Abstract
Development of a nutritious, sustainable food source is essential to address worldwide deficiencies in human micronutrients. Aquatic floating plants (e.g., species in the family Lemnaceae, duckweeds) are uniquely suited for area-efficient productivity with exceptionally high rates of growth and nutritional quality. Here, we provide an overview of the role of dietary micronutrients (with a focus on carotenoids) in human health and the promise of Lemnaceae as sustainable crops. We examine the effect of growth light environment on plant biomass production and levels of the carotenoids zeaxanthin, lutein, and pro-vitamin A (β-carotene), as well as the antioxidant vitamin E (α-tocopherol), and protein. Data on each of these nutrients are reported on a plant dry biomass basis (as relevant for nutrition) as well as relative to the required input of light energy (as relevant to resource-use efficiency).
1. Introduction
Access to nutritious food that is replete in essential human micronutrients (required for vital functions but not synthesized de novo by humans) is urgently needed worldwide to support basic human functioning and lower the risk of many diseases and disorders. To this end, it is necessary to develop crops with superior nutritional traits. Edible floating aquatic plants of the Lemnaceae family (water lens or duckweed) have attractive nutritional traits as well as the potential to support sustainable agriculture in a changing climate.
Here, we present a further evaluation of duckweed nutritional quality by expressing data on carotenoid and protein content (previously reported on frond area and chlorophyll bases) on a biomass basis that is more relevant to human nutrition. We focus on plant protein content as well as carotenoids with emphasis on the xanthophylls zeaxanthin and lutein and their unique and diverse roles in human health. We, furthermore, characterized the influence of growth light intensity on plant biomass accumulation and the content of protein, vitamin E, and carotenoids (β-carotene, lutein, and zeaxanthin) in Lemna gibba.
Rather than expressing nutrient content on a reference basis of area growth, the present study reports nutrient content per dry biomass produced, i.e., as the nutritional quality of the biomass (proportion of biomass consisting of protein and key micronutrients) and as biomass and nutrient production relative to how much light energy is required to support this production (light use efficiency; LUE). The nutritional quality of leafy foods for humans is best evaluated as nutrient content per portion size, i.e., on a biomass (weight) basis, rather than on a chlorophyll or leaf area basis, because humans do not derive more nutrition from a plant-based food when carotenoid-to-chlorophyll ratios are high simply because chlorophyll levels are low. Additionally, we here express carotenoids and vitamin E in mg rather than (as previously reported for these data) on a molar basis because these compounds are generally reported in mg in the nutrition literature. Furthermore, nutrient content per biomass is compared for plants grown under constant low or high growth light intensity (photon flux density; PFD) in environmentally controlled growth chambers versus plants growing on a sun-exposed pond with natural diurnal changes in PFD.
Especially for food production with artificial light supply in urban agriculture [1,2] or in spaceflight environments [3,4], the light energy required for nutrient production is a critical factor for food production. Findings are used to discuss options to improve nutritional quality through a combination of informed crop choice and suitable growing conditions.
Particular attention is given to the carotenoid zeaxanthin in view of its unique roles in supporting human health. Because plants quickly convert zeaxanthin to its precursor violaxanthin upon removal from high light, zeaxanthin levels are reported separately for tissue frozen immediately after harvesting and corresponding samples frozen after a 30-minute recovery period in low light. The extent of nutritional decline post-harvest (with respect to zeaxanthin level) is assessed through the comparison of zeaxanthin level at harvest and after 30 min for a range of growth PFDs.
1.1. Dietary Carotenoids and Human Health
Urbanization and modernization were accompanied by world-wide changes in diet and lifestyle, including a transition to overly energy-dense but nutrient-deficient foods [5,6]. Moreover, rising atmospheric CO2 levels threaten to further lower plant nutritional quality with respect to protein and micronutrient levels [7,8]. The combination of a micronutrient-deficient diet with a sedentary lifestyle and chronic psychological stress can lead to a dysfunctional human immune system with uncontrolled system-wide inflammation and poor immunity against infections [9,10]. Chronic, low-grade inflammation is a root cause for cognitive dysfunctions [11] and mental disorders [12,13]. Uncontrolled system-wide inflammation has also been linked to chronic diseases [14,15,16] as well as infectious diseases [17], including increased risk for severe COVID-19 [18,19] and is associated with long COVID (post-acute sequelae; [19,20,21]). Carotenoids and vitamin E (α-tocopherol) play key roles in opposing chronic inflammation [22] as briefly reviewed below.
System-wide roles associated with membrane fatty acids: Lipid-soluble diet-derived carotenoids and vitamin E become embedded in biological membranes throughout the body and play a unique role in modulating membrane-derived immune regulators (for recent reviews see [22,23]). Zeaxanthin, lutein, and β-carotene can inactivate, and thus detoxify, reactive oxygen species (ROS) and oxidized membrane lipids [24,25], but vary in how susceptible they are to themselves becoming involved in propagating dangerous oxidation cascades [22]. To prevent such pro-oxidant effects, these membrane-associated antioxidants must, furthermore, be recycled (for recent reviews see [4,22]) by water-soluble dietary and endogenous antioxidant systems at the membrane surface. Zeaxanthin’s effect in limiting oxidation cascades was improved when vitamins E and C were present [26,27] (for a recent review see [22]). Conversely, zeaxanthin [26] as well as vitamin C [24,28] improved vitamin E’s detoxifying effect [28,29,30,31,32].
Activity as gene regulators: Carotenoids regulate genes that function in the immune response or in the control of energy balance. The β-carotene cleavage product vitamin A serves as a regulator of key genes of the immune response [23] and impacts the function of multiple organs [28]. Similarly, cleavage products of lutein, zeaxanthin, and other xanthophylls [33] can act as gene regulators [34,35,36]. Some carotenoids (including zeaxanthin) and additional dietary nutraceuticals directly oppose obesity that is also a contributing factor to chronic inflammation [37,38]. Specifically, zeaxanthin [39,40,41] and other nutraceuticals [42] tune the controls of energy balance by triggering emergence of mitochondria in fat cells, enhancing fat burning, and increasing the fraction of energy released as heat (thermogenesis).
Specific to the human eye: The ocular carotenoids, or their derivatives such as the β-carotene cleavage product pro-vitamin A, each have unique roles in the human eye. Vitamin A is required as a component of the vision protein; the xanthophylls zeaxanthin and lutein support visual acuity and reduce glare as well as protect against photodamage by intense light. Whereas zeaxanthin is dominant in the central portions of the human eye that receive the brightest light [23,43,44], lutein is dominant in the peripheral regions of the retina responsible for low-light vision [45,46].
Modulation of other processes: Remarkably, zeaxanthin has an additional, independent effect in lowering the risk for severe COVID-19 by inhibiting viral entry into human cells. Zeaxanthin inhibits one of the two human proteases [47] that cleave the spike protein of SARS-CoV-2 and thereby greatly enhance its binding affinity to the human ACE2 receptor [48].
In summary, dietary antioxidants can be a double-edged sword. A mix of carotenoids, vitamin E, and other dietary micronutrients is needed to combat chronic inflammation and associated diseases effectively because the few dietary antioxidants that can protect biological membranes can turn into damaging pro-oxidants in the absence of synergistically acting water-soluble antioxidants. Such a mix is provided by a diet rich in whole plant-based foods containing different classes of micronutrients [4,22,49].
Whereas leafy greens and other green plant foods contain lutein and β-carotene, such foods typically have little to no zeaxanthin when they reach the human consumer [3,50]. This is because zeaxanthin is typically formed only when leaves are exposed to excess light and accumulates at much lower levels in fast-growing terrestrial crops compared to slow-growing evergreens with inedible leaves. Most diets are thus lacking in zeaxanthin despite this carotenoid’s unique role in health and wellness [51,52]. Currently available whole foods with high, stable levels of zeaxanthin include egg yolk, corn, and orange pepper [49]. As shown here, duckweed may serve as an alternative, less-resource-intensive food source with high levels of both lutein and zeaxanthin.
1.2. Floating Plants with Exceptional Nutritional Quality and Sustainable Production
Aquatic floating plants in the duckweed family Lemnaceae have long been used as crops in Asia [53] and are receiving increasing interest as exceptionally nutritious crops in other parts of the world [54]. Duckweeds are attractive because they exhibit an unusual combination of multiple desirable features, including fast growth, high levels of zeaxanthin [8,55] and synergistically acting antioxidant vitamins and phenolics [4], a high protein content, a healthful fat composition [56,57,58], and a small environmental footprint. We briefly elaborate on each of these points in the following paragraphs.
Antioxidant micronutrients: Duckweed is rich in α-tocopherol [56,57,59] and carotenoids, with exceptionally high levels of zeaxanthin when grown in high light [8,55,59]. Duckweed also shows a unique combination (not seen in land plants) of fast growth while still maintaining high zeaxanthin levels [8,55,59]. In addition, duckweed contains high levels of phenolics [4,60].
Protein content: Duckweeds combine a beneficial amino-acid composition with all essential amino acids required by humans [57,61]. The entire duckweed plant is edible [62] and thus has a higher total protein production (e.g., up to 20× higher) per growing area for a single layer of duckweed (that stores protein throughout the plant) [63] compared to soybean plants (that store protein only in its seed) [64,65,66,67].
Fat composition: Duckweeds have a nutritionally favorable ratio of polyunsaturated omega-6- and omega-3-fatty-acids that have immune-response-initiating and immune-response-terminating effects, respectively [57,68,69,70,71,72,73].
Climate resilience and small environmental footprint: The high growth rates of duckweeds exhibit a lesser responsiveness to certain environmental conditions [8], including elevated CO2 [8,74,75], than those of other species. Furthermore, these aquatic plants exhibit a high nitrogen-use efficiency [76] and a particularly effective synthesis of amino acids and protein [77,78,79]. Moreover, duckweeds are also particularly efficient at taking up inorganic nutrients from their growth medium, which is why they are useful in wastewater recycling [64,65,66,67,80,81,82].
