar] [CrossRef] [PubMed]
- Gal, R.; Deres, L.; Toth, K.; Halmosi, R.; Habon, T. The effect of resveratrol on the cardiovascular system from molecular mechanisms to clinical results. Int. J. Mol. Sci. 2021, 22, 10152. [Google Scholar] [CrossRef] [PubMed]
- Rahman, H.; Akter, R.; Bhattacharya, T.; Abdel-Daim, M.M.; Alkahtani, S.; Arafah, M.W.; Al-Johani, N.S.; Alhoshani, N.M.; Alkeraishan, N.; Alhenaky, A.; et al. Resveratrol and Neuroprotection: Impact and Its Therapeutic Potential in Alzheimer’s Disease. Front. Pharmacol. 2020, 11, 619024. [Google Scholar] [CrossRef] [PubMed]
- Akiyoshi, T.; Uchiyama, M.; Inada, R.; Imaoka, A.; Ohtani, H. Analysis of Inhibition Kinetics of Three Beverage Ingredients Dihydroxybergamottin and Resveratrol, on CYP2C9 Activity. Drug Metab. Pharmacokinet. 2022, 42, 100429. [Google Scholar] [CrossRef] [PubMed]
- Poles, J.; Karhu, E.; McGill, M.; McDaniel, H.R.; Lewis, J.E. The effects of twenty-four nutrients and phytonutrients on immune system function and inflammation: A narrative review. J. Clin. Transl. Res. 2021, 7, 333. [Google Scholar] [CrossRef]
- Delmas, D.; Limagne, E.; Ghiringhelli, F.; Aires, V. Immune Th17 lymphocytes play a critical role in the multiple beneficial properties of resveratrol. Food Chem. Toxicol. 2020, 137, 111091. [Google Scholar] [CrossRef]
- Meng, T.; Xiao, D.; Muhammed, A.; Deng, J.; Chen, L.; He, J. Anti-Inflammatory Action and Mechanisms of Resveratrol. Molecules 2021, 26, 229. [Google Scholar] [CrossRef]
- Zhang, L.-X.; Li, C.-X.; Kakar, M.U.; Khan, M.S.; Wu, P.-F.; Amir, R.M.; Dai, D.-F.; Naveed, M.; Li, Q.-Y.; Saeed, M.; et al. Resveratrol (RV): A pharmacological review and call for further research. Biomed. Pharmacother. 2021, 143, 112164. [Google Scholar] [CrossRef]
- Gambini, J.; Inglés, M.; Olaso, G.; Lopez-Grueso, R.; Bonet-Costa, V.; Gimeno-Mallench, L.; Mas-Bargues, C.; Abdelaziz, K.M.; Gomez-Cabrera, M.C.; Vina, J.; et al. Properties of Resveratrol: In Vitro and In Vivo Studies about Metabolism, Bioavailability, and Biological Effects in Animal Models and Humans. Oxid. Med. Cell Longev. 2015, 2015, 837042. [Google Scholar] [CrossRef]
- de los Reyes Corrales, T.; Losada-Pérez, M.; Casas-Tintó, S. Jnk pathway in CNS pathologies. Int. J. Mol. Sci. 2021, 22, 3883. [Google Scholar] [CrossRef]
- Lee, S.; Rauch, J.; Kolch, W. Targeting MAPK signaling in cancer: Mechanisms of drug resistance and sensitivity. Int. J. Mol. Sci. 2020, 21, 1102. [Google Scholar] [CrossRef] [PubMed]
- Trapp, V.; Parmakhtiar, B.; Papazian, V.; Willmott, L.; Fruehauf, J.P. Anti-angiogenic effects of resveratrol mediated by decreased VEGF and increased TSP1 expression in melanoma-endothelial cell co-culture. Angiogenesis 2010, 13, 305–315. [Google Scholar] [CrossRef] [PubMed]
- Jang, J.Y.; Im, E.; Kim, N.D. Mechanism of Resveratrol-Induced Programmed Cell Death and New Drug Discovery against Cancer: A Review. Int. J. Mol. Sci. 2022, 23, 13689. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Chang, C.-C.; Yang, Y.; Yuan, L.; Xu, L.; Ho, C.-T.; Li, S. Resveratrol Alleviates Rheumatoid Arthritis via Reducing ROS and Inflammation, Inhibiting MAPK Signaling Pathways, and Suppressing Angiogenesis. J. Agric. Food Chem. 2018, 66, 12953–12960. [Google Scholar] [CrossRef] [PubMed]
