Enhanced Bioavailability and Immune Benefits of Liposome-Encapsulated Vitamin C: A Combination of the Effects of Ascorbic Acid and Phospholipid Membranes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Consumable Test Products
2.3. Blood Draws
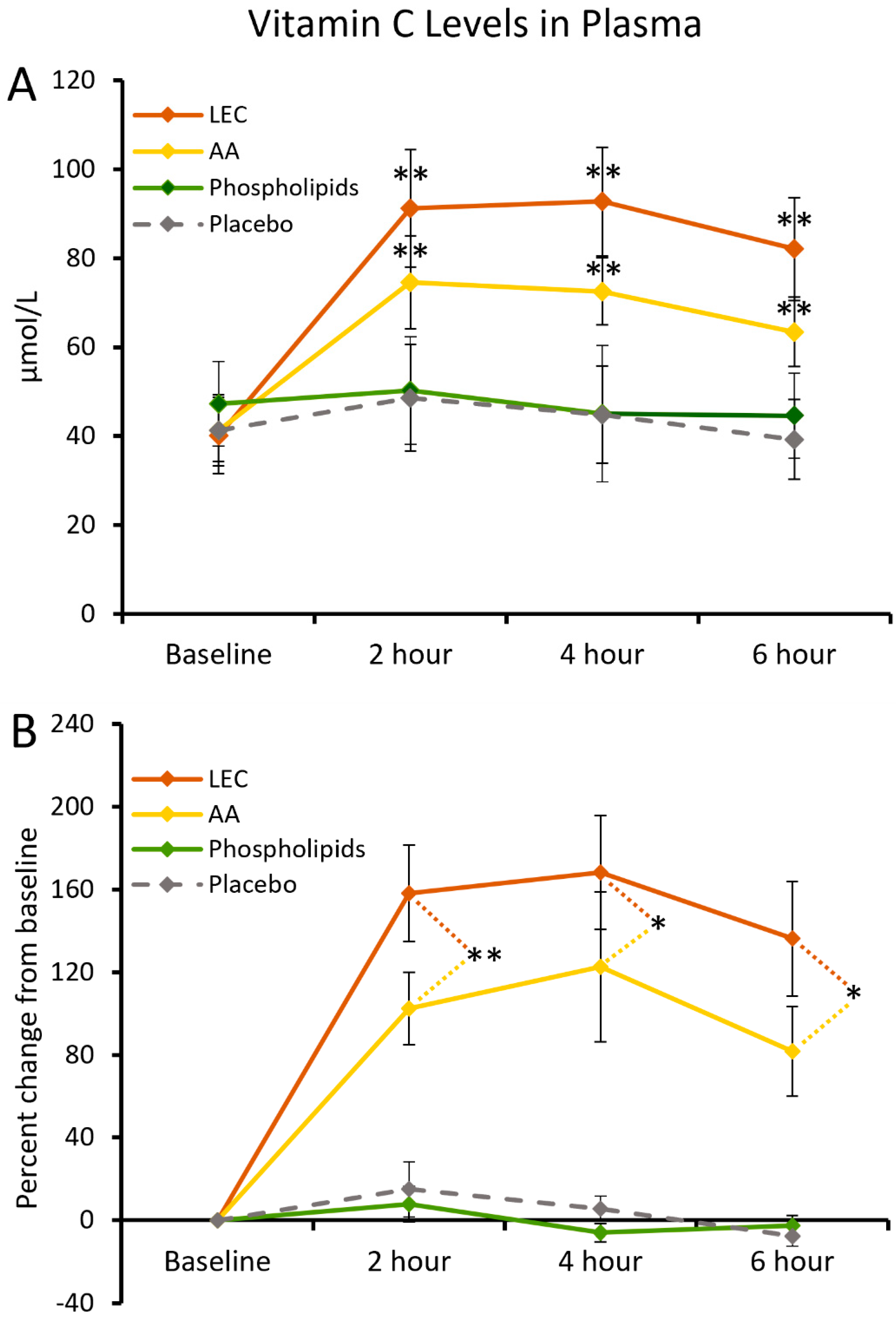
2.4. Vitamin C Levels in Plasma
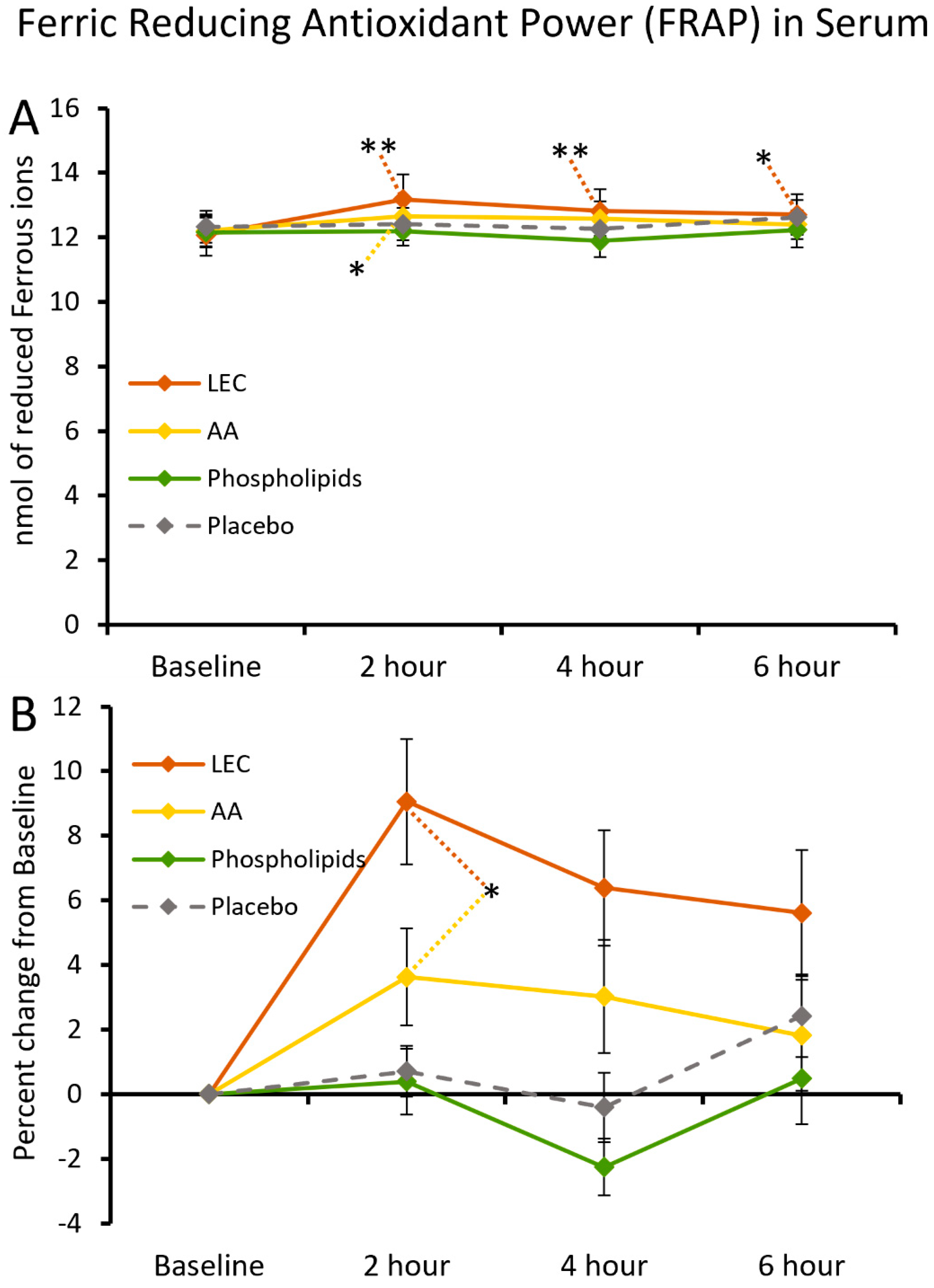
2.5. Ferric-Reducing Antioxidant Power of Serum
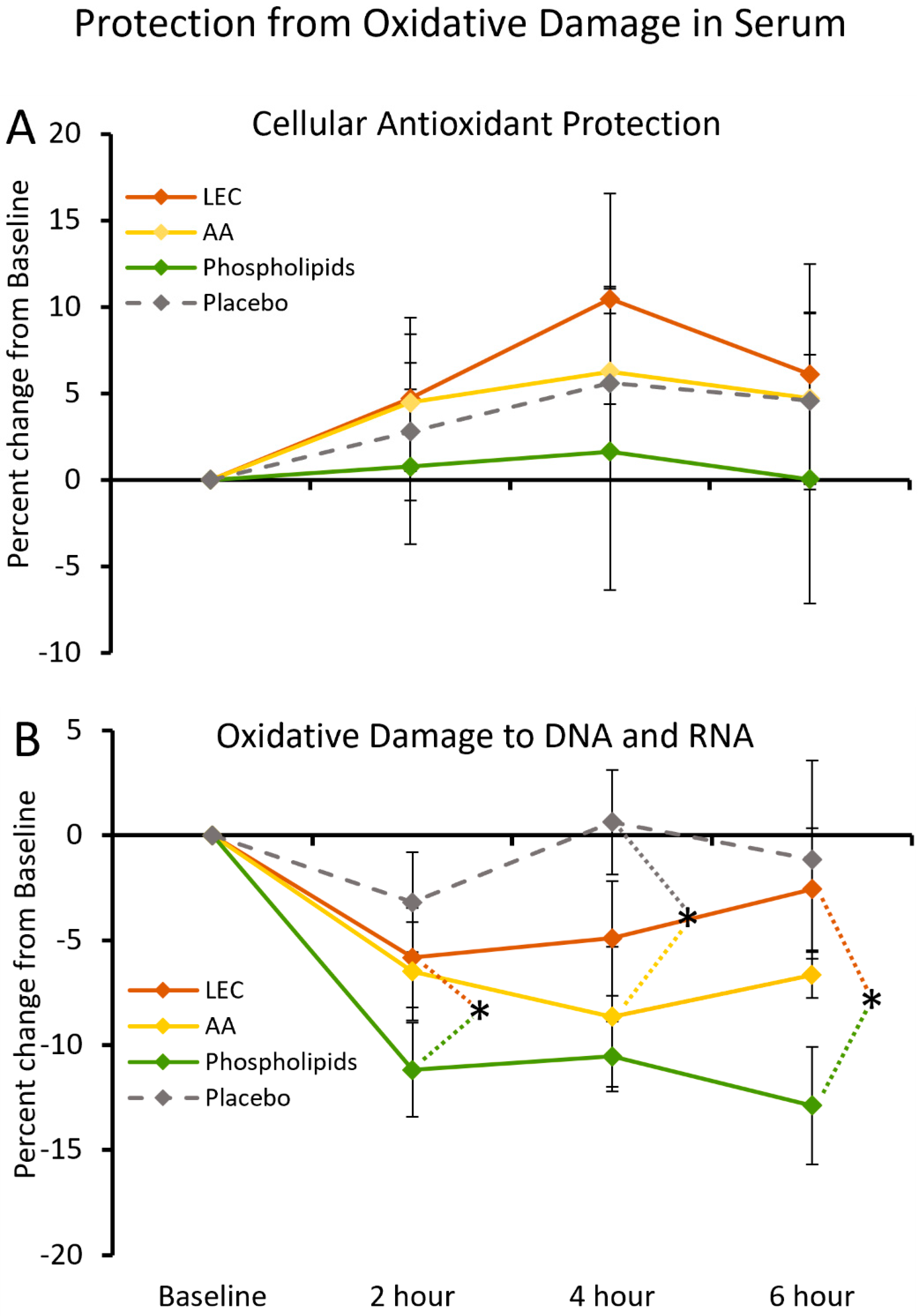
2.6. Cellular Antioxidant Protection by Serum
2.7. DNA and RNA Oxidation in Serum
2.8. Levels of Cytokines, Chemokines, and Growth Factors
2.9. Statistical Analysis
3. Results
3.1. Serum Vitamin C Levels
3.2. Ferric-Reducing Antioxidant Power of Serum
3.3. Antioxidant Protection of Cells and Nucleic Acids
3.4. Modulation of Cytokine Levels
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Drouin, G.; Godin, J.R.; Pagé, B. The genetics of vitamin C loss in vertebrates. Curr. Genom. 2011, 12, 371–378. [Google Scholar] [CrossRef] [PubMed]
- Njus, D.; Kelley, P.M.; Tu, Y.J.; Schlegel, H.B. Ascorbic acid: The chemistry underlying its antioxidant properties. Free Radic. Biol. Med. 2020, 159, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Mansfield, K.D.; Bertozzi, C.C.; Rudenko, V.; Chan, D.A.; Giaccia, A.J.; Simon, M.C. Multiple factors affecting cellular redox status and energy metabolism modulate hypoxia-inducible factor prolyl hydroxylase activity in vivo and in vitro. Mol. Cell. Biol. 2007, 27, 912–925. [Google Scholar] [CrossRef] [PubMed]
- Covarrubias-Pinto, A.; Acuña, A.I.; Beltrán, F.A.; Torres-Díaz, L.; Castro, M.A. Old Things New View: Ascorbic Acid Protects the Brain in Neurodegenerative Disorders. Int. J. Mol. Sci. 2015, 16, 28194–28217. [Google Scholar] [CrossRef]
- May, J.M. Vitamin C transport and its role in the central nervous system. In Water Soluble Vitamins; Subcellular Biochemistry; Springer: Dordrecht, The Netherlands, 2012; Volume 56, pp. 85–103. [Google Scholar]
- Zhang, X.Y.; Xu, Z.P.; Wang, W.; Cao, J.B.; Fu, Q.; Zhao, W.X.; Li, Y.; Huo, X.L.; Zhang, L.M.; Li, Y.F.; et al. Vitamin C alleviates LPS-induced cognitive impairment in mice by suppressing neuroinflammation and oxidative stress. Int. Immunopharmacol. 2018, 65, 438–447. [Google Scholar] [CrossRef]
