Abstract
There has been a rise in popularity of “stimulant-free” or caffeine-free fat loss supplements, but it is not well understood whether those fat loss supplements are effective at enhancing thermogenesis without caffeine’s influence. The purpose of this study was to examine the effects of a caffeinated and non-caffeinated commercially available fat loss supplement on resting energy expenditure (REE), hunger, and hemodynamic variables in healthy adults. Twenty-five healthy male and female participants completed three separate laboratory visits after overnight fasts. Baseline assessments of REE, subjective hunger, heart rate (HR), and blood pressure (BP) were followed by ingestion of a caffeinated (Phoenix, Legion®; CAF), non-caffeinated (Phoenix Caffeine-Free, Legion®; NCAF), or placebo (PL) fat loss supplement. REE, hunger, HR, and BP assessments were repeated at 60-, 120-, and 180-min post-ingestion. CAF, but not NCAF, significantly elevated REE greater than PL at all time points (p < 0.05). NCAF significantly reduced hunger compared to CAF and PL at the 120-min time point (p = 0.006). CAF significantly increased diastolic BP 60-min post-ingestion and significantly increased systolic BP 120- and 180-min post-ingestion compared to NCAF and PL. Further research is warranted with respect to investigating non-caffeinated ingredients and their effects on REE.
1. Introduction
The ability to effectively induce body composition changes is the goal of many diet and exercise intervention programs for those with excess adiposity. For some individuals with overweight and obesity, interventions have extended beyond diet and exercise intervention programs to include the use of dietary supplements (i.e., thermogenic fat burners) [1]. The use of thermogenic supplements (i.e., fat burners) extends beyond this population to fitness-minded individuals and physique athletes that desire extremely low levels of fat mass [2,3]. Recent investigations have identified that commercially available thermogenic supplements and ingredients typically contained in such products positively induce acute changes in energy intake and energy expenditure, and when taken chronically may elicit positive changes in body composition [4,5].
One of the more common ingredients found in thermogenic supplements is caffeine. With decades of research on caffeine, it has a strong scientific backing to support its application to aid in fat loss. Numerous clinical trials have shown acute caffeine ingestion to significantly increase metabolic rate, plasma free fatty acid concentration and fat oxidation in normal weight and obese individuals [6,7]. These observed beneficial adaptations observed are derived through its release of catecholamines which act on adrenergic receptors, catalyzing a critical step in the process of fat oxidation [8], which is reflected in a lower respiratory exchange ratio (RER). For these reasons, caffeine has landed itself as one of the most common ingredients found within commercially available thermogenic supplements. However, additional effects from the stimulatory nature of catecholamine release associated with caffeine consumption include increased heart rate (HR), blood pressure (BP), and feelings of anxiety and restlessness [9]. Due to these drawbacks, dietary supplement manufacturers have formulated “stimulant-free” supplements to meet the demands of individuals who desire a thermogenic effect without additional stimulation of the central nervous system. Since caffeine is typically omitted from stimulant-free supplements, the term “caffeine-free” is often used interchangeably.
A wide variety of ingredients in caffeine-free products have been utilized by supplement manufacturers such as extracts from tree bark, herbs, and various plant compounds. Commercially available supplements commonly use multiple ingredients in conjunction to provide individual unique benefits or work synergistically with one another to aid in weight loss. In the present study, the ingredients under investigation include Caralluma fimbrata, Coleus forskohlii (Forskolin), Mucuna pruriens (L-DOPA), Griffonia simplicifolia (5-Hydroxytryptophan; 5-HTP), Kaempferia parviflora (Black Ginger), Aframomum melegueta (Grains of Paradise), Laminaria jaoponica aresch, and caffeine.
Caralluma fimbrata, a flowering plant native to India, has been consumed in times of famine as it is hypothesized to have appetite suppressant effects [10]. While the results on its effectiveness regarding fat loss are mixed, Caralluma fimbrata supplementation has been shown to be effective in reducing both waist circumference and hip-to-waist ratio [10]. Herbs such as Forskolin have been shown in previous studies to specifically increase cAMP production in animal models [11,12]; cAMP is a regulatory step in the process of fat breakdown (lipolysis) [13]. Mucuna pruriens, a legume from regions of Africa and southwest Asia, contains naturally high levels of L-DOPA. While the primary mechanisms of action attributed to L-DOPA are cognitive, they may provide application to individuals seeking fat loss. Previous investigations have shown supplementation of L-DOPA to be effective in increasing circulating levels of dopamine and serotonin in healthy male participants [14]. Increases in these hormones may aid in mental clarity, alertness and reduction in feelings of anxiety commonly associated with prolonged caloric restriction and energy deficits [15].