- Mittal, M.; Mehta, P.; Rajput, S.; Rajender, S.; Chattopadhyay, N. The pharmacological assessment of resveratrol on preclinical models of rheumatoid arthritis through a systematic review and meta-analysis. Eur. J. Pharmacol. 2021, 910, 174504. [Google Scholar] [CrossRef]
- Fernández-Rodríguez, J.A.; Almonte-Becerril, M.; Ramil-Gómez, O.; Hermida-Carballo, L.; Viñas-Diz, S.; Vela-Anero, Á.; Concha, Á.; Camacho-Encina, M.; Blanco, F.J.; López-Armada, M.J. Autophagy Activation by Resveratrol Reduces Severity of Experimental Rheumatoid Arthritis. Mol. Nutr. Food Res. 2021, 65, e2000377. [Google Scholar] [CrossRef]
- Khojah, H.M.; Ahmed, S.; Abdel-Rahman, M.S.; Elhakeim, E.H. Resveratrol as an effective adjuvant therapy in the management of rheumatoid arthritis: A clinical study. Clin. Rheumatol. 2018, 37, 2035–2042. [Google Scholar] [CrossRef]
- Kotha, R.R.; Luthria, D.L. Curcumin: Biological, pharmaceutical, nutraceutical, and analytical aspects. Molecules 2019, 24, 2930. [Google Scholar] [CrossRef]
- Lestari, M.L.A.D.; Indrayanto, G. Curcumin. In Profiles of Drug Substances, Excipients and Related Methodology; Academic Press: Cambridge, MA, USA, 2014; Volume 39, pp. 113–204. [Google Scholar] [CrossRef]
- Hewlings, S.J.; Kalman, D.S. Curcumin: A review of its effects on human health. Foods 2017, 6, 92. [Google Scholar] [CrossRef]
- Heidari-Beni, M.; Moravejolahkami, A.R.; Gorgian, P.; Askari, G.; Tarrahi, M.J.; Bahreini-Esfahani, N. Herbal formulation “turmeric extract, black pepper, and ginger” versus Naproxen for chronic knee osteoarthritis: A randomized, double-blind, controlled clinical trial. Phytother. Res. 2020, 34, 2067–2073. [Google Scholar] [CrossRef]
- Anand, P.; Kunnumakkara, A.B.; Newman, R.A.; Aggarwal, B.B. Bioavailability of curcumin: Problems and promises. Mol. Pharm. 2007, 4, 807–818. [Google Scholar] [CrossRef] [PubMed]
- Zeng, L.; Yang, T.; Yang, K.; Yu, G.; Li, J.; Xiang, W.; Chen, H. Efficacy and Safety of Curcumin and Curcuma longa Extract in the Treatment of Arthritis: A Systematic Review and Meta-Analysis of Randomized Controlled Trial. Front. Immunol. 2022, 13, 891822. [Google Scholar] [CrossRef]
- Zeng, L.; Yang, T.; Yang, K.; Yu, G.; Li, J.; Xiang, W.; Chen, H. Curcumin and Curcuma longa Extract in the Treatment of 10 Types of Autoimmune Diseases: A Systematic Review and Meta-Analysis of 31 Randomized Controlled Trials. Front. Immunol. 2022, 13, 896476. [Google Scholar] [CrossRef]
- Pourhabibi-Zarandi, F.; Shojaei-Zarghani, S.; Rafraf, M. Curcumin and rheumatoid arthritis: A systematic review of literature. Int. J. Clin. Pract. 2021, 75, e14280. [Google Scholar] [CrossRef]
- Soetikno, V.; Sari, F.R.; Veeraveedu, P.T.; Thandavarayan, R.A.; Harima, M.; Sukumaran, V.; Lakshmanan, A.P.; Suzuki, K.; Kawachi, H.; Watanabe, K. Curcumin ameliorates macrophage infiltration by inhibiting NF-B activation and proinflammatory cytokines in streptozotocin induced-diabetic nephropathy. Nutr. Metab. 2011, 8, 35. [Google Scholar] [CrossRef] [PubMed]