- Lin, J.L.; Huang, Y.H.; Shen, Y.C.; Huang, H.C.; Liu, P.H. Ascorbic acid prevents blood-brain barrier disruption and sensory deficit caused by sustained compression of primary somatosensory cortex. J. Cereb. Blood Flow Metab. 2010, 30, 1121–1136. [Google Scholar] [CrossRef]
- Maekawa, T.; Uchida, T.; Nakata-Horiuchi, Y.; Kobayashi, H.; Kawauchi, S.; Kinoshita, M.; Saitoh, D.; Sato, S. Oral ascorbic acid 2-glucoside prevents coordination disorder induced via laser-induced shock waves in rat brain. PLoS ONE 2020, 15, e0230774. [Google Scholar] [CrossRef]
- Khalili, H.; Abdollahifard, S.; Niakan, A.; Aryaie, M. The effect of Vitamins C and E on clinical outcomes of patients with severe traumatic brain injury: A propensity score matching study. Surg. Neurol. Int. 2022, 13, 548. [Google Scholar] [CrossRef]
- Li, Y.R.; Zhu, H. Vitamin C for sepsis intervention: From redox biochemistry to clinical medicine. Mol. Cell. Biochem. 2021, 476, 4449–4460. [Google Scholar] [CrossRef]
- Traber, M.G.; Stevens, J.F. Vitamins C and E: Beneficial effects from a mechanistic perspective. Free Radic. Biol. Med. 2011, 51, 1000–1013. [Google Scholar] [CrossRef]
- Boo, Y.C. Ascorbic Acid (Vitamin C) as a Cosmeceutical to Increase Dermal Collagen for Skin Antiaging Purposes: Emerging Combination Therapies. Antioxidants 2022, 11, 1663. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Lan, J.; Liu, D.; Backman, L.J.; Zhang, W.; Zhou, Q.; Danielson, P. Ascorbic Acid Promotes the Stemness of Corneal Epithelial Stem/Progenitor Cells and Accelerates Epithelial Wound Healing in the Cornea. Stem Cells Transl. Med. 2017, 6, 1356–1365. [Google Scholar] [CrossRef] [PubMed]
- Cha, J.; Roomi, M.W.; Ivanov, V.; Kalinovsky, T.; Niedzwiecki, A.; Rath, M. Ascorbate supplementation inhibits growth and metastasis of B16FO melanoma and 4T1 breast cancer cells in vitamin C-deficient mice. Int. J. Oncol. 2013, 42, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Maekawa, T.; Miyake, T.; Tani, M.; Uemoto, S. Diverse antitumor effects of ascorbic acid on cancer cells and the tumor microenvironment. Front. Oncol. 2022, 12, 981547. [Google Scholar] [CrossRef]
- Levine, M.; Conry-Cantilena, C.; Wang, Y.; Welch, R.W.; Washko, P.W.; Dhariwal, K.R.; Park, J.B.; Lazarev, A.; Graumlich, J.F.; King, J.; et al. Vitamin C pharmacokinetics in healthy volunteers: Evidence for a recommended dietary allowance. Proc. Natl. Acad. Sci. USA 1996, 93, 3704–3709. [Google Scholar] [CrossRef]
