Saline Extract from Moringa oleifera Leaves Has Antidepressant and Anxiolytic Effects in Mouse Models
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of M. oleifera Leaf Extract (MoLE)
2.2. Characterization of MoLE by Thin Layer Chromatography (TLC)
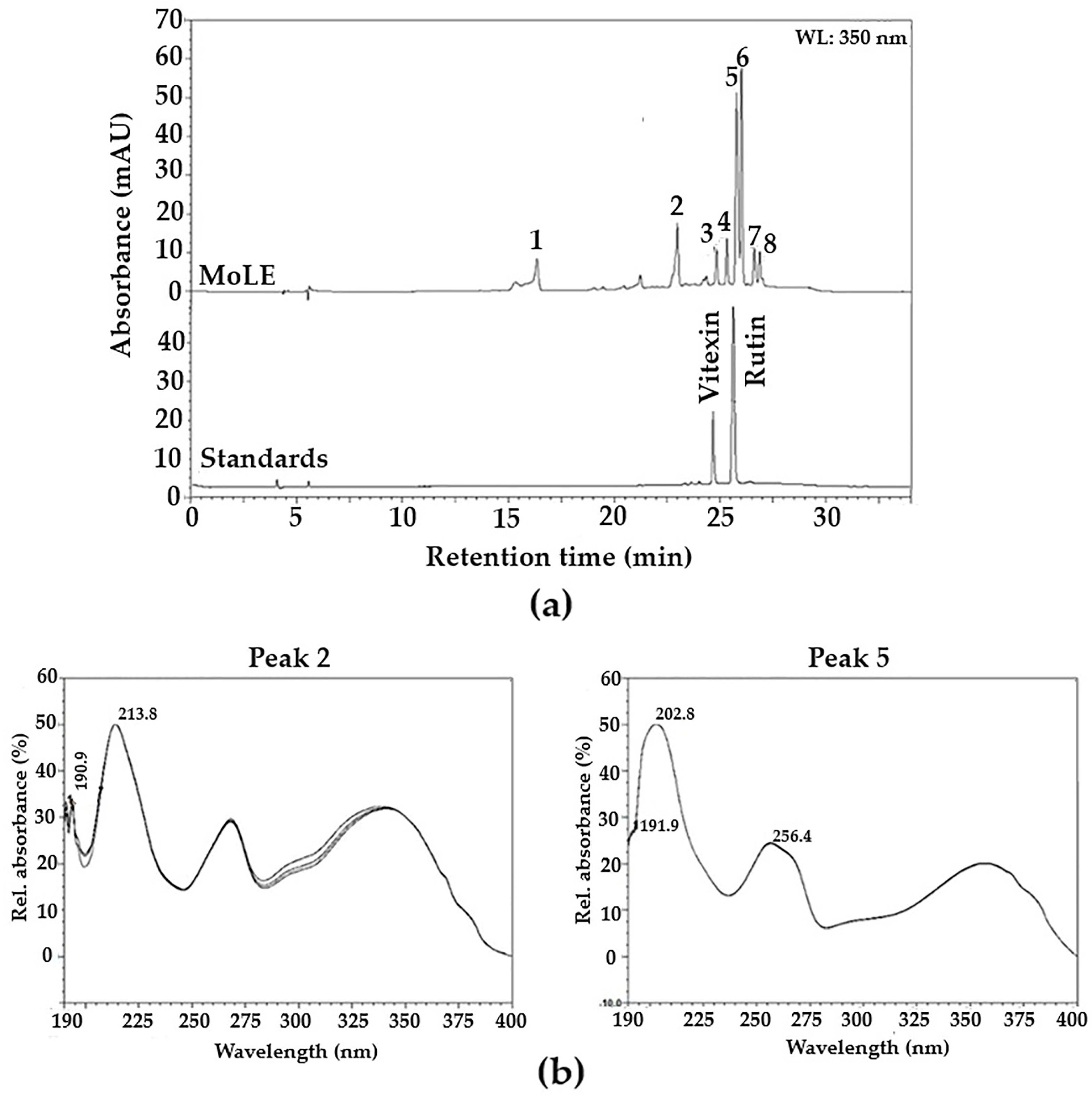
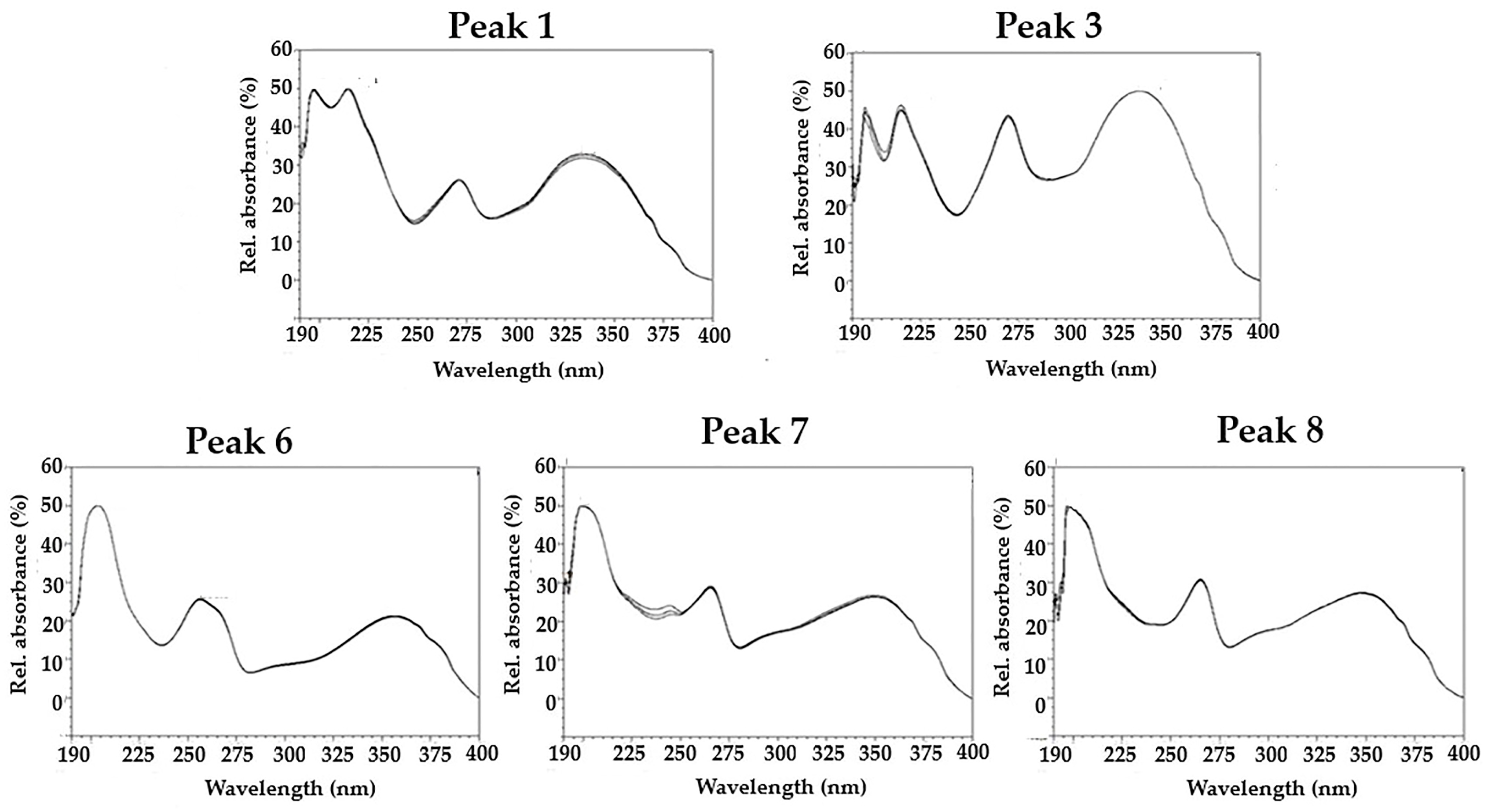
2.3. Characterization of MoLE Using High-Performance Liquid Chromatography (HPLC)
2.4. Investigation of MoLE for Hemagglutinating Activity (Lectin)
2.5. Determination of Trypsin Inhibitor Activity
2.6. Isolation of Peripheral Blood Mononuclear Cells (PBMCs) and Evaluation of the Effect of MoLE on Lymphocyte Viability
2.7. Investigation of MoLE for Hemolytic Activity
2.8. In Vivo Assays
2.8.1. Animals
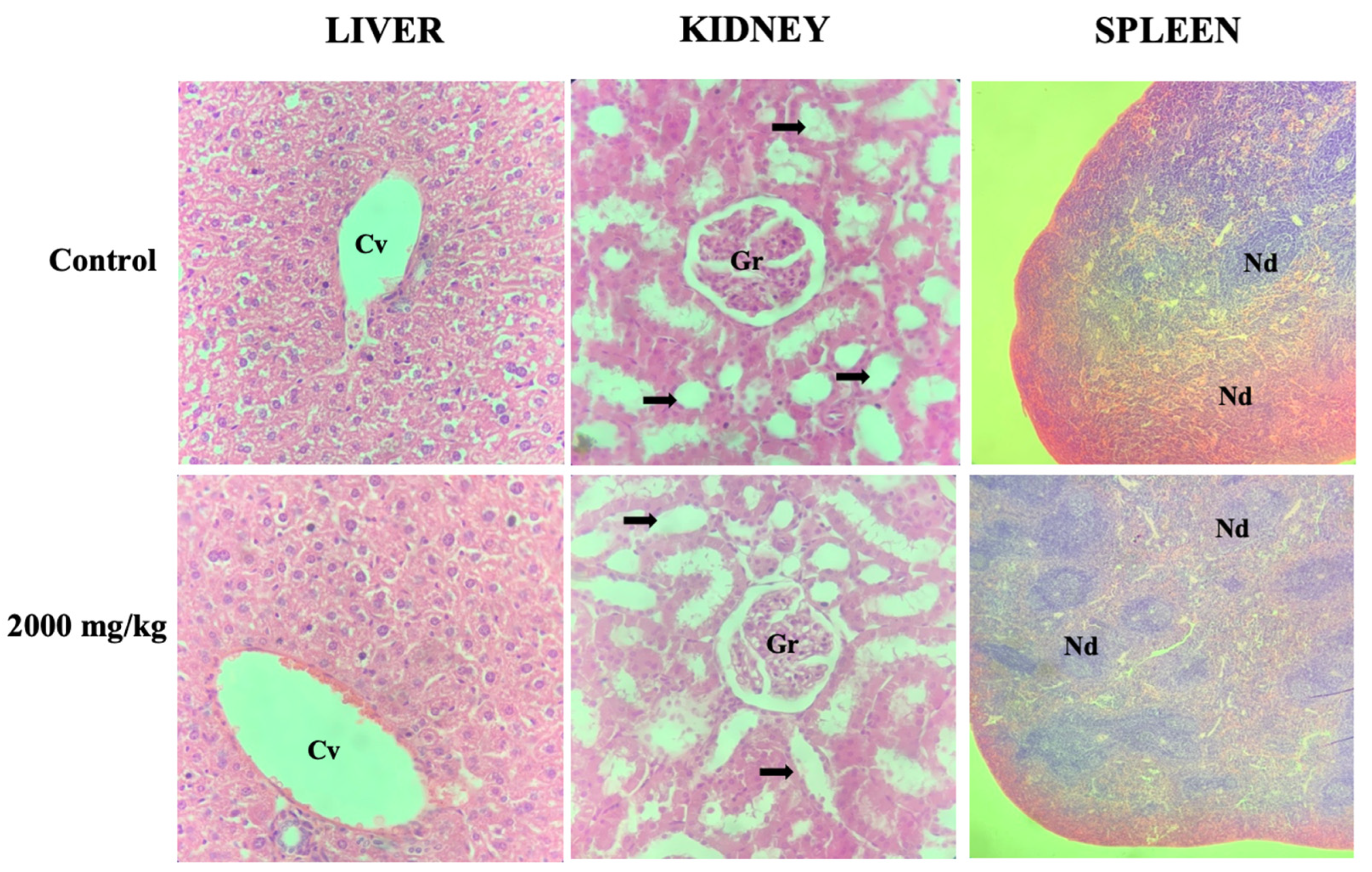
2.8.2. Acute Oral Toxicity
2.8.3. Measurement of Antioxidant Enzyme Levels
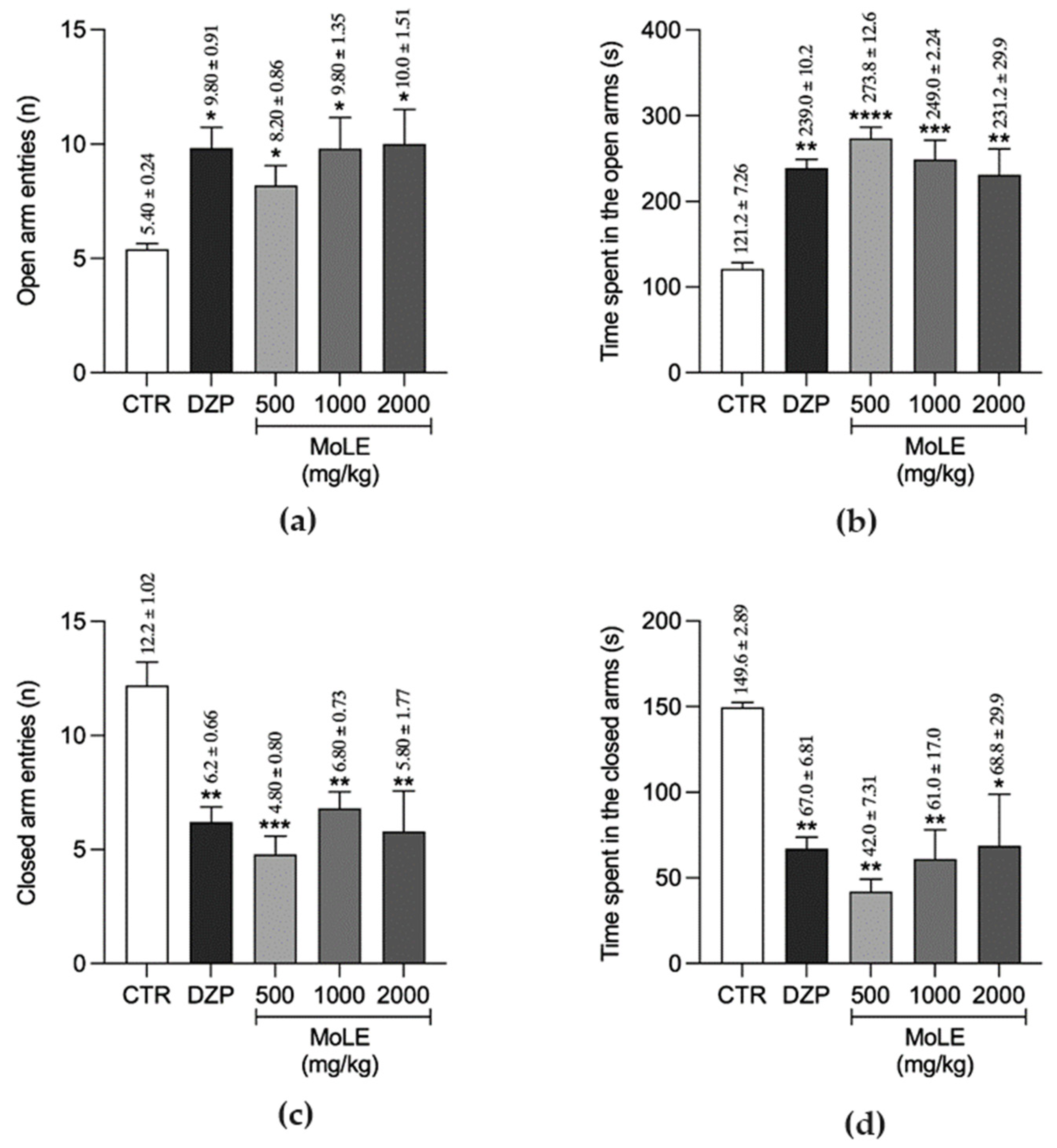
2.8.4. Evaluation of MoLE on Symptoms of Anxiety and Depression in Mice
Elevated plus Maze (EPM) Test
Forced Swimming Test
Tail Suspension Test
2.9. Statistical Analysis
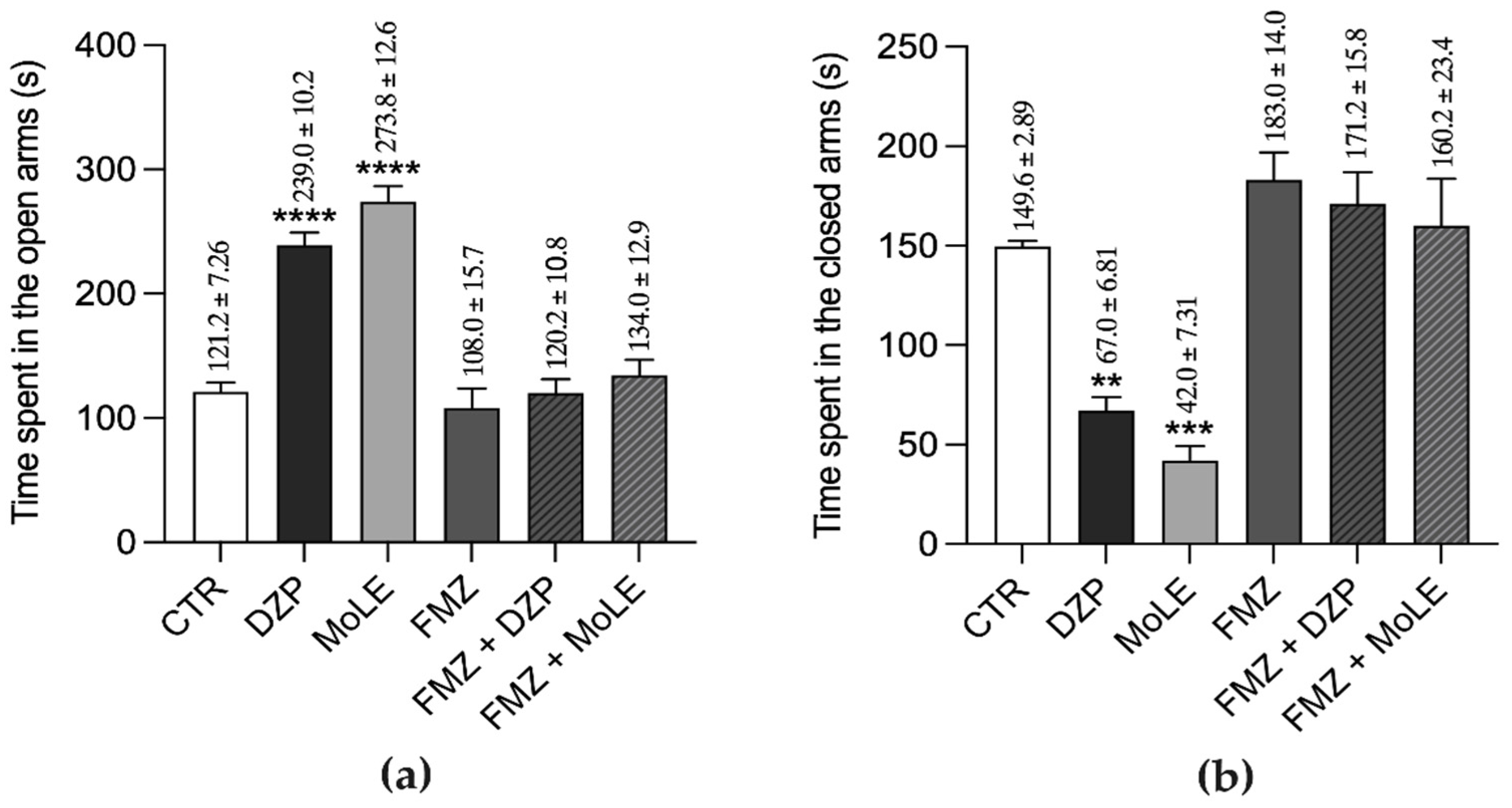
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ingale, S.P.; Gandhi, F.P. Effect of aqueous extract of Moringa oleifera leaves on pharmacological models of epilepsy and anxiety in mice. Int. J. Epilepsy 2016, 3, 12–19. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Epilepsy, Fact Sheet. 2019. Available online: http://www.who.int/mediacentre/factsheets/fs999/en/> (accessed on 15 January 2022).
- Ogunsina, B.S.; Indira, T.N.; Bhatnagar, A.S.; Radha, C.; Debnath, S.; Gopala Krishna, A.G. Quality characteristics and stability of Moringa oleifera seed oil of Indian origin. J. Food Sci. Technol. 2014, 51, 503–510. [Google Scholar] [CrossRef]
- Lindseth, G.; Helland, B.; Caspers, J. The effects of dietary tryptophan on affective disorders. Arch. Psychiatr. Nurs. 2015, 29, 102–107. [Google Scholar] [CrossRef]
- Zheng, Z.H.; Tu, J.L.; Li, X.H.; Hua, Q.; Liu, W.Z.; Liu, Y.; Pan, B.X.; Hu, P.; Zhang, W.H. Neuroinflammation induces anxiety- and depressive-like behavior by modulating neuronal plasticity in the basolateral amygdala. Brain. Behav. Immun. 2021, 91, 505–518. [Google Scholar] [CrossRef]
- Espay, A.J.; Aybek, S.; Carson, A.; Edwards, M.J.; Goldstein, H.; Hallett, M.; Lafaver, K.; Lafrance, W.C., Jr.; Lang, A.E.; Nicholson, T.; et al. Current Concepts in Diagnosis and Treatment of Functional Neurological Disorders. JAMA Neurol. 2018, 75, 1132–1141. [Google Scholar] [CrossRef]