- Nelson, K.M.; Dahlin, J.L.; Bisson, J.; Graham, J.; Pauli, G.F.; Walters, M.A. The Essential Medicinal Chemistry of Curcumin. J. Med. Chem. 2017, 60, 1620–1637. [Google Scholar] [CrossRef]
- Amalraj, A.; Varma, K.; Jacob, J.; Divya, C.; Kunnumakkara, A.B.; Stohs, S.J.; Gopi, S. A Novel Highly Bioavailable Curcumin Formulation Improves Symptoms and Diagnostic Indicators in Rheumatoid Arthritis Patients: A Randomized, Double-Blind, Placebo-Controlled, Two-Dose, Three-Arm, and Parallel-Group Study. J. Med. Food 2017, 20, 1022–1030. [Google Scholar] [CrossRef] [PubMed]
- Chandran, B.; Goel, A. A randomized pilot study to assess the efficacy and safety of curcumin in patients with active rheumatoid arthritis. Phytother. Res. 2012, 26, 1719–1725. [Google Scholar] [CrossRef]
- Jakobsson, P.; Robertson, L.; Welzel, J.; Zhang, M.; Zhihua, Y.; Kaixin, G.; Runyue, H.; Zehuai, W.; Korotkova, M.; Göransson, U. Where traditional Chinese medicine meets Western medicine in the prevention of rheumatoid arthritis. J. Intern. Med. 2022, 292, 745–763. [Google Scholar] [CrossRef]
- Chatterjee, A.; Jayaprakasan, M.; Chakrabarty, A.K.; Lakkaniga, N.R.; Bhatt, B.N.; Banerjee, D.; Narwaria, A.; Katiyar, C.K.; Dubey, S.K. Comprehensive insights into rheumatoid arthritis: Pathophysiology, current therapies and herbal alternatives for effective disease management. Phytother. Res. 2024, 38, 2764–2799. [Google Scholar] [CrossRef]
- Turk, M.A.; Liu, Y.; Pope, J.E. Non-pharmacological interventions in the treatment of rheumatoid arthritis: A systematic review and meta-analysis. Autoimmun. Rev. 2023, 22, 103323. [Google Scholar] [CrossRef] [PubMed]
| Author/Year | Study Population | Intervention | Base/Final | Changes in the Mean | Reference |
|---|---|---|---|---|---|
| Aryaeian N, Shahram F, Mahmoudi M | 70 patients with active RA. | 1500 mg daily as 2 capsules of ginger powder for 12 weeks | NF-κB: 46 vs. 40 | NF-κB ↓ | [20] |
| DAS28: 4.73 vs. 3.44 | DAS28 ↓ |
| Author/Year | Study Population | Intervention | Base/Final | Changes in the Mean | References |
|---|---|---|---|---|---|
| Bahadori B, Uitz E, Thonhofer R et al. (2010) | 23 patients with active RA | 2 weeks of treatment: 0.20 g of fish oil emulsion per kg of body weight 100 mL of this preparation contained 6.5 g of ω-3 PUFA | Swollen joint count: 10 vs. 1 | Swollen joint count ↓ | [38] |
| Rajaei E, Mowla K, Ghorbani A, et al. | 60 patients with active RA | 12 weeks Groups: omega-3, 2 omega 3 capsules per day containing 1.8 and 2.1 g of EPA and DHA | Number of swollen joints: 10 vs. 3 | Number of swollen joints ↓ | [39] |
| Use of analgesics: 25 vs. 7 | Use of analgesics ↓ | ||||
| Geusens P, Wouters C, Nijs J, et al. | 69 patients with active RA | 6 capsules with 1 g of fish oil (2.6 mg of ω-3) 12 months | Ritchie articular index for pain: 27.8 ± 5.1 vs. 13.8 ± 4 | Ritchie articular index for pain ↓ | [40] |
| Painful joints: 20 ± 4 vs. 11 ± 2 | Painful joints ↓ |