- Doseděl, M.; Jirkovský, E.; Macáková, K.; Krčmová, L.K.; Javorská, L.; Pourová, J.; Mercolini, L.; Remião, F.; Nováková, L.; Mladěnka, P.; et al. Vitamin C-Sources, Physiological Role, Kinetics, Deficiency, Use, Toxicity, and Determination. Nutrients 2021, 13, 615. [Google Scholar] [CrossRef]
- Bürzle, M.; Hediger, M.A. Functional and physiological role of vitamin C transporters. Curr. Top. Membr. 2012, 70, 357–375. [Google Scholar]
- Rivas, C.I.; Zúñiga, F.A.; Salas-Burgos, A.; Mardones, L.; Ormazabal, V.; Vera, J.C. Vitamin C transporters. J. Physiol. Biochem. 2008, 64, 357–375. [Google Scholar] [CrossRef]
- Lykkesfeldt, J.; Tveden-Nyborg, P. The Pharmacokinetics of Vitamin C. Nutrients 2019, 11, 2412. [Google Scholar] [CrossRef]
- Sunshine, H.; Iruela-Arispe, M.L. Membrane lipids and cell signaling. Curr. Opin. Lipidol. 2017, 28, 408–413. [Google Scholar] [CrossRef]
- Varshney, P.; Yadav, V.; Saini, N. Lipid rafts in immune signalling: Current progress and future perspective. Immunology 2016, 149, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Fajardo, V.A.; McMeekin, L.; LeBlanc, P.J. Influence of phospholipid species on membrane fluidity: A meta-analysis for a novel phospholipid fluidity index. J. Membr. Biol. 2011, 244, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Treede, I.; Braun, A.; Sparla, R.; Kühnel, M.; Giese, T.; Turner, J.R.; Anes, E.; Kulaksiz, H.; Füllekrug, J.; Stremmel, W.; et al. Anti-inflammatory effects of phosphatidylcholine. J. Biol. Chem. 2007, 282, 27155–27164. [Google Scholar] [CrossRef] [PubMed]
- van der Veen, J.N.; Kennelly, J.P.; Wan, S.; Vance, J.E.; Vance, D.E.; Jacobs, R.L. The critical role of phosphatidylcholine and phosphatidylethanolamine metabolism in health anddisease. Biochim. Biophys. Acta Biomembr. 2017, 1859 Pt B, 1558–1572. [Google Scholar] [CrossRef]
- Ruskamo, S.; Raasakka, A.; Pedersen, J.S.; Martel, A.; Škubník, K.; Darwish, T.; Porcar, L.; Kursula, P. Human myelin proteolipid protein structure and lipid bilayer stacking. Cell. Mol. Life Sci. 2022, 79, 419. [Google Scholar] [CrossRef] [PubMed]