The supplements in the present study (Phoenix and Phoenix Caffeine-Free, Legion®, Clearwater, FL, USA) also contain Griffonia simplicifolia, standardized to 98% 5-HTP. A byproduct of the naturally occurring amino acid L-Tryptophan, in clinical trials 5-HTP has demonstrated an ability to reduce overall calorie consumption, decrease carbohydrate intake, and increase early feelings of satiety in obese individuals consuming a hypocaloric diet [16,17]. Kaempferia parviflora, known as Thai Ginseng or Black Ginger, has been shown in animal models to decrease body weight via a reduction in voluntary feed intake over an 8-week period [18]. Additionally, supplementation of this extract may increase activation of brown adipose tissue and subsequent total body energy expenditure [19]. There is evidence to suggest that chronic supplementation of Grains of Paradise may favorably increase metabolic rate [20]. However, the single investigation that observed these effects included exposure to cold therapy and brown fat as prerequisites for inclusion, which may have impacted the ability to generalize the results [20]. Finally, Laminaria jaoponica aresch is an extract of sea kelp, native to southeast Asia. Occurring naturally within this brown alga is fucoxanthin, a carotenoid suggested to promote numerous health benefits including the inhibition of fat cell proliferation and improvements in glucose control in diabetic populations [21]. In a previous randomized, placebo-controlled, clinical trial, significant reductions in body weight, waist circumference and body fat were observed with chronic supplementation of fucoxanthin [22].
While it is understood that the aforementioned ingredients may have a positive influence on fat loss and resting energy expenditure (REE) individually, the effect of their combination is less clear. Therefore, the primary purpose of this study was to investigate the effects of a commercially available multi-ingredient caffeinated and caffeine-free supplement on resting energy expenditure in metabolically healthy men and women. A secondary aim was to determine whether the multi-ingredient thermogenic supplement had an acute effect on the suppression of hunger. Tertiary objectives for this study were to determine the effects of the thermogenic dietary supplement on resting HR and BP.
2. Materials and Methods
2.1. Participants
Thirty men and women between the ages of 18 and 39 years volunteered to participate in this study. A total of 5 participants dropped out/did not complete all required lab visits due to personal reasons unrelated to the study, leaving only 25 participants (women, n = 18; men, n = 7) included in the data analysis. Characteristics of analyzed participants are presented in Table 1. Participants varied in training and supplementation history; thus, a within-subjects protocol was used. The research protocol was approved by the University of South Florida Institutional Review Board (IRB ID: STUDY001825), and all ethical guidelines set forth in the Declaration of Helsinki were adhered to. All participants provided written informed consent prior to participation in this study. Participants were required to be between the ages of 18 and 50 years and free from metabolic, cardiovascular, and pulmonary diseases. Additionally, participants were excluded if they were pregnant or nursing, actively under treatment for any medical conditions, or had any sensitivities/allergies to the dietary supplements’ ingredients. Participants were instructed to maintain their typical dietary habits throughout the duration of the study.

Table 1.
Participant characteristics.
2.2. Experimental Design
This study utilized a randomized, triple-blind, placebo-controlled cross-over design. Each participant was scheduled to visit the lab on 3 separate days, with each visit being randomly assigned to a supplement condition: caffeinated (CAF, Phoenix, Legion®); non-caffeinated (NCAF, Phoenix Caffeine-Free, Legion®); or placebo (PL; inert ingredients). The ingredients contained in CAF and NCAF are presented in Table 2. All visits were consistently held in the same laboratory and were initiated between 6:15 a.m. and 6:30 a.m. The lab was climate-controlled; the mean temperature, barometric pressure, and humidity were 22.9 °C, 760.1 mmHg, and 46.7%, and these were stable across each of the visits with coefficient of variance values of 1.9%, 0.3%, and 3.1%, respectively. Participants reported to the lab following overnight fast (≥8 h) and abstinence from exercise for at least 24 h.

Table 2.
List of active ingredients for each supplement condition.
2.3. Testing Sessions
Participants were encouraged to use the restroom and void their bladders at the start of each lab visit. Following that, body weight and height were assessed with a physician beam scale (Health-O-Meter™, Model 402KL, McCook, IL, USA). Next, participants rested in a reclined position for 15 min prior to REE assessments. Indirect calorimetry was used to assess REE with a ParvoMedics TrueOne® 2400 Canopy System (ParvoMedics, Inc., Salt Lake City, UT, USA). The device was calibrated immediately preceding the baseline REE assessment and recalibrated between assessments as time allowed. During each REE assessment, participants were instructed to relax, lie motionless, breathe normally, and refrain from speaking or falling asleep for the entirety of the 20 min assessment. The initial 5 min of each assessment were discarded, and the final 15 min of data collected were used to calculate REE [23]. After the REE assessment, participants sat with their legs uncrossed and had their resting HR and BP recorded using a previously validated automated, oscillometric BP measurement device (Omron 5 series Model BP742, Lake Forest, IL, USA) [24]. HR and BP were assessed in triplicate with each measurement separated by 2 min, and the average of the three measurements was recorded.
Figure 1 presents the sequence of events during each testing session. REE was assessed at baseline, 60-, 120-, and 180-min post-ingestion. After the baseline assessment, participants ingested 3 capsules of either CAF, NCAF, or PL, with water under the supervision of research personnel. All capsules were identical in shape, smell, size, color, and taste. Immediately after each REE assessment, the participants had their resting HR and BP assessed along with a hunger VAS scale. The hunger VAS scale was presented in Likert-style, with 7 possible responses on a continuous line ranging from “very hungry” to “full”.