- Heilig, M.; Mackillop, J.; Martinez, D. Addiction as a brain disease revised: Why it still matters, and the need for consilience. Neuropsychopharmacol 2021, 46, 1715–1723. [Google Scholar] [CrossRef]
- Akhlaq, S.; Ara, S.A.; Fazil, M.; Ahmad, B.; Akram, U.; Haque, M.; Khan, A.A. Ethnopharmacology, Phytochemical Analysis, Safety Profile, Prophylactic Aspects, and Therapeutic Potential of Asarum europaeum L. in Unani Medicine: An Evidence-Based Appraisal. Phytomed. Plus 2022, 2, 100226. [Google Scholar] [CrossRef]
- Jafarpoor, N.; Abbasi-Maleki, S.; Asadi-Samani, M.; Khayatnouri, M.H. Evaluation of antidepressant- like effect of hydroalcoholic extract of Passiflora incarnata in animal models of depression in male mice. J. Herb. Med. Pharmacol. 2014, 3, 41–45. [Google Scholar]
- Brilhante, R.S.N.; Sales, J.A.; Pereira, V.S.; Castelo, D.D.S.C.M.; Aguiar-Cordeiro, R.; de Souza Sampaio, C.M.; de Araújo Neto Paiva, M.; Santos, J.B.F.D.; Sidrim, J.J.C.; Rocha, M.F.G. Research advances on the multiple uses of Moringa oleifera: A sustainable alternative for socially neglected population. Asian Pac. J. Trop. Med. 2017, 10, 621–630. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.K.; Rana, H.K.; Tshabalala, T.; Kumar, R.; Gupta, A.; Ndhlala, A.R.; Pandey, A.K. Phytochemical, nutraceutical and pharmacological attributes of a functional crop Moringa oleifera Lam: An overview. J. S. Afr. Bot. 2020, 129, 209–220. [Google Scholar] [CrossRef]
- Lin, M.; Zhang, J.; Chen, X. Bioactive flavonoids in Moringa oleifera and their health-promoting properties. J. Funct. Foods 2018, 47, 469–479. [Google Scholar] [CrossRef]
- González-Trujano, M.E.; Martínez-González, C.L.; Flores-Carrillo, M.; Luna-Nophal, S.I.; Contreras-Murillo, G.; Magdaleno-Madrigal, V.M. Behavioral and electroencephalographic evaluation of the anticonvulsive activity of Moringa oleifera leaf non-polar extracts and one metabolite in PTZ-induced seizures. Phytomedicine 2018, 39, 1–9. [Google Scholar] [CrossRef]
- Kou, X.; Li, B.; Olayanju, J.B.; Drake, J.M.; Chen, N. Nutraceutical or pharmacological potential of Moringa oleifera Lam. Nutrients 2018, 10, 343. [Google Scholar] [CrossRef]
- Fard, M.T.; Arulselvan, P.; Karthivashan, G.; Adam, S.K.; Fakurazi, S. Bioactive extract from Moringa oleifera inhibits the pro-inflammatory mediators in lipopolysaccharide stimulated macrophages. Pharmacogn. Mag. 2015, 11, S556. [Google Scholar] [PubMed]
- Sodvadiya, M.; Patel, H.; Mishra, A.; Nair, S. Emerging insights into anticancer chemopreventive activities of nutraceutical Moringa oleifera: Molecular mechanisms, signal transduction and in vivo efficacy. Curr. Pharmacol. Rep. 2020, 6, 38–51. [Google Scholar] [CrossRef]