- Kister, A.; Kister, I. Overview of myelin, major myelin lipids, and myelin-associated proteins. Front. Chem. 2023, 10, 1041961. [Google Scholar] [CrossRef]
- O’Donnell, V.B.; Rossjohn, J.; Wakelam, M.J. Phospholipid signaling in innate immune cells. J. Clin. Investig. 2018, 128, 2670–2679. [Google Scholar] [CrossRef]
- Lagace, T.A. Phosphatidylcholine: Greasing the Cholesterol Transport Machinery. Lipid Insights 2016, 8 (Suppl. S1), 65–73. [Google Scholar] [CrossRef]
- Kim, M.; Nevado-Holgado, A.; Whiley, L.; Snowden, S.G.; Soininen, H.; Kloszewska, I.; Mecocci, P.; Tsolaki, M.; Vellas, B.; Thambisetty, M.; et al. Association between Plasma Ceramides and Phosphatidylcholines and Hippocampal Brain Volume in Late Onset Alzheimer’s Disease. J. Alzheimers Dis. 2017, 60, 809–817. [Google Scholar] [CrossRef]
- Marcucci, H.; Paoletti, L.; Jackowski, S.; Banchio, C. Phosphatidylcholine biosynthesis during neuronal differentiation and its role in cell fate determination. J. Biol. Chem. 2010, 285, 25382–25393. [Google Scholar] [CrossRef]
- Gurunathan, S.; Kang, M.H.; Kim, J.H. A Comprehensive Review on Factors Influences Biogenesis, Functions, Therapeutic and Clinical Implications of Exosomes. Int. J. Nanomed. 2021, 16, 1281–1312. [Google Scholar] [CrossRef] [PubMed]
- Sheikholeslami, B.; Lam, N.W.; Dua, K.; Haghi, M. Exploring the impact of physicochemical properties of liposomal formulations on their in vivo fate. Life Sci. 2022, 300, 120574. [Google Scholar] [CrossRef] [PubMed]
- Shade, C.W. Liposomes as Advanced Delivery Systems for Nutraceuticals. Integr. Med. 2016, 15, 33–36. [Google Scholar]
- Akbarzadeh, A.; Rezaei-Sadabady, R.; Davaran, S.; Joo, S.W.; Zarghami, N.; Hanifehpour, Y.; Samiei, M.; Kouhi, M.; Nejati-Koshki, K. Liposome: Classification, preparation, and applications. Nanoscale Res. Lett. 2013, 8, 102. [Google Scholar] [CrossRef]
- Wang-Gillam, A.; Hubner, R.A.; Siveke, J.T.; Von Hoff, D.D.; Belanger, B.; de Jong, F.A.; Mirakhur, B.; Chen, L.T. NAPOLI-1 phase 3 study of liposomal irinotecan in metastatic pancreatic cancer: Final overall survival analysis and characteristics of long-term survivors. Eur. J. Cancer 2019, 108, 78–87. [Google Scholar] [CrossRef]