2. Materials and Methods
2.1. Plant Growth and Assessment of Nutrient Content
Data shown are for Lemna gibba L. 7741 (G3) from Rutgers Duckweed Stock Cooperative (https://ruduckweed.org; accessed on 29 September 2022) grown in growth chambers under continuous (24 h per day) light. Lemna minor L. growing on a local pond exposed to natural full sunlight, and three terrestrial species (pumpkin [Cucurbita pepo L. cv. Autumn Gold]; tomato [Solanum lycopersicum L. cv. Brandywine]; sunflower [Helianthus annuus L. ANN 2199]) grown in growth chambers were used for comparison in one instance. Original data were presented previously on a leaf or frond area basis and a chlorophyll basis [55,59,83]. All conditions and procedures for duckweed growth, photosynthesis measurements, and assays of human nutrients (protein, carotenoids, and α-tocopherol) were thus as described in [55,59] and for the terrestrial species as described in [83]. Figure 1 shows images of L. gibba grown under 50, 500, and 1000 μmol photons m–2 s–1.

Figure 1.
Images of Lemna gibba fronds grown under light intensities of 50, 500, and 1000 μmol photons m–2 s–1.
2.2. Light-Use Efficiency
Amounts of carotenoid, α-tocopherol, protein, and dry biomass produced per dish were divided by number of photons received, over the course of an experiment, to calculate light-use efficiency of production (in mg or g of product produced per mol photons provided for plant growth) using [X] (average concentration [mg or g per m2 frond area] of compound X under the growth PFD), FA0 and FAt (m2 of frond area per dish at the beginning and end of the experiment, respectively), and γt (number of photons received by plants over the course of the experiment; for details, see [55]):
2.3. Statistical Analysis
Comparisons of multiple mean values under each growth PFD were made using one-way analysis of variance (ANOVA) and post hoc Tukey–Kramer test for honestly significant differences. Comparisons of two means were made with a Student’s t-test. Sample size for experiments under 50, 200, and 1000 μmol photons m–2 s–1 was 3 or 4 dishes each, and for experiments under 100, 500, and 700 μmol photons m–2 s–1 was 3 dishes each. Sample size of growth-based metrics (e.g., LUE) for experiments under 50 μmol photons m–2 s–1 was 7 dishes since growth was characterized in two separate trials. All statistical tests were conducted with JMP Pro 15 software (SAS Institute Inc., Cary, NC, USA), and data were visualized with R software (https://www.r-project.org; accessed on 9 November 2022) and ggplot2 package [84].
3. Results
3.1. The Impact of Reference Basis on the Assessment of Photosynthetic Performance
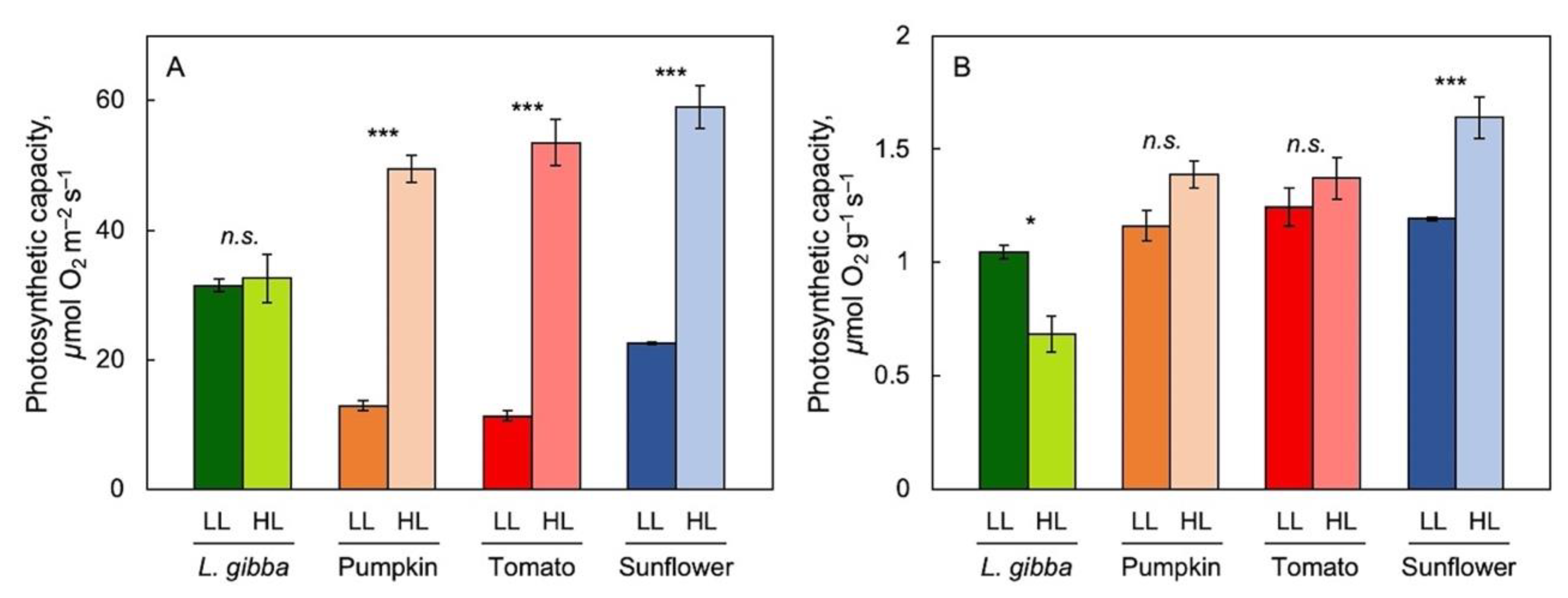
To address the impact of reference basis in revealing species-specific trends in the acclimation to growth light environment, light- and CO2-saturated maximal photosynthetic capacity per area (Figure 2A) and per biomass (Figure 2B) were assessed for fronds of L. gibba in comparison with leaves of three terrestrial species (pumpkin, tomato, and sunflower), all grown under either low (100 μmol photons m–2 s–1) or high (700 μmol photons m–2 s–1 for L. gibba and 750 μmol photons m–2 s–1 for the three land plants) PFD. For all species, biomass per area was significantly higher in plants grown under high versus low PFD. In all three terrestrial species, but not in duckweed, maximal photosynthetic capacity was significantly greater in plants growing under high versus low PFD (Figure 2A). Consequently, maximal photosynthetic capacity expressed on a biomass basis was significantly lower in L. gibba in high versus low growth PFD but was either not significantly different or slightly higher in the three terrestrial species (Figure 2B). The following figures and results focus on the growth-PFD dependence of biomass production and nutritional quality (relative to both biomass and photon input) of duckweed.
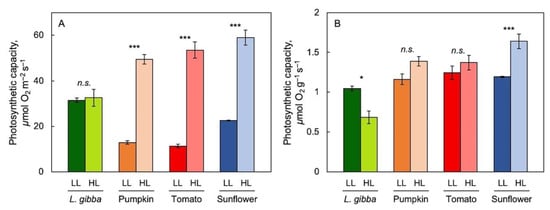
Figure 2.
Light- and CO2-saturated photosynthetic capacity per area (A) as well as per biomass (B) for Lemna gibba (green), pumpkin (orange), tomato (red), and sunflower (blue) grown under low (dark-color columns) and high (light-color columns) PFD. Mean values ± standard errors; n = 3 for all species. Significant differences between PFD conditions are denoted by asterisks *** = p < 0.001; * = p < 0.05; n.s. = not significantly different. Based on recalculation of original data from [55,83].
3.2. Growth Rate, Dry Biomass per Area, and Light-Use Efficiency of Biomass Production as a Function of Growth PFD
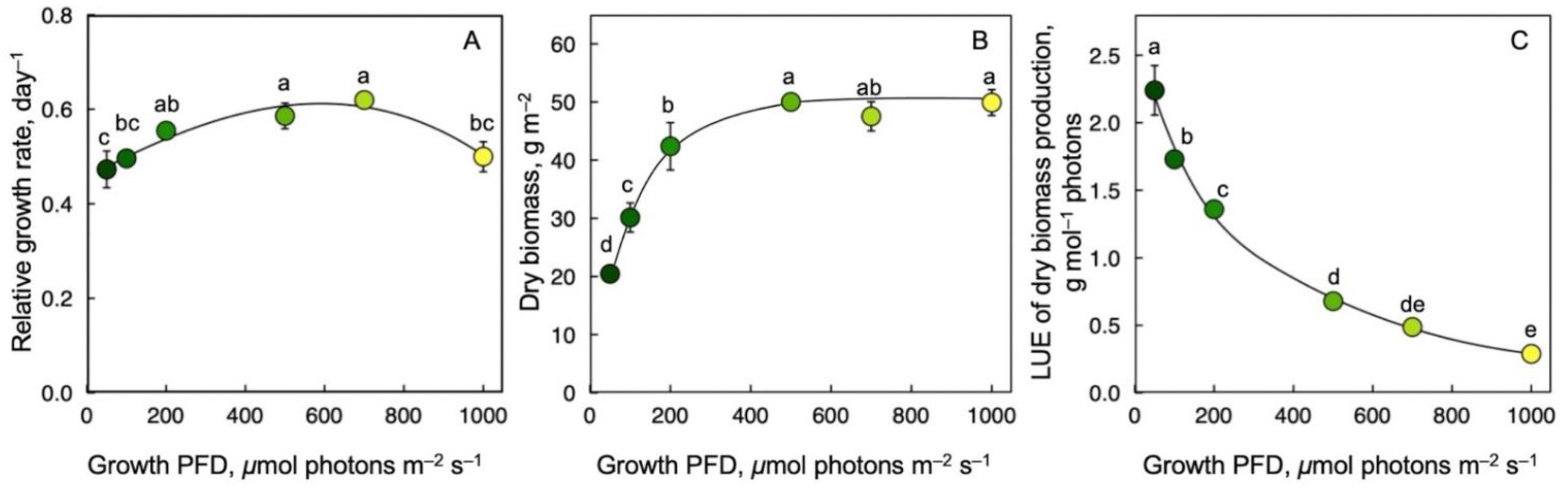
RGR was similar across the range of growth PFD, with a remarkably high RGR even at the lowest growth PFD (Figure 3A). Whereas dry biomass per area doubled between the lowest and the highest growth PFD (Figure 3B), dry biomass production per mol photons received (LUE) of frond dry biomass production exhibited a precipitous decline with increasing growth PFD—by a factor of 20 from lowest to highest intensity (Figure 3C).