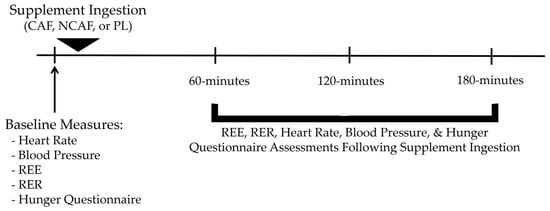
Figure 1.
An overview of testing sessions.
2.4. Statistical Analysis
All statistics were computed using SPSS version 28.0 (IBM Corp., Armonk, NY, USA). The dependent variables of REE, RER, HR, systolic blood pressure (SBP) and diastolic blood pressure (DBP) were analyzed using a 3 (within factor of condition: CAF, NCAF, PL) × 4 (within factor of time: baseline, 60-, 120-, 180-min) repeated measures analysis of variance (RMANOVA). When an interaction was present, changes from baseline were compared at each time point using a RMANOVA. When no interactions were present, the main effects of each condition were analyzed. The total energy expenditure over the 180-min period was also calculated by dividing the individual REE at each time point by 24 and then implementing the trapezoidal method to obtain the area under the curve for the entire 180-min window. An RMANOVA across total energy expenditure during this window was then computed to compare differences in total energy expenditure. Hunger scores were analyzed using a non-parametric Friedman test. Statistical significance was set at p ≤ 0.05.
3. Results
3.1. Resting Energy Expenditure (REE)
There was a significant interaction (p < 0.001) for REE (Figure 2). There was a significant difference for the change in REE the 60-min time point (p = 0.019) with a larger increase in REE resulting from CAF compared to NCAF (p = 0.025) and PL (p = 0.022). At the 120-min time point, there was a greater increase in REE in CAF compared to NCAF (p < 0.001) and PL (p < 0.001). At the 180-min time point, the changes in REE were statistically different (p < 0.001) with CAF increasing to a greater extent than NCAF (p < 0.001) and PL (p = 0.014). At the 180-min time point, PL also increased to a greater extent than NCAF (p = 0.043). When examining total energy expenditure during the 180-min window (Figure 3), there was a statistically significant difference (p = 0.003) with CAF resulting in a greater energy expenditure than PL (p < 0.001). No significant differences were observed between CAF and NCAF (p = 0.070) nor NCAF and PL (p = 0.095).
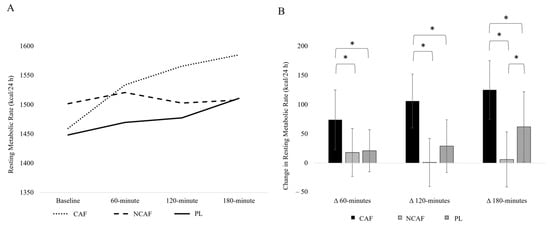
Figure 2.
(A) REE values over the 180-min study period; (B) change from baseline in Resting Energy Expenditure; error bars represent 95% confidence intervals; * indicates statistical significance at the p ≤ 0.05 level.
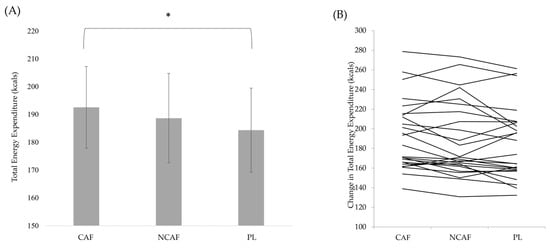
Figure 3.
(A) Total energy expenditure over the 180-min study period; (B) individual responses for changes in total energy expenditure, with each line indicating one individuals REE values across each of the trials; * indicates statistical significance at the p ≤ 0.05 level.
3.2. Respiratory Exchange Ratio (RER)
There was a significant interaction for RER (p = 0.009), but no post hoc analyses were statistically significant. There was no difference in the change in RER at 60-min (p = 0.093), 120-min (p = 0.873), or 180-min (p = 0.735) time points. Table 3 outlines the changes in RER and other secondary outcomes between time points.

Table 3.
Respiratory exchange ratio, hemodynamic variables, and hunger scores.
3.3. Heart Rate and Blood Pressure
There was a significant interaction for HR (p = 0.029). There were no differences in the change in HR at 60-min (p = 0.6540) or 120-min (p = 0.975) time points. There was a difference at the 180-min time point (p = 0.016), with HR decreasing to a greater extent in NCAF than in PL (p = 0.007), but no differences were present between CAF and NCAF (p = 0.160) nor CAF and PL (p = 0.112).
There was a significant interaction for SBP (p = 0.028). While there were no differences in the change in SBP at the 60-min (p = 0.186) time point, there was at both the 120-min (p = 0.007) and 180-min (p = 0.016) time points. At both 120-min and 180-min time points, SBP increased to a greater extent in CAF compared to NCAF (120-min, p = 0.006; 180-min, p = 0.013) and PL (120-min, p = 0.035; 180-min, p = 0.025). No differences were present between NCAF and PL at either 120-min (p = 0.465) or 180-min (p = 0.896) time points.