- Nightingale, S.D.; Saletan, S.L.; Swenson, C.E.; Lawrence, A.J.; Watson, D.A.; Pilkiewicz, F.G.; Silverman, E.G.; Cal, S.X. Liposome-encapsulated gentamicin treatment of Mycobacterium avium-Mycobacterium intracellulare complex bacteremia in AIDS patients. Antimicrob. Agents Chemother. 1993, 37, 1869–1872. [Google Scholar] [CrossRef]
- Calvagno, M.G.; Celia, C.; Paolino, D.; Cosco, D.; Iannone, M.; Castelli, F.; Doldo, P.; Frest, M. Effects of lipid composition and preparation conditions on physical-chemical properties, technological parameters and in vitro biological activity of gemcitabine-loaded liposomes. Curr. Drug Deliv. 2007, 4, 89–101. [Google Scholar] [CrossRef]
- Nsairat, H.; Khater, D.; Sayed, U.; Odeh, F.; Al Bawab, A.; Alshaer, W. Liposomes: Structure, composition, types, and clinical applications. Heliyon 2022, 8, e09394. [Google Scholar] [CrossRef]
- Sinha, R.; Sinha, I.; Calcagnotto, A.; Trushin, N.; Haley, J.S.; Schell, T.D.; Richie, J.P., Jr. Oral supplementation with liposomal glutathione elevates body stores of glutathione and markers of immune function. Eur. J. Clin. Nutr. 2018, 72, 105–111. [Google Scholar] [CrossRef]
- Flory, S.; Sus, N.; Haas, K.; Jehle, S.; Kienhöfer, E.; Waehler, R.; Adler, G.; Venturelli, S.; Frank, J. Increasing Post-Digestive Solubility of Curcumin Is the Most Successful Strategy to Improve its Oral Bioavailability: A Randomized Cross-Over Trial in Healthy Adults and In Vitro Bioaccessibility Experiments. Mol. Nutr. Food Res. 2021, 65, e2100613. [Google Scholar] [CrossRef]
- Jenski, L.J.; Zerouga, M.; Stillwell, W. Omega-3 fatty acid-containing liposomes in cancer therapy. Proc. Soc. Exp. Biol. Med. 1995, 210, 227–233. [Google Scholar] [CrossRef] [PubMed]
- Drapeau, C.; Benson, K.F.; Jensen, G.S. Rapid and selective mobilization of specific stem cell types after consumption of a polyphenol-rich extract from sea buckthorn berries (Hippophae) in healthy human subjects. Clin. Interv. Aging 2019, 4, 253–263. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; McGarry, S.; Cruickshank, D.; Jensen, G.S. Rapid increase in immune surveillance and expression of NKT and γδT cell activation markers after consuming a nutraceutical supplement containing Aloe vera gel, extracts of Poria cocos and rosemary. A randomized placebo-controlled cross-over trial. PLoS ONE 2023, 18, e0291254. [Google Scholar] [CrossRef] [PubMed]