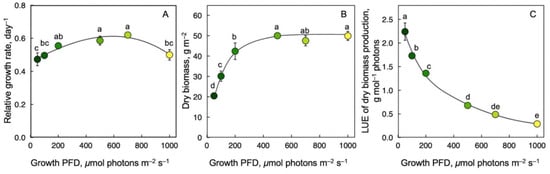
Figure 3.
Relative growth rate, RGR (A), ratio of dry biomass per frond area (B), and light-use efficiency (LUE) of dry biomass production (C) for Lemna gibba grown under a range of seven growth PFDs. Symbol colors from dark green to yellow correspond to frond color under the respective growth PFDs from 50 to 1000 μmol photons m–2 s–1 (see Figure 1). Mean values ± standard deviations; n = 3 for all growth PFDs except for the lowest growth PFD (50 μmol photons m–2 s–1; n = 7). Different lower-case letters represent significant differences at p < 0.05. Data on RGR from [55,59].
3.3. Lutein, β-Carotene, and α-Tocopherol Production on a Dry Biomass Basis and per Photons Received as a Function of Growth PFD
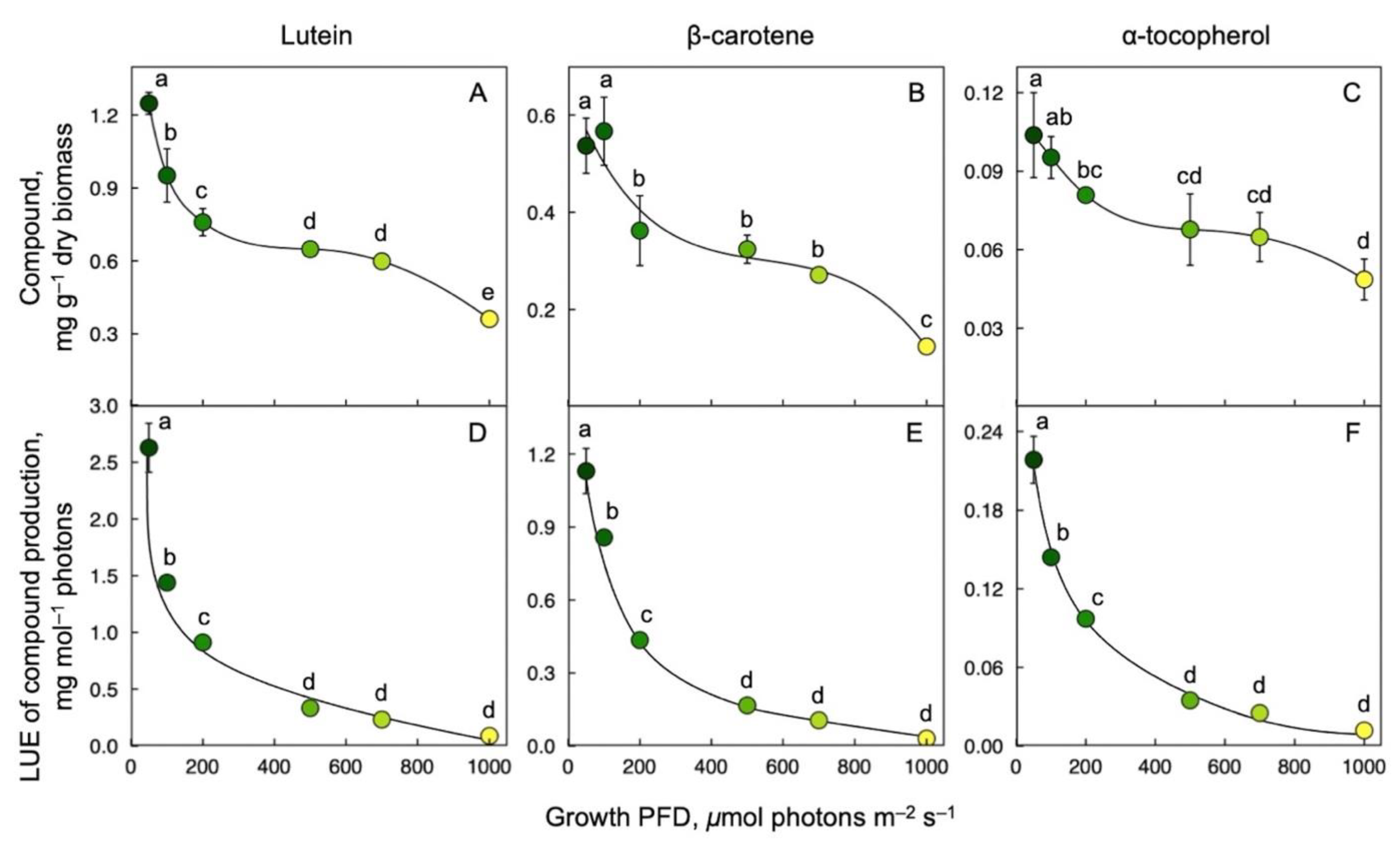
Production of the carotenoids lutein and β-carotene (pro-vitamin A) as well as α-tocopherol (vitamin E) was expressed relative to frond biomass and as nutrient production relative to the amount of light energy used. Lutein (Figure 4A), β-carotene (Figure 4B), and α-tocopherol (Figure 4C) concentration per frond dry biomass and per mol photons received (Figure 4D–F, respectively) were maximal at the lowest growth PFD of 50 μmol photons m–2 s–1 and decreased as growth PFD increased.
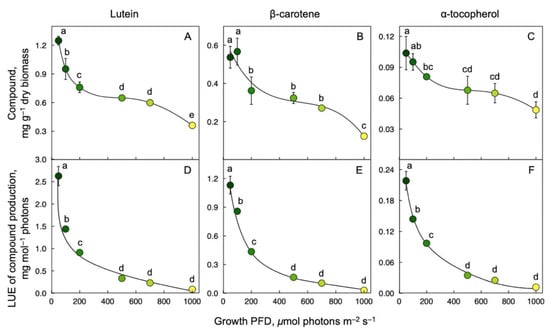
Figure 4.
Production of lutein (A), β-carotene (B), and α-tocopherol (C) per dry biomass, and light-use efficiency (LUE) of lutein (D), β-carotene (E), and α-tocopherol (F) production for fronds grown under a range of growth PFDs. Shades from green to yellow correspond to the respective growth PFDs (see Figure 1). Mean values ± standard deviations, n = 3 for all growth PFDs except for the lowest (50 μmol photons m–2 s–1; n = 3, 4, or 7). Different lower-case letters represent significant differences at p < 0.05. Based on recalculation of original data from [55,59].
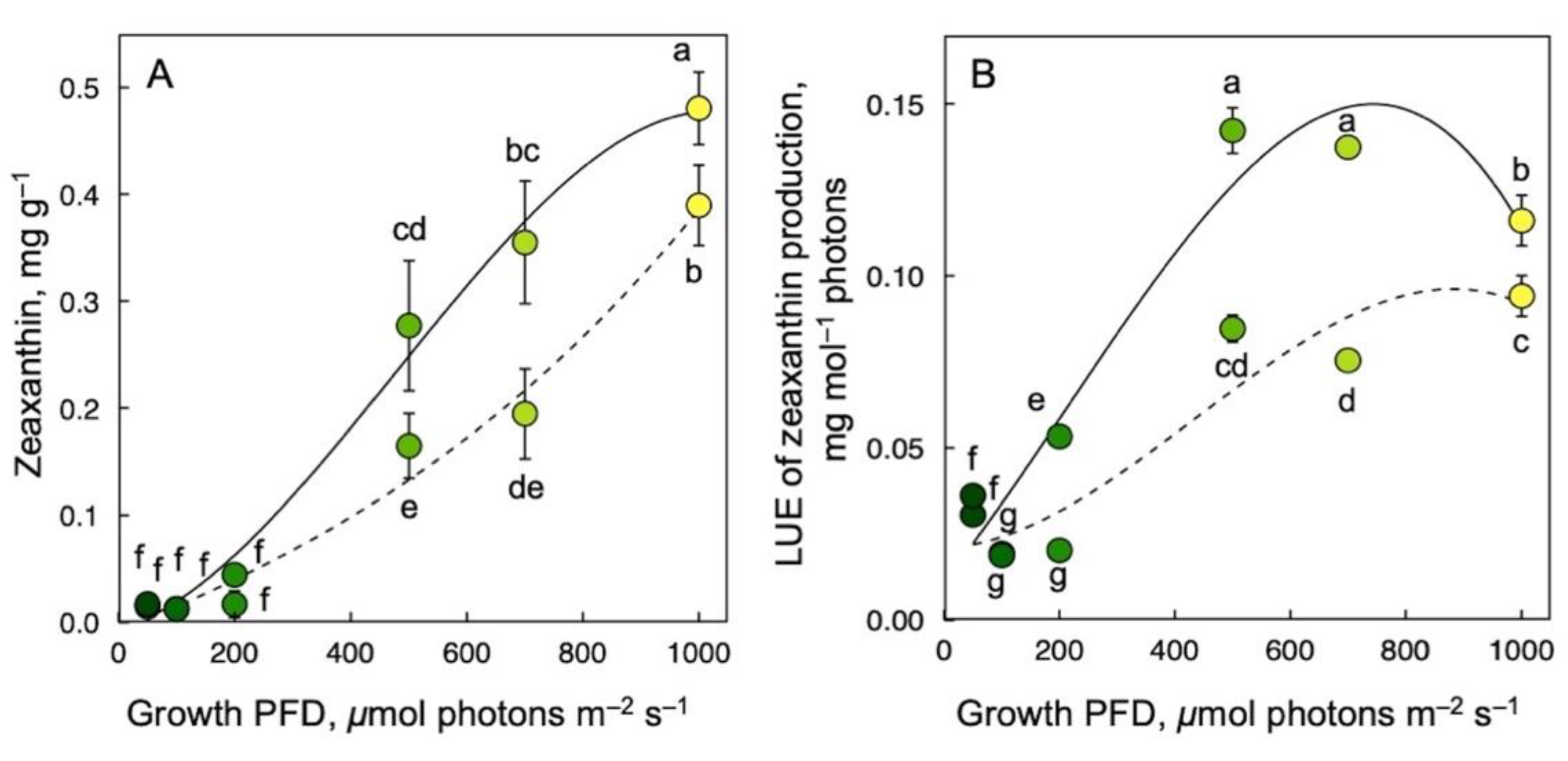
3.4. Zeaxanthin Production on a Dry Biomass Basis and per Photons Received as a Function of Growth PFD
As expected, zeaxanthin production (Figure 5) exhibited a different response to growth PFD than lutein, β-carotene, or α-tocopherol production (Figure 4). For both samples frozen immediately upon harvest (Figure 5; solid lines) and corresponding samples subjected to a 30 min recovery period in low light at room temperature before freezing (Figure 5; dashed lines), zeaxanthin per plant dry biomass increased near-linearly with increasing growth PFD (Figure 5A). After recovery, zeaxanthin concentration per dry biomass was 0.08 mg g–1 lower than immediately after harvest for the highest growth PFD.