- Vergara-Jimenez, M.; Almatrafi, M.M.; Fernandez, M.L. Bioactive components in Moringa oleifera leaves protect against chronic disease. Antioxidants 2017, 6, 91. [Google Scholar] [CrossRef]
- Islam, M.T.; Martins, N.; Imran, M.; Hameed, A.; Ali, S.W.; Salehi, B.; Ahmad, I.; Hussain, A.; Sharifi-Rad, J. Anxiolytic-like effects of Moringa oleifera in Swiss mice. Cell Mol. Biol. 2020, 66, 73–77. [Google Scholar] [CrossRef]
- Mahmoud, M.S.; El-Kott, A.F.; AlGwaiz, H.I.; Fathy, S.M. Protective effect of Moringa oleifera Lam. leaf extract against oxidative stress, inflammation, depression, and apoptosis in a mouse model of hepatic encephalopathy. Environ. Sci. Pollut. Res. 2022, 29, 83783–83796. [Google Scholar] [CrossRef]
- Onasanwo, S.A.; Adamaigbo, V.O.; Adebayo, O.G.; Eleazer, S.E. Moringa oleifera-supplemented diet protect against cortico-hippocampal neuronal degeneration in scopolamine-induced spatial memory deficit in mice: Role of oxido-inflammatory and cholinergic neurotransmission pathway. Metab. Brain Dis. 2021, 36, 2445–2460. [Google Scholar] [CrossRef]
- Patriota, L.L.S.; Lima, B.R.F.; Marinho, A.O.; Costa, J.A.; Coelho, L.C.B.B.; Paiva, P.M.G.; Rosa, M.M.; Napoleão, T.H. The anxiolytic-like activity of water-soluble Moringa oleifera Lam. lectin is mediated via serotoninergic, noradrenergic, and dopaminergic neurotransmission. Brain Disord. 2023, 9, 100066. [Google Scholar] [CrossRef]
- Duong-Ly, K.C.; Gabelli, S.B. Salting out of proteins using ammonium sulfate precipitation. Methods Enzymol. 2014, 541, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.R.; Vasconcelos Alves, R.R.; da Silva, S.P.; Branco, S.J.D.S.C.; de Oliveira Marinho, A.; dos Santos Souza, T.G.; Chagas, C.A.; Paiva, P.M.G.; de Oliveira, A.M.; Napoleão, T.H. Acute toxicity and genotoxicity assessment of PgTeL, a lectin from pomegranate sarcotesta, in mice. S. Afr. J. Bot. 2022, 151, 301–308. [Google Scholar] [CrossRef]
- Ramos, D.D.B.M.; Araújo, M.T.D.M.F.; de Lima Araújo, T.C.; dos Santos Neto, O.G.; e Silva, M.G.; Silva, Y.A.; Torres, D.J.L.; Patriota, L.L.d.S.; de Melo, C.M.L.; de Lorena, V.M.B.; et al. Evaluation of antitumor activity and toxicity of Schinus terebinthifolia leaf extract and lectin (SteLL) in sarcoma 180-bearing mice. J. Ethnopharmacol. 2019, 233, 148–157. [Google Scholar] [CrossRef] [PubMed]
- Patriota, L.L.S.; do Nascimento Santos, D.K.; da Silva Barros, B.R.; de Souza Aguiar, L.M.; Silva, Y.A.; Dos Santos, A.C.L.A.; e Silva, M.G.; Coelho, L.C.B.B.; Paiva, P.M.G.; Pontual, E.V.; et al. Evaluation of the in vivo acute toxicity and in vitro hemolytic and immunomodulatory activities of the Moringa oleifera flower trypsin inhibitor (MoFTI). Protein Pept. Lett. 2021, 28, 665–674. [Google Scholar] [CrossRef] [PubMed]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin-phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef] [PubMed]