| Author/Year | Study Population | Intervention | Base/Final | Changes in the Mean | Reference |
|---|---|---|---|---|---|
| Khojah H, Ahmed S, Abdel-Rahman M, et al. | 100 Egyptian patients with RA | Test group: daily capsule with 1 g of resveratrol for 3 months. | DAS28: 4.98 ± 1.07 vs. 3.02 ± 0.77 | DAS28- ESR ↓ | [57] |
| SJC-28: 3.8 ± 1.0 vs. 1.5 ± 0.8 | SJC-28 ↓ | ||||
| RF positivity (% of patients): 92.7 vs. 85.4 | RF positivity (% of patients) ↓ | ||||
| PCR: 2.9 ± 0.9 vs. 1.8 ± 0.5 | PCR ↓ |
| Author/Year | Study Population | Intervention | Base/Final | Changes in the Mean | References |
|---|---|---|---|---|---|
| Amalraj A, Varma K, Jacob J, et al. | 36 patients with active RA | 250 mg of curcumin product as the low dose | Low-dose | Low-dose | [68] |
| DAS28: 4.51 ± 0.64 vs. 2.14 ± 0.16 | DAS28 ↓ | ||||
| PCR: 0.97 ± 0.15 vs. 0.68 ± 0.10 | PCR ↓ | ||||
| RF: 123.3 ± 36.9 vs. 24.4 ± 5.7 | RF ↓ | ||||
| VAS: 7.01 ± 0.86 vs. 2.63 ± 0.74 | VAS ↓ | ||||
| 500 mg of curcumin product as the high dose 3 months | High-dose | High-dose | |||
| DAS28: 5.29 ± 0.54 vs. 1.80 ± 0.36 | DAS28 ↓ | ||||
| PCR: 1.21 ± 0.18 vs. 0.59 ± 0.08 | PCR ↓ | ||||
| RF: 150.6 ± 41.7 vs. 23.8 ± 6.0 | RF ↓ | ||||
| VAS: 7.99 ± 0.71 vs. 2.21 ± 0.45 | VAS ↓ | ||||
| Chandran B, Goel A. | 45 patients in India diagnosed with active RA | 500 mg of curcumin 8 weeks | DAS28: 6.40 ± 0.73 vs. 3.55 ± 0.73 | DAS28 ↓ | [69] |
| VAS: 68.5 ± 17.14 vs. 27.5 ± 9.35 | VAS ↓ | ||||
| PCR: 5.34 ± 4.12 vs. 2.56 ± 1.8 | PCR ↓ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
González Heredia, T.; Hernández Corona, D.M.; Méndez del Villar, M.; Guzmán Ornelas, M.O.; Corona Meraz, F.I.; Chávez Tostado, M.; Diosdado Pardo, G.E.; Pérez Padilla, A.J.; Pérez Villalobos, F.B.; Montaño Vargas, P.Y.; et al. Adjunctive Therapies in Rheumatoid Arthritis. Nutraceuticals 2024, 4, 643-657. https://doi.org/10.3390/nutraceuticals4040035
González Heredia T, Hernández Corona DM, Méndez del Villar M, Guzmán Ornelas MO, Corona Meraz FI, Chávez Tostado M, Diosdado Pardo GE, Pérez Padilla AJ, Pérez Villalobos FB, Montaño Vargas PY, et al. Adjunctive Therapies in Rheumatoid Arthritis. Nutraceuticals. 2024; 4(4):643-657. https://doi.org/10.3390/nutraceuticals4040035
Chicago/Turabian StyleGonzález Heredia, Tonatiuh, Diana Mercedes Hernández Corona, Miriam Méndez del Villar, Milton Omar Guzmán Ornelas, Fernanda Isadora Corona Meraz, Mariana Chávez Tostado, Grecia Elizabeth Diosdado Pardo, Arely Jaqueline Pérez Padilla, Fátima Berenice Pérez Villalobos, Perla Yareli Montaño Vargas, and et al. 2024. "Adjunctive Therapies in Rheumatoid Arthritis" Nutraceuticals 4, no. 4: 643-657. https://doi.org/10.3390/nutraceuticals4040035
APA StyleGonzález Heredia, T., Hernández Corona, D. M., Méndez del Villar, M., Guzmán Ornelas, M. O., Corona Meraz, F. I., Chávez Tostado, M., Diosdado Pardo, G. E., Pérez Padilla, A. J., Pérez Villalobos, F. B., Montaño Vargas, P. Y., & Morales García, P. (2024). Adjunctive Therapies in Rheumatoid Arthritis. Nutraceuticals, 4(4), 643-657. https://doi.org/10.3390/nutraceuticals4040035