- Benzie, I.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Honzel, D.; Carter, S.G.; Redman, K.A.; Schauss, A.G.; Endres, J.R.; Jensen, G.S. Comparison of chemical and cell-based antioxidant methods for evaluation of foods and natural products: Generating multifaceted data by parallel testing using erythrocytes and polymorphonuclear cells. J. Agric. Food Chem. 2008, 56, 8319–8325. [Google Scholar] [CrossRef]
- Lim, C.Y.; In, J. Considerations for crossover design in clinical study. Korean J. Anesthesiol. 2021, 74, 293–299. [Google Scholar] [CrossRef]
- Genser, B.; Cooper, P.J.; Yazdanbakhsh, M.; Barreto, M.L.; Rodrigues, L.C. A guide to modern statistical analysis of immunological data. BMC Immunol. 2007, 8, 27. [Google Scholar] [CrossRef]
- Kietzmann, T. Vitamin C: From nutrition to oxygen sensing and epigenetics. Redox Biol. 2023, 63, 102753. [Google Scholar] [CrossRef]
- Liu, X.; Khan, A.; Li, H.; Wang, S.; Chen, X.; Huang, H. Ascorbic Acid in Epigenetic Reprogramming. Curr. Stem Cell Res. Ther. 2022, 17, 13–25. [Google Scholar] [CrossRef]
- Camarena, V.; Wang, G. The epigenetic role of vitamin C in health and disease. Cell. Mol. Life Sci. 2016, 73, 1645–1658. [Google Scholar] [CrossRef]
- Brabson, J.P.; Leesang, T.; Mohammad, S.; Cimmino, L. Epigenetic Regulation of Genomic Stability by Vitamin C. Front. Genet. 2021, 12, 675780. [Google Scholar] [CrossRef] [PubMed]
- Bowie, A.G.; O’Neill, L.A. Vitamin C inhibits NF-kappa B activation by TNF via the activation of p38 mitogen-activated protein kinase. J. Immunol. 2000, 165, 7180–7188. [Google Scholar] [CrossRef] [PubMed]
- Morante-Palacios, O.; Godoy-Tena, G.; Calafell-Segura, J.; Ciudad, L.; Martínez-Cáceres, E.M.; Sardina, J.L.; Ballestar, E. Vitamin C enhances NF-κB-driven epigenomic reprogramming and boosts the immunogenic properties of dendritic cells. Nucleic Acids Res. 2022, 50, 10981–10994. [Google Scholar] [CrossRef] [PubMed]
- Esteban, M.A.; Wang, T.; Qin, B.; Yang, J.; Qin, D.; Cai, J.; Li, W.; Weng, Z.; Chen, J.; Ni, S.; et al. Vitamin C enhances the generation of mouse and human induced pluripotent stem cells. Cell Stem Cell 2010, 6, 71–79. [Google Scholar] [CrossRef]
- Saha, B.; Jyothi Prasanna, S.; Chandrasekar, B.; Nandi, D. Gene modulation and immunoregulatory roles of interferon gamma. Cytokine 2010, 50, 1–14. [Google Scholar] [CrossRef]