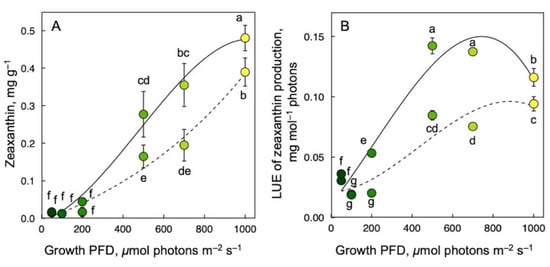
Figure 5.
Zeaxanthin production per dry biomass (A) as well as light-use efficiency of zeaxanthin production (B) immediately upon removal from growth conditions (without recovery; solid lines) or with a 30 min recovery period in low light (dashed lines). Symbol shades from green to yellow correspond to frond color under the respective growth PFDs (see Figure 1). Mean values ± standard deviations, n = 3 for all growth PFDs except for the lowest growth PFD (n = 4 or 7 for 50 μmol photons m–2 s–1). Different lower-case letters represent significant differences at p < 0.05. Based on recalculation of original data for zeaxanthin content at harvest from [55,59] with additional data for zeaxanthin content 30 min post-harvest.
The growth-PFD dependency of LUE of zeaxanthin production was also affected by recovery. LUE of zeaxanthin content as assessed immediately after harvest increased quickly with increasing growth PFD, peaked at 500 µmol photons m–2 s–1, and then declined somewhat as growth PFD increased further, resulting in an arc-shaped response (Figure 5B; solid line). On the other hand, LUE of zeaxanthin content as assessed after recovery increased more steadily and formed a plateau at the higher growth PFDs (Figure 5B; dashed line). As was the case for zeaxanthin concentration on a dry biomass basis, LUE of zeaxanthin production was lower at each respective growth PFD after the recovery period compared to immediately after harvest.
3.5. Variations in Carotenoids and Vitamin E Dynamics
We include here a comparison of the pigment composition of L. gibba exposed to continuous light with L. minor fronds growing in a sun-exposed pond with naturally increasing (peak PFD of 1600 µmol m−2 s−1 at midday) and decreasing PFD. Sun-grown L. minor maintained higher concentrations of lutein, β-carotene, and α-tocopherol, but had lower concentrations of zeaxanthin at midday compared to L. gibba fronds grown under controlled conditions with continuous high PFD (1000 µmol photons m−2 s−1; Table 1). At the same time, the L. gibba plants grown under continuous low PFD (50 µmol photons m−2 s−1) had higher levels of lutein and β-carotene than either L. gibba grown under high continuous PFD under controlled conditions or L. minor grown in full sun outdoors (Table 1). The two compounds that exhibited prominence under high light were zeaxanthin (produced exclusively under either continuous high PFD or in sun-exposed leaves) and α-tocopherol (produced at a particularly high ratio relative to carotenoids in sun-exposed leaves).

Table 1.
Individual compounds (given as concentrations in mg g−1 dry mass) and their ratios (given in g g−1) in fronds of L. gibba grown under PFDs of 50 and 1000 µmol photons m−2 s−1 (continuous light 24 h per day) under controlled conditions or L. minor growing in a natural setting (sun-exposed) in Superior, CO, USA. Based on recalculation of original data from [59].
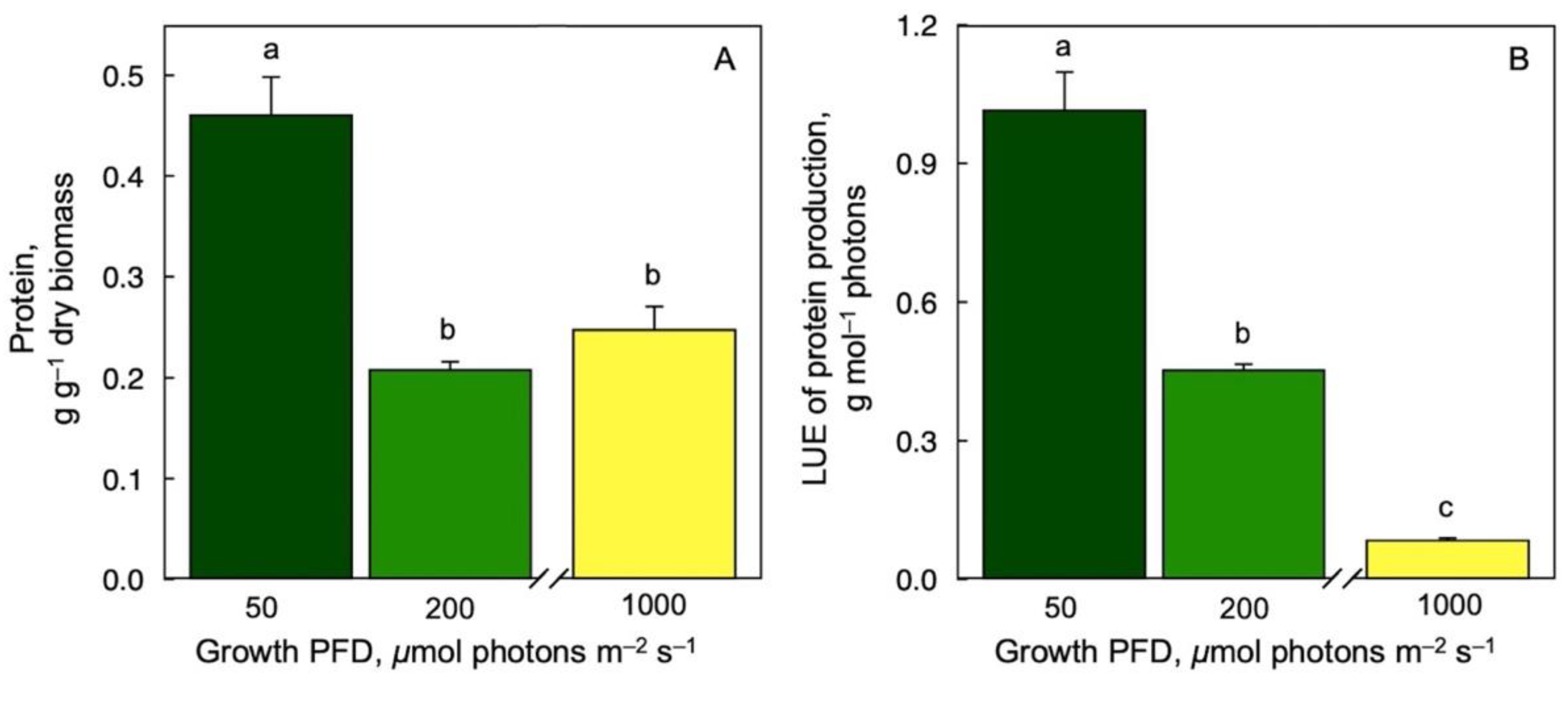
3.6. Protein Production on a Dry Biomass Basis and per Photons Received as a Function of Growth PFD
Protein content per dry biomass (Figure 6A) and per mol photons (Figure 6B) were highest at the lowest growth PFD and lower at higher growth PFDs under controlled growth conditions with continuous light.
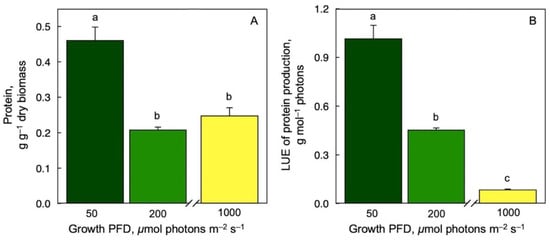
Figure 6.
Protein production per dry biomass (A) as well as light-use efficiency (LUE) of protein production (B) for Lemna gibba grown under three growth PFDs. Symbol colors from green to yellow correspond to frond color under the respective growth PFDs (see Figure 1). Mean values ± standard deviations; n = 4 for (A) and n = 3 (200 and 1000 μmol photons m–2 s–1) or 7 (50 μmol photons m–2 s–1) for (B). Different lower-case letters represent significant differences at p < 0.05. Based on recalculation of original data from [59] with additional data for 200 μmol photons m–2 s–1.
4. Discussion and Recommendations
Plant growth environment affects multiple aspects of plant form and function [85,86,87] and can thus potentially be used to produce desired outcomes with respect to specific individual crop traits. The finding that duckweed featured a remarkably high maximal photosynthetic capacity, as well as a remarkably high relative growth rate, even when grown in a low-light environment may, in part, be explained by the fact that duckweed fronds are thin and non-overlapping. Chloroplasts in these fronds thus likely experience a minimal level of self-shading and contribute to photosynthetic productivity to a greater degree than chloroplasts in leaves of terrestrial plants with multiple palisade layers and a high degree of self-shading that can be exacerbated in tiered plant canopies. Further discussion of the photosynthetic performance and relative growth rate of L. gibba grown under low PFD is presented in the context of protein content. The following sections focus on the nutritional quality of duckweed fronds on a biomass basis (as well as relative to light-energy input) as the most relevant reference bases from the standpoint for nutrient production for the human consumer.