There was a significant interaction for DBP (p = 0.013). There was a significant difference in the increase in DBP at the 60-min time point (p = 0.002) with greater increases present in CAF compared to NCAF (p = 0.015) and PL (p = 0.001). No differences were present between NCAF and PL (p = 0.597). There were no differences in the change in DBP at 120-min (p = 0.052) or 180-min (p = 0.138) time points.
3.4. Hunger Scores
There was no difference in the change in hunger at 60-min (p = 0.949) or 180-min (p = 0.302) time points. At the 120-min time point, the change in hunger did differ (p = 0.006) with hunger scores decreasing to a greater extent in NCAF relative to both CAF (p = 0.018) and PL (p = 0.014). No differences were present between CAF and PL (p = 0.648).
4. Discussion
The primary results of this study were that the CAF treatment was able to elevate REE when compared to NCAF and PL at all time points, and that the NCAF was able to suppress perceptions of hunger two hours post-ingestion compared to CAF and PL treatments. Presumably, with an increase in energy expenditure and a decrease in energy intake (resulting from a reduction in hunger), ingestion of the multi-ingredient thermogenic supplements used in this study would promote fat loss outcomes over longer periods of supplementation. Relative to REE, the CAF treatment significantly elevated REE by 5.1%, 7.2%, and 8.6% at 60-min, 120-min, and 180-min post-ingestion respectively. This is consistent with previous research with respect to both effect and magnitude [6,25]. Previous work from our laboratory using similar caffeine-containing multi-ingredient thermogenic supplements reported nearly identical increases in REE three hours post-ingestion, with increases of approximately 9% compared to pre-supplementation baseline levels [26,27,28]. NCAF did not impact energy expenditure differently than the PL condition at any time point, despite limited evidence for ingredients found in both CAF and NCAF supplements to increase energy expenditure [19,20,29]. A potential explanation for this observed phenomenon may be an interaction effect, or lack thereof. CAF and NCAF contained identical ingredients and dosages with the sole exception of 200 mg caffeine anhydrous present in CAF but not NCAF. This single-ingredient deletion, coupled with the strength of randomized, placebo-controlled, triple-blinded, cross-over study design, allows for NCAF’s lack of effect on REE to be reasonably attributed to the absence of caffeine.
Previous literature on the ingredients of the present thermogenic supplements, as outlined earlier, suggest that each ingredient was included to either suppress appetite, increase energy expenditure, or upregulate fat metabolism. Caffeine is well understood to achieve all three of those desired outcomes on its own [7,8,25,30] and thus could still potentially bring about the observed effects of CAF without any of the other ingredients. For the ingredients in the NCAF treatment, consideration of their efficacy without the presence of caffeine, as well as their dosing, is warranted.
Forskolin is understood to upregulate lipolysis by increasing activation of cAMP. Interestingly, Forskolin’s ability to increase cAMP activation is maximized when in the presence of both adenosine deaminase and a methylxanthine [11]. This is due to adenosine deaminase’s ability to degrade adenosine, an inhibitor of cAMP, as well as methylxanthines’ ability to block adenosine from binding to adenylate cyclase and to inhibit the degradation of cAMP [11]. Caffeine is a methylxanthine [31], meaning Forskolin’s ability to upregulate lipolysis is less robust when caffeine is not present. Black Ginger has evidence supporting its efficacy in increasing brown adipose tissue activation when containing a concentration of 4.07% dimethoxyflavone, which is the primary polymethoxyflavonoid in Black Ginger extract and is responsible for inhibiting cAMP phosphodiesterase, an enzyme that degrades cAMP [19]. CAF and NCAF contain a concentration of 2.5% dimethyoxyflavone but, given NCAF’s lack of effect on REE, it is likely that caffeine is able to inhibit cAMP phosphodiesterase in CAF regardless of Black Ginger [11].
Grains of Paradise was another ingredient contained in both CAF and NCAF treatments. Previous research in healthy women reported that chronic (4 weeks) Grains of Paradise supplementation at a dosage of 30 mg/day, which was the same amount contained in CAF and NCAF treatments, significantly increased whole-body energy expenditure [29]. In the present study, only an acute dosage was assessed for its effectiveness of increasing resting energy expenditure. Considering NCAF’s lack of an REE response, it is plausible that repeated daily dosages of Grains of Paradise across several weeks are needed to induce an increase in resting energy expenditure. Mucuna pruriens has some evidence supporting its effect on increasing levels of epinephrine and norepinephrine in humans with 5 g of crushed seed powder ingested daily [14]. L-DOPA is the active ingredient in Mucuna pruriens, and although both CAF and NCAF contain 153 mg of Mucuna pruriens standardized at 98% L-DOPA, it is not possible to determine whether the dose contained in the present supplements is optimal compared to the doses in previous research due to the unknown concentration of L-DOPA in 5 g of crushed seed powder. Nonetheless, caffeine is understood to increase the release of epinephrine and norepinephrine itself, which may help further explain the increase in REE observed in CAF but not in NCAF, especially in the possible event of the L-DOPA dose in CAF and NCAF being suboptimal.