- Schoenborn, J.R.; Wilson, C.B. Regulation of interferon-gamma during innate and adaptive immune responses. Adv. Immunol. 2007, 96, 41–101. [Google Scholar]
- Kim, S.T.; Kyung, E.J.; Suh, J.S.; Lee, H.S.; Lee, J.H.; Chae, S.I.; Park, E.S.; Chung, Y.H.; Bae, J.; Lee, T.J.; et al. Phosphatidylcholine attenuated docetaxel-induced peripheral neurotoxicity in rats. Drug Chem. Toxicol. 2018, 41, 476–485. [Google Scholar] [CrossRef]
- Halliwell, B. Understanding mechanisms of antioxidant action in health and disease. Nat. Rev. Mol. Cell. Biol. 2024, 25, 13–33. [Google Scholar] [CrossRef]
- Liu, Y.Z.; Wang, Y.X.; Jiang, C.L. Inflammation: The Common Pathway of Stress-Related Diseases. Front. Hum. Neurosci. 2017, 11, 316. [Google Scholar] [CrossRef]
- Frost, D.M.; Meyer, I.H. Minority stress theory: Application, critique, and continued relevance. Curr. Opin. Psychol. 2023, 51, 101579. [Google Scholar] [CrossRef]
- Tyrrell, R.M. Ultraviolet radiation and free radical damage to skin. Biochem. Soc. Symp. 1995, 61, 47–53. [Google Scholar] [PubMed]
- Pan, B.; Li, H.; Lang, D.; Xing, B. Environmentally persistent free radicals: Occurrence, formation mechanisms and implications. Environ. Pollut. 2019, 248, 320–331. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Zalma, R.; Pezerat, H. Chemical reactivity of the carbon-centered free radicals and ferrous iron in coals: Role of bioavailable Fe2+ in coal workers pneumoconiosis. Free Radic. Res. 1999, 30, 439–451. [Google Scholar] [CrossRef] [PubMed]
- Pohjoismäki, J.L.O.; Goffart, S. Adaptive and Pathological Outcomes of Radiation Stress-Induced Redox Signaling. Antioxid. Redox Signal. 2022, 37, 336–348. [Google Scholar] [CrossRef] [PubMed]
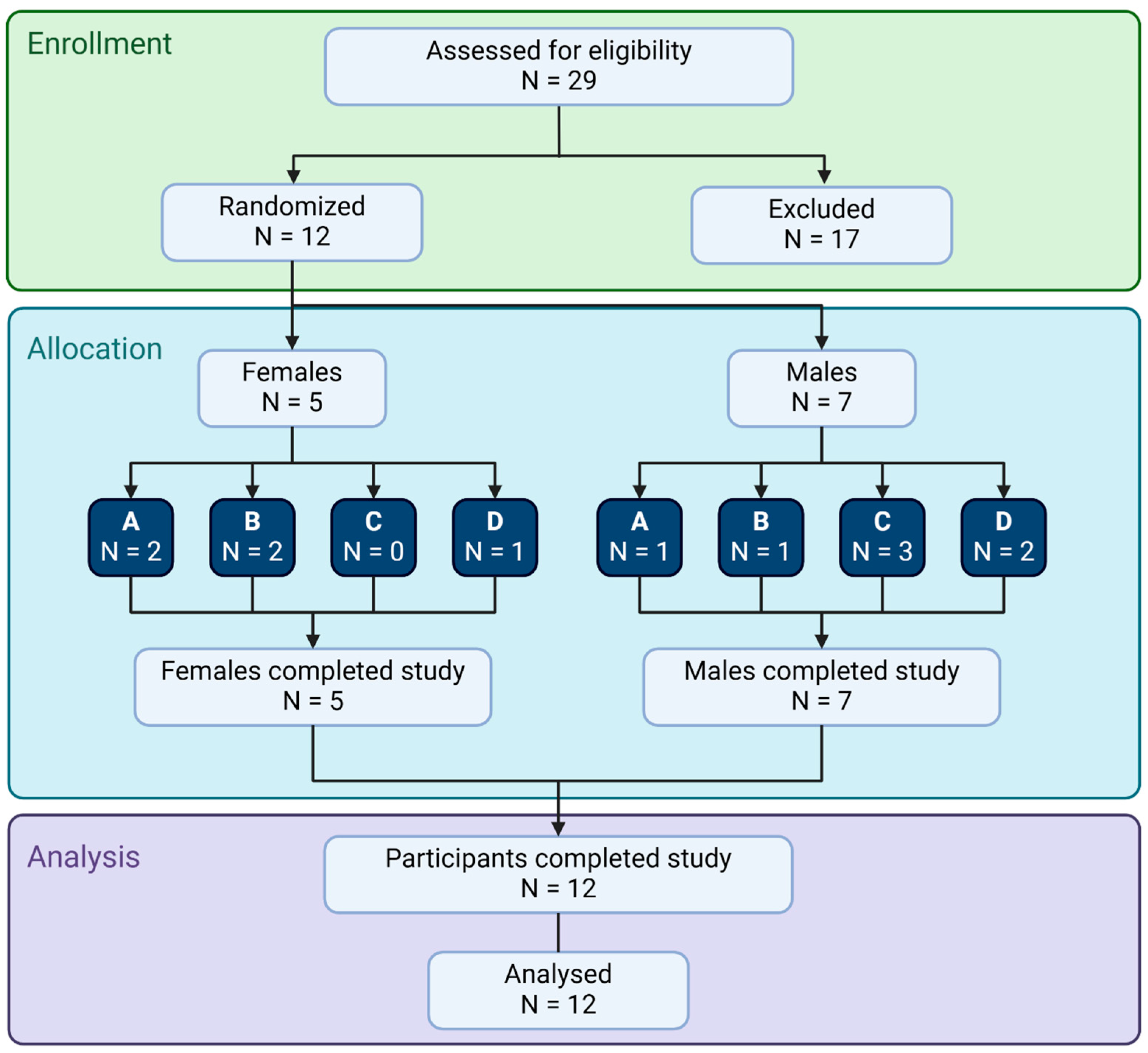



| Gender | N | Age Average a | Age Range | BMI Average a | BMI Range | Vitamin C Average b | Vitamin C Range b |
|---|---|---|---|---|---|---|---|
| Females | 5 | 64.3 ± 6.4 | 57.3–71.4 | 29.7 ± 3.4 | 25.1–34.4 | 38.6 ± 29.2 | 11.0–87.0 |
| Males | 7 | 56.7 ± 18.9 | 29.7–75 | 26 ± 5.5 | 19.6–34.7 | 44.0 ± 34.7 | 16.0–118.0 |
| Role | Effects | References |
|---|---|---|
| Antioxidant | Protection from free radical damage: | [2,3,4,5,6,7,8,9] |
| Mitochondrial energy production | ||