Duckweeds, such as L. gibba, stand out for combining exceptional protein and micronutrient content, a low negative environmental impact, and climate resilience (for recent reviews, see [7,8,57,88]). Plant growth rate expressed as area expansion rate is—as typically observed for Lemnaceae [89,90]—very high and was, furthermore, remarkably independent of growth PFDs over a wide range from 50 to 1000 μmol photons m–2 s–1 [55,59].
Due to a 2.5-fold increase in dry biomass per frond area between 50 and 500 μmol photons m–2 s–1, the decrease in the levels of lutein, β-carotene, α-tocopherol, and protein was even more pronounced on a biomass basis (as reported here) than on a frond area basis (as previously reported in [55]). The associated precipitous decline in LUE of nutrient production demonstrates that nutritional yield relative to the investment of light energy became less and less favorable as growth PFD increased.
In stark contrast, nutritional quality with respect to the essential human micronutrient zeaxanthin increased strongly with increasing growth PFD both relative to the biomass produced and to the light energy used (as LUE of production). This was expected for zeaxanthin because green plant organs produce this carotenoid only when the amount of absorbed light exceeds what can be utilized in photochemistry (for recent reviews, see [8,23]). Because zeaxanthin has a unique role in the removal of excitation energy from chlorophyll (in a process that is protective under excess energy), zeaxanthin presence under low PFD can, conversely, compete with efficient light utilization in photochemistry [8]. Because plants remove zeaxanthin in low light, fronds kept in low light for 30 min post-harvest exhibited some loss of zeaxanthin content across the range of growth PFDs. This also caused LUE to saturate at about 500 μmol photons m–2 s–1, whereas absolute zeaxanthin production per dry biomass continued to increase. In other words, zeaxanthin accumulation was also lower on a biomass versus frond area basis but zeaxanthin levels per biomass nevertheless exhibited a remarkable near-linear increase with increasing growth PFD. The contrasting response of the other human micronutrients, with declines on all reference bases with increasing growth PFD, are explained by plant protective functions across a wide PFD range that were characterized for α-tocopherol [91], lutein [92], and β-carotene [93].
Furthermore, our findings include differences between L. minor growing naturally on a sun-exposed pond versus the closely related L. gibba growing under continuous high growth PFD in environmentally controlled chambers. The higher levels of chlorophyll, lutein, β-carotene, and α-tocopherol in L. minor grown under natural sun-exposed conditions with diurnal changes suggest higher levels of chlorophyll/carotenoid-binding complexes compared to L. gibba grown under continuous very high PFD that had evidently resulted in a strong downregulation of chlorophyll content (and thus chlorophyll/carotenoid-binding complexes). While species-dependent differences cannot be excluded, the protective roles of carotenoids and α-tocopherol, which include removal of chlorophyll-related excess excitation [91,94], should indeed be expected to be more important in the greener sun-grown (L. minor) plants compared to the yellower (L. gibba) plants grown under continuous high PFD. The fact that zeaxanthin exhibited the opposite trend suggests that a considerable portion of zeaxanthin was dissolved in the chloroplast membrane phospholipid bilayer [95,96] in plants grown under continuous high PFD. Such membrane-dissolved zeaxanthin can make an equal contribution to human nutrition without causing removal of light energy from photosynthesis [97]. Our findings suggest that controlled light environments may be more effective at producing a significant zeaxanthin pool not associated with chlorophyll than natural sun exposure.
Our finding of an exceptionally high protein content (45% of dry biomass) specifically under low growth PFD was similar to the unusually high maximal photosynthetic capacity, as well as a remarkably high relative growth rate, under the growth PFD of 100 versus 700 μmol photons m–2 s–1. These findings are consistent with the observation that ribulose bisphosphate carboxylase-oxygenase (RUBISCO)—the carboxylating protein of photosynthesis and the vegetative storage protein in duckweeds—could be fully activated for engagement in photosynthesis in duckweed grown under light-limiting conditions [98]. It is thought that a lowering of leaf protein content in terrestrial plants growing in light-limiting environments is important for lowering metabolic costs of protein turnover in support of shade tolerance. The fact that duckweeds use RUBISCO as their vegetative storage protein (possibly with associated low turnover rates) may allow them to accumulate and maintain biomass high in protein, resulting in an exceptional nutritional quality, even in low-light environments. Conversely, growth under high PFD decreases duckweed nutritional quality, especially on a biomass basis, with respect to not only micronutrients but also protein.
5. Conclusions
These findings reported here can further inform the design of suitable growth protocols that optimize nutritional quality for the human consumer relative to the required light input for plant cultivation in controlled environments, including in locations with extreme climates or high levels of urbanization [1,2]. Duckweed is an attractive candidate for controlled growth environments and limited space, where this diminutive plant can be grown in shallow trays stacked vertically in multiple layers and supplied with lighting from energy-efficient light-emitting diodes [99]. To optimize production of multiple essential human nutrients, a growing procedure would be desirable that includes growth in low/non-excessive light combined with approaches that increase zeaxanthin content via either a sudden pre-harvest increase in growth PFD and/or via engineering of the xanthophyll cycle. Duckweeds provide an attractive mix of carotenoids and polyphenols [4,55,57,60] and duckweed consumption has benefits for human health [100,101,102,103,104,105,106,107]. Future studies of the impact of growth light environment on duckweed’s nutritional quality should combine evaluation of carotenoid, antioxidant, vitamin, and phenolics production in consideration of their synergistic actions. In addition to varying growth PFD as done in the present study, variation of light quality may serve to enhance phenolics content (see [108]) as well as carotenoid and vitamin E content [109].
Author Contributions
B.D.-A., S.K.P., W.W.A.III and J.J.S. conceptualized the manuscript and designed the figures with contributions from M.M.; J.J.S. and M.L.-P. carried out the experiments and biochemical assays with contributions from N.D.G., and M.M.; S.K.P. prepared the figures with input from J.J.S. and B.D.-A.; B.D.-A. and S.K.P. prepared the first draft of the manuscript with contributions from M.M.; J.J.S. and W.W.A.III edited the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by the Translational Research Institute for Space Health through Cooperative Agreement NNX16AO69A, the National Science Foundation award number IOS-1907338, and the University of Colorado.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available from the corresponding author upon reasonable request.
Acknowledgments
We thank Cedric Zeller and Gabrielle Glime for assistance with data collection and Christine Escobar for valuable discussions.
Conflicts of Interest
The authors declare no conflict of interest.
References
- McCartney, L.; Lefsrud, M. Protected Agriculture in Extreme Environments: A Review of Controlled Environment Agriculture in Tropical, Arid, Polar, and Urban Locations. Appl. Eng. Agric. 2018, 34, 455–473. [Google Scholar] [CrossRef]
- Gómez, C.; Currey, C.J.; Dickson, R.W.; Kim, H.-J.; Hernández, R.; Sabeh, N.C.; Raudales, R.E.; Brumfield, R.G.; Laury-Shaw, A.; Wilke, A.K.; et al. Controlled Environment Food Production for Urban Agriculture. HortScience 2019, 54, 1448–1458. [Google Scholar] [CrossRef]