RER was not different between any conditions, despite CAF’s impact on REE. Caffeine has been previously shown to support weight loss and fat loss [32], suggesting caffeine favors increasing lipid metabolism. A recent meta-analysis by Conger and colleagues [33] suggests caffeine’s effect on lipid metabolism is more robustly measured via blood biomarkers rather than via whole-body gas exchange, which may explain a lack of observed difference in RER from the CAF group to NCAF or PL.
There were minimal differences in hunger scores between the groups, with the only difference observed being a decrease in hunger at the 120-min time point for the NCAF group. Caralluma fimbriata found in both CAF and NCAF supplements has limited evidence to suggest appetite suppression [10]; however, the inclusion of this ingredient in both supplements does not explain the sole observed difference during the experiment. There is evidence that caffeine exerts modest reductions in hunger, but the data are not consistent [30,34,35], suggesting high interindividual variability. Griffonia simplicifolia is understood to decrease energy intake, predominantly through a reduction in carbohydrate consumption, at dosages of 750 mg or 900 mg of 5-HTP. Standardized to 98% 5-HTP, CAF and NCAF both provide 153 mg of Griffonia simplicifolia, which may be suboptimal and provide some explanation of CAF and NCAF’s ability to impact hunger throughout each of the three-hour post-supplementation time frame. Furthermore, it may be possible that the interaction of caffeine with Caralluma fimbriata, Griffonia simplicifolia, and the other included ingredients may have produced a null effect on hunger that was not observed in NCAF due to its exclusion of caffeine.
Additional outcomes for this study included BP and HR responses between supplement conditions. The CAF condition had no effect on HR at any time point. This finding is consistent with prior work in our laboratory reporting that caffeine-containing thermogenic supplements had no effect on resting heart rate three hours post-ingestion [26,27,28]. The only observed difference in HR was a significant decrease in the NCAF group at the 180-min time point when compared to PL. Considering this significant decrease in tandem with the lack of observed increase in REE, there is plausibility in the combined effect of NCAF’s ingredients being somewhat depressive if not inert. More research on the interactive mechanistic pathways of each ingredient is needed to better elucidate the cause behind the observed significant decrease in HR at the 180-min time point. Focusing on BP, CAF induced a significant increase in systolic blood pressure at 120- and 180-min time points compared to NCAF and PL. This observation was also consistent with previous work from our laboratory relative to consumption of a caffeine-containing thermogenic supplement and systolic blood pressure [26,28]. This is likely due to caffeine’s known effect on cardiac contractility; it can be speculated that although not measured, stroke volume may have increased, leading to an increase in BP despite the absence of significant change in HR. While caffeine is well understood to acutely increase BP, caffeine ingestion has been shown to have positive effects on overall long-term cardiometabolic health and other diseases [36].
Despite the inherent strengths of the triple-blind placebo-controlled design, the present study is not without limitations. Inclusion of a caffeine-only condition would have better elucidated the impact caffeine has on desired outcome variables when combined, alone, and absent from the other active ingredients in CAF and NCAF. Future studies investigating caffeinated and non-caffeinated thermogenic supplements would benefit from including a caffeine-only condition. Another potential limitation of the design was not accounting for training status nor caffeine habituation differences among the subjects. A recent meta-analysis by Carvalho and colleagues [37] concluded that caffeine habituation did not influence the ergogenic potential of caffeine; however, more research is needed on the effects of caffeine habituation on metabolic response. Furthermore, the cross-over nature of the present study design suggests that any existing effect of caffeine habituation would have been equally present between all three supplement conditions. Additionally, the present study did not compare the results of the male participants to that of the female participants in a separate analysis, as that was not the purpose of this study. Some limited previous literature has shown sex differences in caffeine metabolism [38], making this an interesting area for future research. One delimitation of the present study is the lack of body weight adjusted dosing. Given that the purpose of this study was to examine the effects of a commercially available thermogenic supplement, it is important to recognize that the general population has abundant access to these types of pre-blended commercially available products. Therefore, the use of this supplement with pre-determined dosing provides greater generalizability of results for the average consumer. Finally, the time points chosen for data collection could be considered both a limitation and delimitation. The present study captured the results at 60-, 120-, and 180-min post-ingestion of the supplements. Potential differences between conditions could have remained unobserved between time points; however, the use of intermittent rather than continuous testing allowed for additional data (HR, BP, hunger scores) to be collected and therefore resulted in a more robust overall design.
5. Conclusions
The primary results of this study were that the CAF treatment was able to elevate REE when compared to NCAF and PL at all time points, and NCAF was able to suppress perceptions of hunger two hours post-ingestion compared to CAF and PL treatments. Additionally, the caffeinated supplement did not influence subjective hunger or heart rate responses but did increase acute systolic blood pressure responses. The non-caffeinated supplement exhibited a modest effect via hunger suppression and may find utility as a fat loss aid through that mechanism. However, further research is warranted for the ingredients of both thermogenic supplements, specifically focused on the impact of caffeine, varying ingredient dosages, and ingredient interaction effects on resting energy expenditure, fat metabolism, and appetite suppression in humans. Future research examining adaptations to chronic supplementation with Phoenix and Phoenix Caffeine-Free would also be beneficial for further elucidating the overall efficacy of these commercially available supplements.