| Neuronal health during myelin formation and synaptic activity | ||
| Free radicals produced during active immune defense activity | ||
| Enzyme cofactor | Resilience against tissue invasion and wounding: | [10,11,12,13,14,15] |
| Collagen synthesis | ||
| Tissue integrity | ||
| Immune responses | ||
| Anti-aging, tissue rejuvenation | ||
| Epigenetic regulator | Regulation of gene expression: | [49,50,51,52,53,54,55] |
| Gene regulation in hypoxia, oxygen-sensing | ||
| Stem cell biology, maintaining or inducing stemness | ||
| Immune cell regulation of inflammation via NF-κB, Nrf2 |
| Role | Effects | References |
|---|---|---|
| Building blocks for cell membranes | Cell membrane mechanics: | [21,22,23] |
| Membrane fluidity for efficient cell signaling | ||
| Membrane cholesterol homeostasis | ||
| Prevention of cholesterol over-accumulation | ||
| Cognition | Nerve system support: | [24,25,26,27,28,29] |
| Nerve insulation through myelin | ||
| Neuronal regeneration | ||
| Maintaining brain health | ||
| Memory |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
McGarry, S.V.; Cruickshank, D.; Iloba, I.; Jensen, G.S. Enhanced Bioavailability and Immune Benefits of Liposome-Encapsulated Vitamin C: A Combination of the Effects of Ascorbic Acid and Phospholipid Membranes. Nutraceuticals 2024, 4, 626-642. https://doi.org/10.3390/nutraceuticals4040034
McGarry SV, Cruickshank D, Iloba I, Jensen GS. Enhanced Bioavailability and Immune Benefits of Liposome-Encapsulated Vitamin C: A Combination of the Effects of Ascorbic Acid and Phospholipid Membranes. Nutraceuticals. 2024; 4(4):626-642. https://doi.org/10.3390/nutraceuticals4040034
Chicago/Turabian StyleMcGarry, Sage V., Dina Cruickshank, Ifeanyi Iloba, and Gitte S. Jensen. 2024. "Enhanced Bioavailability and Immune Benefits of Liposome-Encapsulated Vitamin C: A Combination of the Effects of Ascorbic Acid and Phospholipid Membranes" Nutraceuticals 4, no. 4: 626-642. https://doi.org/10.3390/nutraceuticals4040034
APA StyleMcGarry, S. V., Cruickshank, D., Iloba, I., & Jensen, G. S. (2024). Enhanced Bioavailability and Immune Benefits of Liposome-Encapsulated Vitamin C: A Combination of the Effects of Ascorbic Acid and Phospholipid Membranes. Nutraceuticals, 4(4), 626-642. https://doi.org/10.3390/nutraceuticals4040034