- Procópio, T.F.; de Siqueira Patriota, L.L.; de Moura, M.C.; da Silva, P.M.; de Oliveira, A.P.S.; do Nascimento Carvalho, L.V.; Lima, T.d.A.; Soares, T.; da Silva, T.D.; Coelho, L.C.B.B.; et al. CasuL: A new lectin isolated from Calliandra surinamensis leaf pinnulae with cytotoxicity to cancer cells, antimicrobial activity and antibiofilm effect. Int. J. Biol. Macromol. 2017, 98, 419–429. [Google Scholar] [CrossRef] [PubMed]
- Pontual, E.V.; Napoleão, T.H.; Dias De Assis, C.R.; De Souza Bezerra, R.; Xavier, H.S.; Navarro, D.M.D.A.F.; Paiva, P.M.G. Effect of Moringa oleifera flower extract on larval trypsin and acethylcholinesterase activities in Aedes aegypti. Arch. Insect Biochem. Physiol. 2012, 79, 135–152. [Google Scholar] [CrossRef]
- OECD—Organization for Economic Cooperation and Development. Guidelines for the Testing of Chemicals, OECD 423. In Acute Oral Toxicity-Acute Toxic Class Method; Organization for Economic Cooperation and Development: Paris, France, 2001. [Google Scholar]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Ohkawa, H.; Ohishi, N.; Yagi, K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 1979, 95, 351–358. [Google Scholar] [CrossRef]
- Beers, R.F.; Sizer, I.W. A spectrophotometric method for measuring the breakdown of hydrogen peroxide by catalase. J. Biol. chem. 1952, 195, 133–140. [Google Scholar] [CrossRef]
- Barros, M.C.; Silva, A.G.B.; dos Santos Souza, T.G.; Chagas, C.A.; Machado, J.C.B.; Ferreira, M.R.A.; Soares, L.A.L.; Xavier, V.L.; de Araújo, L.C.C.; Borba, E.F.d.O.; et al. Evaluation of acute toxicity, 28-day repeated dose toxicity, and genotoxicity of Moringa oleifera leaves infusion and powder. J. Ethnopharmacol. 2022, 296, 115504. [Google Scholar] [CrossRef] [PubMed]
- Lister, R.G. The use of a plus-maze to measure anxiety in the mouse. Psychopharmacology 1987, 92, 180–185. [Google Scholar] [CrossRef] [PubMed]
- Almeida, E.R.D.; Rafael, K.R.D.O.; Couto, G.B.L.; Ishigami, A.B.M. Anxiolytic and anticonvulsant effects on mice of flavonoids, linalool, and α-tocopherol presents in the extract of leaves of Cissus sicyoides L. (Vitaceae). BioMed Res. Int. 2009, 2009, 274740. [Google Scholar] [CrossRef]
- Gersner, R.; Dar, D.E.; Shabat-Simon, M.; Zangen, A. Behavioral analysis during the forced swimming test using a joystick device. J. Neurosci. Methods 2005, 143, 117–121. [Google Scholar] [CrossRef] [PubMed]