Author Contributions
Conceptualization, B.I.C.; methodology, B.I.C.; formal analysis, S.D.; investigation, B.I.C., K.L., J.B., K.P., Y.A., W.A.A.-C., Y.O., J.S., C.B. and A.P.; resources, B.I.C., K.L., J.B. and K.P.; data curation, B.I.C., K.L., J.B., K.P. and J.S.; writing—original draft preparation, B.I.C., K.L., J.B. and K.P.; writing—review and editing, B.I.C., K.L., J.B., K.P., Y.A., W.A.A.-C., Y.O., J.S., C.B., A.P. and S.D.; visualization, B.I.C., K.L. and S.D.; supervision, B.I.C., K.L., J.B. and K.P.; project administration, B.I.C.; funding acquisition, B.I.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by The Florida High Tech Corridor and Legion Athletics, Inc., grant number 1776108900.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Review Board of the University of South Florida (ID: STUDY001825, approved 25 February 2022).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are available on request from the corresponding author, BIC (ethical reasons).
Acknowledgments
The authors would like to acknowledge Karina Noboa for her assistance with piloting this study, as well as Alexis Belcher, Malena Sellen, Zachary Warhul, Rashed Daher, Eric Velazquez, Savannah Ericksen, Gretchen Shelton, Indira Alur, Cassandra Resler, and Andrew Heath for their assistance with data collection.
Conflicts of Interest
BIC has received grants and contracts to conduct research on dietary supplements; has served as a paid consultant for industry; has received honoraria for speaking at conferences and writing lay articles about sports nutrition ingredients and topics; is a member of the International Protein Board that disseminates knowledge on protein and protein products; has served as an expert witness on behalf of the plaintiff and defense in cases involving dietary supplements; and receives compensation for writing and providing educational services related to exercise and nutrition-related topics. No other authors have conflicts of interest to report. All researchers involved in this study independently collected, analyzed, and interpreted the results from this study, and have no financial interests concerning the outcome of the study. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Clark, J.E.; Welch, S. Comparing effectiveness of fat burners and thermogenic supplements to diet and exercise for weight loss and cardiometabolic health: Systematic review and meta-analysis. Nutr. Health 2021, 27, 445–459. [Google Scholar] [CrossRef]
- Chappell, A.J.; Simper, T.; Barker, M.E. Nutritional strategies of high level natural bodybuilders during competition preparation. J. Int. Soc. Sports Nutr. 2018, 15, 4. [Google Scholar] [CrossRef]
- Rukstela, A.; Lafontant, K.; Helms, E.; Escalante, G.; Phillips, K.; Campbell, B.I. Bodybuilding Coaching Strategies Meet Evidence-Based Recommendations: A Qualitative Approach. J. Funct. Morphol. Kinesiol. 2023, 8, 84. [Google Scholar] [CrossRef]
- Nagao, T.; Komine, Y.; Soga, S.; Meguro, S.; Hase, T.; Tanaka, Y.; Tokimitsu, I. Ingestion of a tea rich in catechins leads to a reduction in body fat and malondialdehyde-modified LDL in men. Am. J. Clin. Nutr. 2005, 81, 122–129. [Google Scholar] [CrossRef]
- Nagao, T.; Hase, T.; Tokimitsu, I. A green tea extract high in catechins reduces body fat and cardiovascular risks in humans. Obesity (Silver Spring) 2007, 15, 1473–1483. [Google Scholar] [CrossRef]
- Acheson, K.J.; Zahorska-Markiewicz, B.; Pittet, P.; Anantharaman, K.; Jequier, E. Caffeine and coffee: Their influence on metabolic rate and substrate utilization in normal weight and obese individuals. Am. J. Clin. Nutr. 1980, 33, 989–997. [Google Scholar] [CrossRef] [PubMed]
- Dulloo, A.G.; Geissler, C.A.; Horton, T.; Collins, A.; Miller, D.S. Normal caffeine consumption: Influence on thermogenesis and daily energy expenditure in lean and postobese human volunteers. Am. J. Clin. Nutr. 1989, 49, 44–50. [Google Scholar] [CrossRef] [PubMed]