- Cohu, C.M.; Lombardi, E.; Adams, W.W., III; Demmig-Adams, B. Increased Nutritional Quality of Plants for Long-Duration Spaceflight Missions through Choice of Plant Variety and Manipulation of Growth Conditions. Acta Astronaut. 2014, 94, 799–806. [Google Scholar] [CrossRef]
- Polutchko, S.K.; Adams, W.W., III; Escobar, C.M.; Demmig-Adams, B. Conquering Space with Crops That Produce Ample Oxygen and Antioxidants. Oxygen 2022, 2, 211–226. [Google Scholar] [CrossRef]
- Popkin, B.M. The Nutrition Transition: An Overview of World Patterns of Change. Nutr. Rev. 2004, 62, S140–S143. [Google Scholar] [CrossRef]
- Baker, P.; Machado, P.; Santos, T.; Sievert, K.; Backholer, K.; Hadjikakou, M.; Russell, C.; Huse, O.; Bell, C.; Scrinis, G.; et al. Ultra-processed Foods and the Nutrition Transition: Global, Regional and National Trends, Food Systems Transformations and Political Economy Drivers. Obes. Rev. 2020, 21, e13126. [Google Scholar] [CrossRef]
- Myers, S.S.; Zanobetti, A.; Kloog, I.; Huybers, P.; Leakey, A.D.; Bloom, A.J.; Carlisle, E.; Dietterich, L.H.; Fitzgerald, G.; Hasegawa, T.; et al. Increasing CO2 Threatens Human Nutrition. Nature 2014, 510, 139–142. [Google Scholar] [CrossRef]
- Demmig-Adams, B.; López-Pozo, M.; Polutchko, S.K.; Fourounjian, P.; Stewart, J.J.; Zenir, M.C.; Adams, W.W., III. Growth and Nutritional Quality of Lemnaceae Viewed Comparatively in an Ecological and Evolutionary Context. Plants 2022, 11, 145. [Google Scholar] [CrossRef]
- Furman, D.; Campisi, J.; Verdin, E.; Carrera-Bastos, P.; Targ, S.; Franceschi, C.; Ferrucci, L.; Gilroy, D.W.; Fasano, A.; Miller, G.W.; et al. Chronic Inflammation in the Etiology of Disease across the Life Span. Nat. Med. 2019, 25, 1822–1832. [Google Scholar] [CrossRef]
- Schett, G.; Neurath, M.F. Resolution of Chronic Inflammatory Disease: Universal and Tissue-Specific Concepts. Nat. Commun. 2018, 9, 3261. [Google Scholar] [CrossRef]
- Ashraf-Ganjouei, A.; Moradi, K.; Bagheri, S.; Aarabi, M.H. The Association between Systemic Inflammation and Cognitive Performance in Healthy Adults. J. Neuroimmunol. 2020, 345, 577272. [Google Scholar] [CrossRef] [PubMed]
- Vogelzangs, N.; Beekman, A.T.F.; De Jonge, P.; Penninx, B. Anxiety Disorders and Inflammation in a Large Adult Cohort. Transl. Psychiatry 2013, 3, e249. [Google Scholar] [CrossRef] [PubMed]
- Slavich, G.M.; Irwin, M.R. From Stress to Inflammation and Major Depressive Disorder: A Social Signal Transduction Theory of Depression. Psychol. Bull. 2014, 140, 774–815. [Google Scholar] [CrossRef] [PubMed]
- Danesh, J.; Whincup, P.; Walker, M.; Lennon, L.; Thomson, A.; Appleby, P.; Gallimore, J.R.; Pepys, M.B. Low Grade Inflammation and Coronary Heart Disease: Prospective Study and Updated Meta-Analyses. BMJ 2000, 321, 199–204. [Google Scholar] [CrossRef]
- Coussens, L.M.; Werb, Z. Inflammation and Cancer. Nature 2002, 420, 860–867. [Google Scholar] [CrossRef]
- Tsoupras, A.; Lordan, R.; Zabetakis, I. Inflammation, Not Cholesterol, Is a Cause of Chronic Disease. Nutrients 2018, 10, 604. [Google Scholar] [CrossRef]
- Enichen, E.; Adams, R.B.; Demmig-Adams, B. Physical Activity as an Adjunct Treatment for People Living with HIV? Am. J. Lifestyle Med. 2022, 155982762210782. [Google Scholar] [CrossRef]
- Wu, D.; Yang, X.O. TH17 Responses in Cytokine Storm of COVID-19: An Emerging Target of JAK2 Inhibitor Fedratinib. J. Microbiol. Immunol. Infect. 2020, 53, 368–370. [Google Scholar] [CrossRef]
- Enichen, E.; Harvey, C.; Demmig-Adams, B. COVID-19 Spotlights Connections between Disease and Multiple Lifestyle Factors. Am. J. Lifestyle Med. 2022, 15598276221123005. [Google Scholar] [CrossRef]
- Haran, J.P.; Bradley, E.; Zeamer, A.L.; Cincotta, L.; Salive, M.-C.; Dutta, P.; Mutaawe, S.; Anya, O.; Meza-Segura, M.; Moormann, A.M.; et al. Inflammation-Type Dysbiosis of the Oral Microbiome Associates with the Duration of COVID-19 Symptoms and Long COVID. JCI Insight 2021, 6, e152346. [Google Scholar] [CrossRef]
- Evans, R.A.; Leavy, O.C.; Richardson, M.; Elneima, O.; McCauley, H.J.C.; Shikotra, A.; Singapuri, A.; Sereno, M.; Saunders, R.M.; Harris, V.C.; et al. Clinical Characteristics with Inflammation Profiling of Long COVID and Association with 1-Year Recovery Following Hospitalisation in the UK: A Prospective Observational Study. Lancet Respir. Med. 2022, 10, 761–775. [Google Scholar] [CrossRef]
- Polutchko, S.K.; Glime, G.N.; Demmig-Adams, B. Synergistic Action of Membrane-Bound and Water-Soluble Antioxidants in Neuroprotection. Molecules 2021, 26, 5385. [Google Scholar] [CrossRef] [PubMed]
- Demmig-Adams, B.; Polutchko, S.K.; Adams, W.W., III. Structure-Function-Environment Relationship of the Isomers Zeaxanthin and Lutein. Photochem 2022, 2, 308–325. [Google Scholar] [CrossRef]
- Tapiero, H.; Townsend, D.M.; Tew, K.D. The Role of Carotenoids in the Prevention of Human Pathologies. Biomed. Pharmacother. 2004, 58, 100–110. [Google Scholar] [CrossRef]
- Woodall, A.A.; Britton, G.; Jackson, M.J. Carotenoids and Protection of Phospholipids in Solution or in Liposomes against Oxidation by Peroxyl Radicals: Relationship between Carotenoid Structure and Protective Ability. Biochim. Biophys. Acta BBA—Gen. Subj. 1997, 1336, 575–586. [Google Scholar] [CrossRef]
- Wrona, M.; Korytowski, W.; Różanowska, M.; Sarna, T.; Truscott, T.G. Cooperation of Antioxidants in Protection against Photosensitized Oxidation. Free Radic. Biol. Med. 2003, 35, 1319–1329. [Google Scholar] [CrossRef]
- Wrona, M.; Różanowska, M.; Sarna, T. Zeaxanthin in Combination with Ascorbic Acid or α-Tocopherol Protects ARPE-19 Cells against Photosensitized Peroxidation of Lipids. Free Radic. Biol. Med. 2004, 36, 1094–1101. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.M.; Zwart, S.R. Nutritional Biochemistry of Spaceflight. In Advances in Clinical Chemistry; Makowski, G.S., Ed.; Elsevier: Amsterdam, The Netherlands, 2008; Volume 46, pp. 87–130. [Google Scholar] [CrossRef]
- Mocchegiani, E.; Costarelli, L.; Giacconi, R.; Malavolta, M.; Basso, A.; Piacenza, F.; Ostan, R.; Cevenini, E.; Gonos, E.S.; Franceschi, C.; et al. Vitamin E-Gene Interactions in Aging and Inflammatory Age-Related Diseases: Implications for Treatment. A Systematic Review. Ageing Res. Rev. 2014, 14, 81–101. [Google Scholar] [CrossRef]
- Peh, H.Y.; Tan, W.D.; Liao, W.; Wong, W.F. Vitamin E Therapy beyond Cancer: Tocopherol versus Tocotrienol. Pharmacol. Ther. 2016, 162, 152–169. [Google Scholar] [CrossRef]
- Traber, M.G.; Atkinson, J. Vitamin E, Antioxidant and Nothing More. Free Radic. Biol. Med. 2007, 43, 4–15. [Google Scholar] [CrossRef]
- Zareba, M.; Widomska, J.; Burke, J.M.; Subczynski, W.K. Nitroxide Free Radicals Protect Macular Carotenoids against Chemical Destruction (Bleaching) during Lipid Peroxidation. Free Radic. Biol. Med. 2016, 101, 446–454. [Google Scholar] [CrossRef] [PubMed]
- Thomas, L.D.; Bandara, S.; Parmar, V.M.; Srinivasagan, R.; Khadka, N.; Golczak, M.; Kiser, P.D.; von Lintig, J. The Human Mitochondrial Enzyme BCO2 Exhibits Catalytic Activity toward Carotenoids and Apocarotenoids. J. Biol. Chem. 2020, 295, 15553–15565. [Google Scholar] [CrossRef] [PubMed]
- Sayo, T.; Sugiyama, Y.; Inoue, S. Lutein, a Nonprovitamin A, Activates the Retinoic Acid Receptor to Induce HAS3-Dependent Hyaluronan Synthesis in Keratinocytes. Biosci. Biotechnol. Biochem. 2013, 77, 1282–1286. [Google Scholar] [CrossRef] [PubMed]
- Ahn, Y.J.; Kim, H. Lutein as a Modulator of Oxidative Stress-Mediated Inflammatory Diseases. Antioxidants 2021, 10, 1448. [Google Scholar] [CrossRef] [PubMed]
- Uppal, S.; Dergunov, S.A.; Zhang, W.; Gentleman, S.; Redmond, T.M.; Pinkhassik, E.; Poliakov, E. Xanthophylls Modulate Palmitoylation of Mammalian β-Carotene Oxygenase 2. Antioxidants 2021, 10, 413. [Google Scholar] [CrossRef]
- Coppack, S.W. Pro-Inflammatory Cytokines and Adipose Tissue. Proc. Nutr. Soc. 2001, 60, 349–356. [Google Scholar] [CrossRef]
- Trayhurn, P.; Beattie, J.H. Physiological Role of Adipose Tissue: White Adipose Tissue as an Endocrine and Secretory Organ. Proc. Nutr. Soc. 2001, 60, 329–339. [Google Scholar] [CrossRef]