- Steru, L.; Chermat, R.; Thierry, B.; Simon, P. The tail suspension test: A new method for screening antidepressants in mice. Psychopharmacology 1985, 85, 367–370. [Google Scholar] [CrossRef]
- Fernandez, S.P.; Nguyen, M.; Yow, T.T.; Chu, C.; Johnston, G.A.; Hanrahan, J.R.; Chebib, M. The flavonoid glycosides, myricitrin, gossypin and naringin exert anxiolytic action in mice. Neurochem. Res. 2009, 34, 1867–1875. [Google Scholar] [CrossRef]
- Liu, Z.; Silva, J.; Shao, A.S.; Liang, J.; Wallner, M.; Shao, X.M.; Li, M.; Olsen, R.W. Flavonoid compounds isolated from Tibetan herbs, binding to GABAA receptor with anxiolytic property. J. Ethnopharmacol. 2021, 267, 113630. [Google Scholar] [CrossRef]
- Foudah, A.I.; Alqarni, M.H.; Alam, A.; Devi, S.; Salkini, M.A.; Alam, P. Rutin improves anxiety and reserpine-induced depression in rats. Molecules 2022, 27, 7313. [Google Scholar] [CrossRef]
- Pourahmad, J.; Salimi, A. Isolated human peripheral blood mononuclear cell (PBMC), a cost effective tool for predicting immunosuppressive effects of drugs and xenobiotics. Iran. J. Pharm. Res. 2015, 14, 979. [Google Scholar]
- Araújo, L.C.C.; Aguiar, J.S.; Napoleão, T.H.; Mota, F.V.B.; Barros, A.L.S.; Moura, M.C.; Coriolano, M.C.; Coelho, L.C.B.B.; Silva, T.G.; Paiva, P.M.G. Evaluation of cytotoxic and anti-inflammatory activities of extracts and lectins from Moringa oleifera seeds. PLoS ONE 2013, 8, e81973. [Google Scholar] [CrossRef]
- Tiloke, C.; Phulukdaree, A.; Gengan, R.M.; Chuturgoon, A.A. Moringa oleifera aqueous leaf extract induces cell-cycle arrest and apoptosis in human liver hepatocellular carcinoma cells. Nutr. Cancer 2019, 71, 1165–1174. [Google Scholar] [CrossRef] [PubMed]
- Qi, M.; Wang, X.; Chen, J.; Liu, Y.; Liu, Y.; Jia, J.; Li, L.; Yue, T.; Gao, L.; Yan, B.; et al. Transformation, absorption and toxicological mechanisms of silver nanoparticles in the gastrointestinal tract following oral exposure. ACS Nano 2023, 17, 8851–8865. [Google Scholar] [CrossRef]
- Lama, S.; Merlin-Zhang, O.; Yang, C. In vitro and in vivo models for evaluating the oral toxicity of nanomedicines. Nanomaterials 2020, 10, 2177. [Google Scholar] [CrossRef]
- Madukwe, E.U.; Ezeugwu, J.O.; Eme, P.E. Nutrient composition and sensory evaluation of dry Moringa oleifera aqueous extract. Int. J. Basic Appl. Sci. 2013, 13, 100–102. [Google Scholar]
- Okumu, M.O.; Ochola, F.O.; Mbaria, J.M. Mitigative effects of Moringa oleifera against liver injury induced by artesunate amodiaquine antimalarial combination in Wistar rats. Clin. Phytosci. 2017, 3, 18. [Google Scholar] [CrossRef]