- Acheson, K.J.; Gremaud, G.; Meirim, I.; Montigon, F.; Krebs, Y.; Fay, L.B.; Gay, L.J.; Schneiter, P.; Schindler, C.; Tappy, L. Metabolic effects of caffeine in humans: Lipid oxidation or futile cycling? Am. J. Clin. Nutr. 2004, 79, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Temple, J.L.; Bernard, C.; Lipshultz, S.E.; Czachor, J.D.; Westphal, J.A.; Mestre, M.A. The safety of ingested caffeine: A comprehensive review. Front. Psychiatry 2017, 8, 80. [Google Scholar] [CrossRef] [PubMed]
- Kuriyan, R.; Raj, T.; Srinivas, S.K.; Vaz, M.; Rajendran, R.; Kurpad, A.V. Effect of Caralluma fimbriata extract on appetite, food intake and anthropometry in adult Indian men and women. Appetite 2007, 48, 338–344. [Google Scholar] [CrossRef] [PubMed]
- Litosch, I.; Hudson, T.H.; Mills, I.; Li, S.Y.; Fain, J.N. Forskolin as an activator of cyclic AMP accumulation and lipolysis in rat adipocytes. Mol. Pharmacol. 1982, 22, 109–115. [Google Scholar]
- Okuda, H.; Morimoto, C.; Tsujita, T. Relationship between cyclic AMP production and lipolysis induced by forskolin in rat fat cells. J. Lipid Res. 1992, 33, 225–231. [Google Scholar] [CrossRef] [PubMed]
- Lass, A.; Zimmermann, R.; Oberer, M.; Zechner, R. Lipolysis—A highly regulated multi-enzyme complex mediates the catabolism of cellular fat stores. Prog. Lipid Res. 2011, 50, 14–27. [Google Scholar] [CrossRef]
- Shukla, K.K.; Mahdi, A.A.; Ahmad, M.K.; Shankhwar, S.N.; Rajender, S.; Jaiswar, S.P. Mucuna pruriens improves male fertility by its action on the hypothalamus-pituitary-gonadal axis. Fertil. Steril. 2009, 92, 1934–1940. [Google Scholar] [CrossRef]
- Perez-Cornago, A.; Ramirez, M.J.; Zulet, M.A.; Martinez, J.A. Effect of dietary restriction on peripheral monoamines and anxiety symptoms in obese subjects with metabolic syndrome. Psychoneuroendocrinology 2014, 47, 98–106. [Google Scholar] [CrossRef] [PubMed]
- Cangiano, C.; Ceci, F.; Cascino, A.; Del Ben, M.; Laviano, A.; Muscaritoli, M.; Antonucci, F.; Rossi-Fanelli, F. Eating behavior and adherence to dietary prescriptions in obese adult subjects treated with 5-hydroxytryptophan. Am. J. Clin. Nutr. 1992, 56, 863–867. [Google Scholar] [CrossRef]
- Cangiano, C.; Laviano, A.; Del Ben, M.; Preziosa, I.; Angelico, F.; Cascino, A.; Rossi-Fanelli, F. Effects of oral 5-hydroxy-tryptophan on energy intake and macronutrient selection in non-insulin dependent diabetic patients. Int. J. Obes. Relat. Metab. Disord. 1998, 22, 648–654. [Google Scholar] [CrossRef][Green Version]
- Yoshino, S.; Kim, M.; Awa, R.; Kuwahara, H.; Kano, Y.; Kawada, T. Kaempferia parviflora extract increases energy consumption through activation of BAT in mice. Food Sci. Nutr. 2014, 2, 634–637. [Google Scholar] [CrossRef] [PubMed]
- Matsushita, M.; Yoneshiro, T.; Aita, S.; Kamiya, T.; Kusaba, N.; Yamaguchi, K.; Takagaki, K.; Kameya, T.; Sugie, H.; Saito, M. Kaempferia parviflora extract increases whole-body energy expenditure in humans: Roles of brown adipose tissue. J. Nutr. Sci. Vitaminol. 2015, 61, 79–83. [Google Scholar] [CrossRef]
- Sugita, J.; Yoneshiro, T.; Hatano, T.; Aita, S.; Ikemoto, T.; Uchiwa, H.; Iwanaga, T.; Kameya, T.; Kawai, Y.; Saito, M. Grains of paradise (Aframomum melegueta) extract activates brown adipose tissue and increases whole-body energy expenditure in men. Br. J. Nutr. 2013, 110, 733–738. [Google Scholar] [CrossRef]
- Maeda, H.; Hosokawa, M.; Sashima, T.; Murakami-Funayama, K.; Miyashita, K. Anti-obesity and anti-diabetic effects of fucoxanthin on diet-induced obesity conditions in a murine model. Mol. Med. Rep. 2009, 2, 897–902. [Google Scholar] [CrossRef]
- Abidov, M.; Ramazanov, Z.; Seifulla, R.; Grachev, S. The effects of Xanthigen in the weight management of obese premenopausal women with non-alcoholic fatty liver disease and normal liver fat. Diabetes Obes. Metab. 2010, 12, 72–81. [Google Scholar] [CrossRef]
- Horner, N.K.; Lampe, J.W.; Patterson, R.E.; Neuhouser, M.L.; Beresford, S.A.; Prentice, R.L. Indirect calorimetry protocol development for measuring resting metabolic rate as a component of total energy expenditure in free-living postmenopausal women. J. Nutr. 2001, 131, 2215–2218. [Google Scholar] [CrossRef][Green Version]
- Coleman, A.; Freeman, P.; Steel, S.; Shennan, A. Validation of the Omron MX3 Plus oscillometric blood pressure monitoring device according to the European Society of Hypertension international protocol. Blood Press. Monit. 2005, 10, 165–168. [Google Scholar] [CrossRef]