- Liu, M.; Zheng, M.; Cai, D.; Xie, J.; Jin, Z.; Liu, H.; Liu, J. Zeaxanthin Promotes Mitochondrial Biogenesis and Adipocyte Browning via AMPKα1 Activation. Food Funct. 2019, 10, 2221–2233. [Google Scholar] [CrossRef]
- Xie, J.; Liu, M.; Liu, H.; Jin, Z.; Guan, F.; Ge, S.; Yan, J.; Zheng, M.; Cai, D.; Liu, J. Zeaxanthin Ameliorates Obesity by Activating the β3-Adrenergic Receptor to Stimulate Inguinal Fat Thermogenesis and Modulating the Gut Microbiota. Food Funct. 2021, 12, 12734–12750. [Google Scholar] [CrossRef]
- Zhao, B.; Liu, M.; Liu, H.; Xie, J.; Yan, J.; Hou, X.; Liu, J. Zeaxanthin Promotes Browning by Enhancing Mitochondrial Biogenesis through the PKA Pathway in 3T3-L1 Adipocytes. Food Funct. 2021, 12, 6283–6293. [Google Scholar] [CrossRef]
- El Hadi, H.; Di Vincenzo, A.; Vettor, R.; Rossato, M. Food Ingredients Involved in White-to-Brown Adipose Tissue Conversion and in Calorie Burning. Front. Physiol. 2019, 9, 1954. [Google Scholar] [CrossRef] [PubMed]
- Schalch, W.; Landrum, J.T.; Bone, R.A. The eye. In Carotenoids. Nutrition and Health; Britton, G., Pfander, H., Liaaen-Jensen, S., Eds.; Birkhäuser Verlag: Basel, Switzerland, 2009; Volume 5, pp. 301–334. [Google Scholar] [CrossRef]
- Li, B.; George, E.W.; Rognon, G.T.; Gorusupudi, A.; Ranganathan, A.; Chang, F.-Y.; Shi, L.; Frederick, J.M.; Bernstein, P.S. Imaging Lutein and Zeaxanthin in the Human Retina with Confocal Resonance Raman Microscopy. Proc. Natl. Acad. Sci. USA 2020, 117, 12352–12358. [Google Scholar] [CrossRef] [PubMed]
- Krinsky, N.I.; Landrum, J.T.; Bone, R.A. Biologic Mechanisms of the Protective Role of Lutein and Zeaxanthin in the Eye. Annu. Rev. Nutr. 2003, 23, 171–201. [Google Scholar] [CrossRef] [PubMed]
- Richer, S.P.; Stiles, W.; Graham-Hoffman, K.; Levin, M.; Ruskin, D.; Wrobel, J.; Park, D.-W.; Thomas, C. Randomized, Double-Blind, Placebo-Controlled Study of Zeaxanthin and Visual Function in Patients with Atrophic Age-Related Macular Degeneration: The Zeaxanthin and Visual Function Study (ZVF) FDA IND# 78, 973. Optom.-J. Am. Optom. Assoc. 2011, 82, 667–680. [Google Scholar] [CrossRef]
- Zaragoza-Huesca, D.; Martínez-Cortés, C.; Banegas-Luna, A.J.; Pérez-Garrido, A.; Vegara-Meseguer, J.M.; Peñas-Martínez, J.; Rodenas, M.C.; Espín, S.; Pérez-Sánchez, H.; Martínez-Martínez, I. Identification of Kukoamine A, Zeaxanthin, and Clexane as New Furin Inhibitors. Int. J. Mol. Sci. 2022, 23, 2796. [Google Scholar] [CrossRef]
- Bestle, D.; Heindl, M.R.; Limburg, H.; Pilgram, O.; Moulton, H.; Stein, D.A.; Hardes, K.; Eickmann, M.; Dolnik, O.; Rohde, C.; et al. TMPRSS2 and Furin Are Both Essential for Proteolytic Activation of SARS-CoV-2 in Human Airway Cells. Life Sci. Alliance 2020, 3, e202000786. [Google Scholar] [CrossRef]
- Demmig-Adams, B.; Adams, W.W., III. Overview of Diet-Gene Interaction and the Example of Xanthophylls. Adv. Exp. Med. Biol. 2010, 698, 17–26. [Google Scholar] [CrossRef]
- Demmig-Adams, B.; Stewart, J.J.; López-Pozo, M.; Polutchko, S.K.; Adams, W.W., III. Zeaxanthin, a Molecule for Photoprotection in Many Different Environments. Molecules 2020, 25, 5825. [Google Scholar] [CrossRef]
- Mares-Perlman, J.A.; Millen, A.E.; Ficek, T.L.; Hankinson, S.E. The Body of Evidence to Support a Protective Role for Lutein and Zeaxanthin in Delaying Chronic Disease. Overview. J. Nutr. 2002, 132, 518S–524S. [Google Scholar] [CrossRef]
- Ribaya-Mercado, J.D.; Blumberg, J.B. Lutein and Zeaxanthin and Their Potential Roles in Disease Prevention. J. Am. Coll. Nutr. 2004, 23, 567S–587S. [Google Scholar] [CrossRef]
- Bhanthumnavin, K.; McGarry, M.G. Wolffia arrhiza as a Possible Source of Inexpensive Protein. Nature 1971, 232, 495. [Google Scholar] [CrossRef] [PubMed]
- De Beukelaar, M.F.; Zeinstra, G.G.; Mes, J.J.; Fischer, A.R. Duckweed as Human Food. The Influence of Meal Context and Information on Duckweed Acceptability of Dutch Consumers. Food Qual. Prefer. 2019, 71, 76–86. [Google Scholar] [CrossRef]
- Stewart, J.J.; Adams, W.W., III; Escobar, C.M.; López-Pozo, M.; Demmig-Adams, B. Growth and Essential Carotenoid Micronutrients in Lemna gibba as a Function of Growth Light Intensity. Front. Plant Sci. 2020, 11, 480. [Google Scholar] [CrossRef]
- Appenroth, K.-J.; Sree, K.S.; Bog, M.; Ecker, J.; Seeliger, C.; Böhm, V.; Lorkowski, S.; Sommer, K.; Vetter, W.; Tolzin-Banasch, K.; et al. Nutritional Value of the Duckweed Species of the Genus Wolffia (Lemnaceae) as Human Food. Front. Chem. 2018, 6, 483. [Google Scholar] [CrossRef] [PubMed]
- Appenroth, K.-J.; Sree, K.S.; Böhm, V.; Hammann, S.; Vetter, W.; Leiterer, M.; Jahreis, G. Nutritional Value of Duckweeds (Lemnaceae) as Human Food. Food Chem. 2017, 217, 266–273. [Google Scholar] [CrossRef] [PubMed]
- Sree, K.S.; Dahse, H.-M.; Chandran, J.N.; Schneider, B.; Jahreis, G.; Appenroth, K.J. Duckweed for Human Nutrition: No Cytotoxic and No Anti-Proliferative Effects on Human Cell Lines. Plant Foods Hum. Nutr. 2019, 74, 223–224. [Google Scholar] [CrossRef] [PubMed]
- Stewart, J.J.; Adams, W.W., III; López-Pozo, M.; Doherty Garcia, N.; McNamara, M.; Escobar, C.M.; Demmig-Adams, B. Features of the Duckweed Lemna That Support Rapid Growth under Extremes of Light Intensity. Cells 2021, 10, 1481. [Google Scholar] [CrossRef]
- Yaskolka Meir, A.; Tuohy, K.; von Bergen, M.; Krajmalnik-Brown, R.; Heinig, U.; Zelicha, H.; Tsaban, G.; Rinott, E.; Kaplan, A.; Aharoni, A.; et al. The Metabolomic-Gut-Clinical Axis of Mankai Plant-Derived Dietary Polyphenols. Nutrients 2021, 13, 1866. [Google Scholar] [CrossRef]
- Appenroth, K.-J.; Augsten, H.; Liebermann, B.; Feist, H. Effects of Light Quality on Amino Acid Composition of Proteins in Wolffia arrhiza (L.) Wimm. Using a Specially Modified Bradford Method. Biochem. Physiol. Pflanz. 1982, 177, 251–258. [Google Scholar] [CrossRef]
- Skillicorn, P.; Spira, W.; Journey, W. Duckweed Aquaculture: A New Aquatic Farming System for Developing Countries; The World Bank: Washington, DC, USA, 1993. [Google Scholar]
- Mohedano, R.A.; Costa, R.H.; Tavares, F.A.; Belli Filho, P. High Nutrient Removal Rate from Swine Wastes and Protein Biomass Production by Full-Scale Duckweed Ponds. Bioresour. Technol. 2012, 112, 98–104. [Google Scholar] [CrossRef]
- Oron, G.; Wildschut, L.; Porath, D. Waste Water Recycling by Duckweed for Protein Production and Effluent Renovation. Water Sci. Technol. 1985, 17, 803–817. [Google Scholar] [CrossRef]
- Oron, G.; Porath, D.; Jansen, H. Performance of the Duckweed Species Lemna gibba on Municipal Wastewater for Effluent Renovation and Protein Production. Biotechnol. Bioeng. 1987, 29, 258–268. [Google Scholar] [CrossRef] [PubMed]
- Oron, G. Duckweed Culture for Wastewater Renovation and Biomass Production. Agric. Water Manag. 1994, 26, 27–40. [Google Scholar] [CrossRef]
- Elshafai, S.; Elgohary, F.; Nasr, F.; Petervandersteen, N.; Gijzen, H. Nutrient Recovery from Domestic Wastewater Using a UASB-Duckweed Ponds System. Bioresour. Technol. 2007, 98, 798–807. [Google Scholar] [CrossRef] [PubMed]
- Simopoulos, A.P. Omega-3 Fatty Acids and Cancer. Indoor Built Environ. 2003, 12, 405–412. [Google Scholar] [CrossRef]
- Simopoulos, A.P. Omega-6/Omega-3 Essential Fatty Acid Ratio and Chronic Diseases. Food Rev. Int. 2004, 20, 77–90. [Google Scholar] [CrossRef]
- Simopoulos, A.P. The Omega-6/Omega-3 Fatty Acid Ratio, Genetic Variation, and Cardiovascular Disease. Asia Pac. J. Clin. Nutr. 2008, 17, 131–134. [Google Scholar]
- Calder, P.C.; Ahluwalia, N.; Brouns, F.; Buetler, T.; Clement, K.; Cunningham, K.; Esposito, K.; Jönsson, L.S.; Kolb, H.; Lansink, M.; et al. Dietary Factors and Low-Grade Inflammation in Relation to Overweight and Obesity. Br. J. Nutr. 2011, 106, S1–S78. [Google Scholar] [CrossRef]
- Saini, R.K.; Keum, Y.-S. Omega-3 and Omega-6 Polyunsaturated Fatty Acids: Dietary Sources, Metabolism, and Significance—A Review. Life Sci. 2018, 203, 255–267. [Google Scholar] [CrossRef]
- Innes, J.K.; Calder, P.C. Omega-6 Fatty Acids and Inflammation. Prostaglandins Leukot. Essent. Fatty Acids 2018, 132, 41–48. [Google Scholar] [CrossRef]
- Andersen, I.H.; Dons, C.; Nilsen, S.; Haugstad, M.K. Growth, Photosynthesis and Photorespiration of Lemna gibba: Response to Variations in CO2 and O2 Concentrations and Photon Flux Density. Photosynth. Res. 1985, 6, 87–96. [Google Scholar] [CrossRef] [PubMed]