- Krishnaiah, D.; Khiari, M.; Klibet, F.; Kechrid, Z. Oxidative Stress Toxicity Effect of Potential Metal Nanoparticles on Human cells. In Toxicology; Academic Press: Cambridge, MA, USA, 2021; pp. 107–117. [Google Scholar]
- Gbadamosi, I.T.; Omotoso, G.O.; Arogundade, T.; Alabi, A.S.; Balogun, R.B.; Yawson, E.O. Moringa regimen corrects nicotine-induced deficits in behaviour, altered energy metabolism and neurotransmitter processing in rat brain. J. Krishna Inst. Medical Sci. Univ. 2019, 8, 1–13. [Google Scholar]
- Shousha, W.G.; Aboulthana, W.M.; Salama, A.H.; Saleh, M.H.; Essawy, E.A. Evaluation of the biological activity of Moringa oleifera leaves extract after incorporating silver nanoparticles, in vitro study. Bull. Natl. Res. 2019, 43, 212. [Google Scholar] [CrossRef]
- Fakurazi, S.; Sharifudin, S.A.; Arulselvan, P. Moringa oleifera hydroethanolic extracts effectively alleviate acetaminophen-induced hepatotoxicity in experimental rats through their antioxidant nature. Molecules 2012, 17, 8334–8350. [Google Scholar] [CrossRef]
- David, B.; Wolfender, J.L.; Dias, D.A. The pharmaceutical industry and natural products: Historical status and new trends. Phytochem. Rev. 2015, 14, 299–315. [Google Scholar] [CrossRef]
- Karim, N.; Khan, I.; Khan, H.; Ayub, B.; Abdel-Halim, H.; Gavande, N. Anxiolytic potential of natural flavonoids. SM J. Steroids Horm. 2018, 1, 1001. [Google Scholar]
- Gazola, A.C.; Costa, G.M.; Zucolotto, S.M.; Castellanos, L.; Ramos, F.A.; de Lima, T.C.M.; Schenkel, E.P. The sedative activity of flavonoids from Passiflora quadrangularis is mediated through the GABAergic pathway. Biomed. Pharmacother. 2018, 100, 388–393. [Google Scholar] [CrossRef]
- Can, Ö.D.; Özkay, Ü.D.; Üçel, U.İ. Anti-depressant-like effect of vitexin in BALB/c mice and evidence for the involvement of monoaminergic mechanisms. Eur. J. Pharmacol. 2013, 699, 250–257. [Google Scholar] [CrossRef]
- Sakaguchi, M.; Arruda-Carvalho, M.; Kang, N.H.; Imaizumi, Y.; Poirier, F.; Okano, H.; Frankland, P.W. Impaired spatial and contextual memory formation in galectin-1 deficient mice. Mol. Brain. 2011, 4, 33. [Google Scholar] [CrossRef]
- Lima, B.R.F.; Patriota, L.L.S.; Marinho, A.O.; Costa, J.A.; Napoleão, T.H.; Rosa, M.M.; Paiva, P.M.G. The lectin from Schinus terebinthifolia leaf (SteLL) reduces immobility of mice on the tail suspension test dependent on the monoaminergic and nitric oxide signaling. Neurosci. Lett. 2023, 801, 137092. [Google Scholar] [CrossRef]
- Kaur, G.; Invally, M.; Sanzagiri, R.; Buttar, H.S. Evaluation of the antidepressant activity of Moringa oleifera alone and in combination with fluoxetine. J. Ayurveda Integr. Med. 2015, 6, 273. [Google Scholar] [CrossRef]