- Poehlman, E.T.; Despres, J.P.; Bessette, H.; Fontaine, E.; Tremblay, A.; Bouchard, C. Influence of caffeine on the resting metabolic rate of exercise-trained and inactive subjects. Med. Sci. Sports Exerc. 1985, 17, 689–694. [Google Scholar] [CrossRef]
- Campbell, B.I.; Colquhoun, R.J.; Zito, G.; Martinez, N.; Kendall, K.; Buchanan, L.; Lehn, M.; Johnson, M.; St Louis, C.; Smith, Y.; et al. The effects of a fat loss supplement on resting metabolic rate and hemodynamic variables in resistance trained males: A randomized, double-blind, placebo-controlled, cross-over trial. J. Int. Soc. Sports Nutr. 2016, 13, 14. [Google Scholar] [CrossRef]
- Campbell, B.I.; Perry, R.; Horsley, J.; Aguilar, D.; Shimshock, T.; Fox, C.; Vargas, A.; Colenso-Semple, L. A commercially available thermogenic dietary supplement increases resting metabolic rate in physically active males: A randomized, double-blind, placebo-controlled investigation. J. Diet Suppl. 2020, 17, 150–160. [Google Scholar] [CrossRef]
- Campbell, B.I.; Zito, G.; Colquhoun, R.; Martinez, N.; Kendall, K.; Buchanan, L.; Lehn, M.; Johnson, M.; St Louis, C.; Smith, Y.; et al. The effects of a single-dose thermogenic supplement on resting metabolic rate and hemodynamic variables in healthy females—A randomized, double-blind, placebo-controlled, cross-over trial. J. Int. Soc. Sports Nutr. 2016, 13, 13. [Google Scholar] [CrossRef] [PubMed]
- Sugita, J.; Yoneshiro, T.; Sugishima, Y.; Ikemoto, T.; Uchiwa, H.; Suzuki, I.; Saito, M. Daily ingestion of grains of paradise (Aframomum melegueta) extract increases whole-body energy expenditure and decreases visceral fat in humans. J. Nutr. Sci. Vitaminol. 2014, 60, 22–27. [Google Scholar] [CrossRef] [PubMed]
- Schubert, M.M.; Grant, G.; Horner, K.; King, N.; Leveritt, M.; Sabapathy, S.; Desbrow, B. Coffee for morning hunger pangs. An examination of coffee and caffeine on appetite, gastric emptying, and energy intake. Appetite 2014, 83, 317–326. [Google Scholar] [CrossRef] [PubMed]
- Saboury, A.A.; Divsalar, A.; Ataie, G.; Amanlou, M.; Moosavi-Movahedi, A.A.; Hakimelahi, G.H. Inhibition study of adenosine deaminase by caffeine using spectroscopy and isothermal titration calorimetry. Acta Biochim. Pol. 2003, 50, 849–855. [Google Scholar] [CrossRef]
- Tabrizi, R.; Saneei, P.; Lankarani, K.B.; Akbari, M.; Kolahdooz, F.; Esmaillzadeh, A.; Nadi-Ravandi, S.; Mazoochi, M.; Asemi, Z. The effects of caffeine intake on weight loss: A systematic review and dos-response meta-analysis of randomized controlled trials. Crit. Rev. Food Sci. Nutr. 2019, 59, 2688–2696. [Google Scholar] [CrossRef]
- Conger, S.A.; Tuthill, L.M.; Millard-Stafford, M.L. Does caffeine increase fat metabolism? a systematic review and meta-analysis. Int. J. Sport Nutr. Exerc. Metab. 2023, 33, 112–120. [Google Scholar] [CrossRef]
- Grant, C.L.; Coates, A.M.; Dorrian, J.; Paech, G.M.; Pajcin, M.; Della Vedova, C.; Johnson, K.; Kamimori, G.H.; Fidock, J.; Aidman, E.; et al. The impact of caffeine consumption during 50 hr of extended wakefulness on glucose metabolism, self-reported hunger and mood state. J. Sleep Res. 2018, 27, e12681. [Google Scholar] [CrossRef] [PubMed]
- Schubert, M.M.; Irwin, C.; Seay, R.F.; Clarke, H.E.; Allegro, D.; Desbrow, B. Caffeine, coffee, and appetite control: A review. Int. J. Food Sci. Nutr. 2017, 68, 901–912. [Google Scholar] [CrossRef] [PubMed]
- Grosso, G.; Godos, J.; Galvano, F.; Giovannucci, E.L. Coffee, caffeine, and health outcomes: An umbrella review. Annu. Rev. Nutr. 2017, 37, 131–156. [Google Scholar] [CrossRef]
- Carvalho, A.; Marticorena, F.M.; Grecco, B.H.; Barreto, G.; Saunders, B. Can I have my coffee and drink it? A systematic review and meta-analysis to determine whether habitual caffeine consumption affects the ergogenic effect of caffeine. Sports Med. 2022, 52, 2209–2220. [Google Scholar] [CrossRef]
- Lassen, M.L.; Byrne, C.; Sheykhzade, M.; Wissenberg, M.; Hurry, P.K.; Schmedes, A.V.; Kjaer, A.; Hasbak, P. Sex differences and caffeine impact in adenosine-induced hyperemia. J. Nucl. Med. 2022, 63, 431–437. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).