- Bakre, A.G.; Aderibigbe, A.O.; Ademowo, O.G. Studies on neuropharmacological profile of ethanol extract of Moringa oleifera leaves in mice. J. Ethnopharmacol. 2013, 149, 783–789. [Google Scholar] [CrossRef]
- Nuss, P. Anxiety disorders and GABA neurotransmission: A disturbance of modulation. Neuropsychiatr. Dis. Treat. 2015, 11, 165–175. [Google Scholar] [CrossRef] [PubMed]
- Whitwam, J.G.; Amrein, R. Pharmacology of flumazenil. Acta Anaesthesiol. Scand. Suppl. 1995, 108, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, E.J.; Warren, E.W. Flumazenil: A benzodiazepine antagonist. Clin. Pharm. 1993, 12, 641–656. [Google Scholar] [PubMed]
- Bhat, S.K.; Joy, A.E. Antianxiety effect of ethanolic extract of leaves of Moringa oleifera in Swiss albino mice. Arch. Med. Health 2014, 2, 5–7. [Google Scholar] [CrossRef]
- Pratiwi, R.D.; Utomo, R.B.; Kuswandari, S. Anxiolytic effect of aqueous extract of Moringa oleifera leaves in balb/c mice. Dental J. 2023, 10, 1–6. [Google Scholar] [CrossRef]
- Araújo, J.R.C.; Campos, A.R.; Barros, M.V.; Damasceno, M.; Santos, S.; Ferreira, M.K.A.; Azevedo, M.R.; Monteiro-Moreira, A.C.O. Neuropharmacological Characterization of Dioclea altissima Seed Lectin (DAL) in Mice: Evidence of Anxiolytic-like Effect Mediated by Serotonergic, GABAergic Receptors and NO Pathway. Curr. Pharm. Des. 2020, 26, 3895–3904. [Google Scholar] [CrossRef]
- Pannu, A.; Sharma, P.C.; Thakur, V.K.; Goyal, R.K. Emerging Role of Flavonoids as the Treatment of Depression. Biomolecules. 2021, 11, 1825. [Google Scholar] [CrossRef]

| Protein (mg/mL) | HA (Titer−1) | SHA | TIA (U/mg) | STIA | |
|---|---|---|---|---|---|
| MoLE | 8.12 | 16 | 1.97 | 9.81 | 54.5 |
| Treatment | Parameters | ||
|---|---|---|---|
| Weight (g) | Food Consumption (g) | Water Consumption (mL) | |
| Control | 36.45 ± 2.58 | 13.04 ± 1.23 | 25.11 ± 2.24 |
| MoLE | 37.33 ± 3.02 | 14.10 ± 1.10 | 24.16 ± 2.08 |
| Parameter | Control | MoLE (2000 mg/kg) |
|---|---|---|
| Red cells (106/mm3) | 5.21 ± 0.58 | 5.74 ± 0.39 |
| Hematocrit (%) | 34.23 ± 2.09 | 35.69 ± 3.34 |
| Hemoglobin (g/dL) | 14.11 ± 0.26 | 14.10 ± 0.24 |
| Mean corpuscular volume (%) | 46.45 ± 3.98 | 43.70 ± 4.55 |
| Mean corpuscular hemoglobin (%) | 16.52 ± 1.31 | 16.34 ± 1.15 |
| Mean corpuscular hemoglobin concentration (%) | 36.10 ± 3.16 | 37.28 ± 3.22 |
| Leukocytes (103/mm3) | 7.65 ± 0.56 | 7.34 ± 0.45 |
| Segmented (%) | 68.89 ± 4.66 | 72.25 ± 5.74 |
| Lymphocytes (%) | 27.94 ± 1.35 | 26.03 ± 1.74 |
| Monocytes (%) | 3.43 ± 0.30 | 3.33 ± 0.35 |
| Basophils (%) | 0.25 ± 0.05 | 0.20 ± 0.05 |
| Eosinophils (%) | 1.34 ± 0.19 | 1.38 ± 0.18 |
| Parameter | Control | MoLE (2000 mg/kg) |
|---|---|---|
| Albumin (g/dL) | 39.19 ± 3.76 | 38.75 ± 3.65 |
| Alanine aminotransferase (U/L) | 67.32 ± 4.51 | 68.57 ± 4.75 |
| Aspartate aminotransferase (U/L) | 89.10 ± 4.31 | 90.04 ± 5.79 |
| Alkaline phosphatase (U/L) | 13.24 ± 0.45 | 13.52 ± 0.45 |
| Gamma-glutamyl transferase (U/L) | 12.44 ± 0.41 | 12.17 ± 0.42 |
| Total protein (g/dL) | 70.26 ± 5.14 | 71.33 ± 5.48 |
| Urea (mg/dL) | 0.35 ± 0.04 | 0.34 ± 0.05 |
| Creatinine (mg/dL) | 4.57 ± 0.50 | 4.42 ± 0.39 |
| Bilirubin (mg/dL) | 0.42 ± 0.09 | 0.43 ± 0.09 |
| Total cholesterol (mg/dL) | 70.65 ± 6.34 | 74.84 ± 6.11 |
| Triglycerides (mg/dL) | 90.12 ± 7.53 | 92.35 ± 8.20 |
| Treatment | Parameter | ||
|---|---|---|---|
| Malondialdehyde (nM/mg of Protein) | Superoxide Dismutase (U/mg of Protein) | Catalase (nM/mg of Protein) | |
| Control | 6.19 ± 0.52 | 10.12 ± 1.25 | 3.10 ± 0.15 |
| MoLE | 6.10 ± 0.43 | 10.27 ± 1.12 | 3.17 ± 0.26 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fidelis, K.R.; Alves, R.R.d.V.; Patriota, L.L.d.S.; Coelho, L.C.B.B.; Ferreira, M.R.A.; Soares, L.A.L.; Oliveira, A.M.d.; Napoleão, T.H.; Paiva, P.M.G. Saline Extract from Moringa oleifera Leaves Has Antidepressant and Anxiolytic Effects in Mouse Models. Nutraceuticals 2024, 4, 65-81. https://doi.org/10.3390/nutraceuticals4010005
Fidelis KR, Alves RRdV, Patriota LLdS, Coelho LCBB, Ferreira MRA, Soares LAL, Oliveira AMd, Napoleão TH, Paiva PMG. Saline Extract from Moringa oleifera Leaves Has Antidepressant and Anxiolytic Effects in Mouse Models. Nutraceuticals. 2024; 4(1):65-81. https://doi.org/10.3390/nutraceuticals4010005
Chicago/Turabian StyleFidelis, Kleber Ribeiro, Robson Raion de Vasconcelos Alves, Leydianne Leite de Siqueira Patriota, Luana Cassandra Breitenbach Barroso Coelho, Magda Rhayanny Assunção Ferreira, Luiz Alberto Lira Soares, Alisson Macário de Oliveira, Thiago Henrique Napoleão, and Patrícia Maria Guedes Paiva. 2024. "Saline Extract from Moringa oleifera Leaves Has Antidepressant and Anxiolytic Effects in Mouse Models" Nutraceuticals 4, no. 1: 65-81. https://doi.org/10.3390/nutraceuticals4010005
APA StyleFidelis, K. R., Alves, R. R. d. V., Patriota, L. L. d. S., Coelho, L. C. B. B., Ferreira, M. R. A., Soares, L. A. L., Oliveira, A. M. d., Napoleão, T. H., & Paiva, P. M. G. (2024). Saline Extract from Moringa oleifera Leaves Has Antidepressant and Anxiolytic Effects in Mouse Models. Nutraceuticals, 4(1), 65-81. https://doi.org/10.3390/nutraceuticals4010005