- Mohedano, R.A.; Costa, R.H.R.; Filho, P.B. Effects of CO2 Concentration on Nutrient Uptake and Starch Accumulation by Duckweed Used for Wastewater Treatment and Bioethanol Production. Rev. Latinoam. Biotecnol. Ambient. Algal 2016, 7, 3. [Google Scholar] [CrossRef]
- Kittiwongwattana, C.; Thawai, C. Rhizobium lemnae Sp. Nov., a Bacterial Endophyte of Lemna aequinoctialis. Int. J. Syst. Evol. Microbiol. 2014, 64, 2455–2460. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Haberer, G.; Gundlach, H.; Gläßer, C.; Nussbaumer, T.; Luo, M.C.; Lomsadze, A.; Borodovsky, M.; Kerstetter, R.A.; Shanklin, J. The Spirodela Polyrhiza Genome Reveals Insights into Its Neotenous Reduction Fast Growth and Aquatic Lifestyle. Nat. Commun. 2014, 5, 3311. [Google Scholar] [CrossRef]
- Zahran, H.H. Rhizobium-Legume Symbiosis and Nitrogen Fixation under Severe Conditions and in an Arid Climate. Microbiol. Mol. Biol. Rev. 1999, 63, 968–989. [Google Scholar] [CrossRef]
- Zhou, Y.; Kishchenko, O.; Stepanenko, A.; Chen, G.; Wang, W.; Zhou, J.; Pan, C.; Borisjuk, N. The Dynamics of NO3− and NH4+ Uptake in Duckweed Are Coordinated with the Expression of Major Nitrogen Assimilation Genes. Plants 2021, 11, 11. [Google Scholar] [CrossRef]
- Hammouda, O.; Gaber, A.; Abdel-Hameed, M.S. Assessment of the Effectiveness of Treatment of Wastewater-Contaminated Aquatic Systems with Lemna gibba. Enzyme Microb. Technol. 1995, 17, 317–323. [Google Scholar] [CrossRef]
- Ozengin, N.; Elmaci, A. Performance of Duckweed (Lemna minor L.) on Different Types of Wastewater Treatment. J. Environ. Biol. 2007, 28, 307–314. [Google Scholar]
- Panfili, I.; Bartucca, M.L.; Del Buono, D. The Treatment of Duckweed with a Plant Biostimulant or a Safener Improves the Plant Capacity to Clean Water Polluted by Terbuthylazine. Sci. Total Environ. 2019, 646, 832–840. [Google Scholar] [CrossRef]
- Polutchko, S.K.; Stewart, J.J.; Adams, W.W., III; Demmig-Adams, B. Photosynthesis and Foliar Vascular Adjustments to Growth Light Intensity in Summer Annual Species with Symplastic and Apoplastic Phloem Loading. J. Plant Physiol. 2021, 267, 153532. [Google Scholar] [CrossRef]
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis; Springer: Cham, Switzerland, 2016. [Google Scholar] [CrossRef]
- Adams, W.W., III; Stewart, J.J.; Demmig-Adams, B. Photosynthetic Modulation in Response to Plant Activity and Environment. In The Leaf: A Platform for Performing Photosynthesis, Advances in Photosynthesis and Respiration; Adams, W.W., III, Terashima, I., Eds.; Springer: Cham, Switzerland, 2018; Volume 44, pp. 493–563. [Google Scholar] [CrossRef]
- Adams, W.W., III; Stewart, J.J.; Polutchko, S.K.; Demmig-Adams, B. Leaf Vasculature and the Upper Limit of Photosynthesis. In The Leaf: A Platform for Performing Photosynthesis, Advances in Photosynthesis and Respiration; Adams, W.W., III, Terashima, I., Eds.; Springer: Cham, Switzerland, 2018; Volume 44, pp. 27–54. [Google Scholar] [CrossRef]
- Dusenge, M.E.; Duarte, A.G.; Way, D.A. Plant Carbon Metabolism and Climate Change: Elevated CO2 and Temperature Impacts on Photosynthesis, Photorespiration and Respiration. New Phytol. 2019, 221, 32–49. [Google Scholar] [CrossRef] [PubMed]
- Acosta, K.; Appenroth, K.J.; Borisjuk, L.; Edelman, M.; Heinig, U.; Jansen, M.A.; Oyama, T.; Pasaribu, B.; Schubert, I.; Sorrels, S.; et al. Return of the Lemnaceae: Duckweed as a Model Plant System in the Genomics and Postgenomics Era. Plant Cell 2021, 33, 3207–3234. [Google Scholar] [CrossRef] [PubMed]
- Sree, K.S.; Sudakaran, S.; Appenroth, K.-J. How Fast Can Angiosperms Grow? Species and Clonal Diversity of Growth Rates in the Genus Wolffia (Lemnaceae). Acta Physiol. Plant. 2015, 37, 204. [Google Scholar] [CrossRef]
- Ziegler, P.; Adelmann, K.; Zimmer, S.; Schmidt, C.; Appenroth, K.-J. Relative in Vitro Growth Rates of Duckweeds (Lemnaceae)—The Most Rapidly Growing Higher Plants. Plant Biol. 2015, 17, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Munné-Bosch, S.; Alegre, L. The Function of Tocopherols and Tocotrienols in Plants. Crit. Rev. Plant Sci. 2002, 21, 31–57. [Google Scholar] [CrossRef]
- Dall’Osto, L.; Lico, C.; Alric, J.; Giuliano, G.; Havaux, M.; Bassi, R. Lutein Is Needed for Efficient Chlorophyll Triplet Quenching in the Major LHCII Antenna Complex of Higher Plants and Effective Photoprotection in Vivo under Strong Light. BMC Plant Biol. 2006, 6, 32. [Google Scholar] [CrossRef]
- Cazzaniga, S.; Li, Z.; Niyogi, K.K.; Bassi, R.; Dall’Osto, L. The Arabidopsis szl1 Mutant Reveals a Critical Role of β-Carotene in Photosystem I Photoprotection. Plant Physiol. 2012, 159, 1745–1758. [Google Scholar] [CrossRef]
- Krieger-Liszkay, A.; Fufezan, C.; Trebst, A. Singlet Oxygen Production in Photosystem II and Related Protection Mechanism. Photosynth. Res. 2008, 98, 551–564. [Google Scholar] [CrossRef]
- Havaux, M.; Niyogi, K.K. The violaxanthin cycle protects plants from photooxidative damage by more than one mechanism. Proc. Natl. Acad. Sci. USA 1999, 96, 8762–8767. [Google Scholar] [CrossRef]
- Havaux, M.; García-Plazaola, J.I. Beyond Non-Photochemical Fluorescence Quenching: The Overlapping Antioxidant Functions of Zeaxanthin and Tocopherols. In Non-Photochemical Quenching and Energy Dissipation in Plants, Algae and Cyanobacteria; Demmig-Adams, B., Garab, G., Adams, W.W., III, Govindjee, Eds.; Springer: Dordrecht, The Netherlands, 2014; Volume 40, pp. 583–603. [Google Scholar] [CrossRef]
- Davison, P.A.; Hunter, C.N.; Horton, P. Overexpression of β-carotene hydroxylase enhances stress tolerance in Arabidopsis. Nature 2002, 418, 203–206. [Google Scholar] [CrossRef]
- Martindale, W.; Bowes, G. The effects of irradiance and CO2 on the activity and activation of ribulose-1,5-bisphosphate carboxylase/oxygenase in the aquatic plant Spirodela polyrhiza. J. Exp. Bot. 1996, 47, 781–784. [Google Scholar] [CrossRef]
- Escobar, C.M.; Escobar, A.C.; Power, G.J.; Nabity, J.A. µG-LilyPondTM: Preliminary Design of a Floating Plant Pond for Microgravity. In Proceedings of the 50th International Conference on Environmental Systems, Lisbon, Portugal, 12–16 July 2020. [Google Scholar]
- Rinott, E.; Youngster, I.; Meir, A.Y.; Tsaban, G.; Zelicha, H.; Kaplan, A.; Knights, D.; Tuohy, K.; Fava, F.; Scholz, M.U.; et al. Effects of Diet-Modulated Autologous Fecal Microbiota Transplantation on Weight Regain. Gastroenterology 2021, 160, 158–173. [Google Scholar] [CrossRef]
- Rinott, E.; Meir, A.Y.; Tsaban, G.; Zelicha, H.; Kaplan, A.; Knights, D.; Tuohy, K.; Scholz, M.U.; Koren, O.; Stampfer, M.J.; et al. The Effects of the Green-Mediterranean Diet on Cardiometabolic Health Are Linked to Gut Microbiome Modifications: A Randomized Controlled Trial. Genome Med. 2022, 14, 29. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, A.; Zelicha, H.; Yaskolka Meir, A.; Rinott, E.; Tsaban, G.; Levakov, G.; Prager, O.; Salti, M.; Yovell, Y.; Ofer, J.; et al. The Effect of a High-Polyphenol Mediterranean Diet (Green-MED) Combined with Physical Activity on Age-Related Brain Atrophy: The Dietary Intervention Randomized Controlled Trial Polyphenols Unprocessed Study (DIRECT PLUS). Am. J. Clin. Nutr. 2022, 115, 1270–1281. [Google Scholar] [CrossRef] [PubMed]
- Sela, I.; Yaskolka Meir, A.; Brandis, A.; Krajmalnik-Brown, R.; Zeibich, L.; Chang, D.; Dirks, B.; Tsaban, G.; Kaplan, A.; Rinott, E.; et al. Wolffia globosa–Mankai Plant-Based Protein Contains Bioactive Vitamin B12 and Is Well Absorbed in Humans. Nutrients 2020, 12, 3067. [Google Scholar] [CrossRef]
- Tsaban, G.; Meir, A.Y.; Rinott, E.; Zelicha, H.; Kaplan, A.; Shalev, A.; Katz, A.; Rudich, A.; Tirosh, A.; Shelef, I.; et al. The Effect of Green Mediterranean Diet on Cardiometabolic Risk; a Randomised Controlled Trial. Heart 2020, 107, 1054–1061. [Google Scholar] [CrossRef]
- Tsaban, G.; Yaskolka Meir, A.; Zelicha, H.; Rinott, E.; Kaplan, A.; Shalev, A.; Katz, A.; Brikner, D.; Blüher, M.; Ceglarek, U.; et al. Diet-Induced Fasting Ghrelin Elevation Reflects the Recovery of Insulin Sensitivity and Visceral Adiposity Regression. J. Clin. Endocrinol. Metab. 2022, 107, 336–345. [Google Scholar] [CrossRef]
- Zelicha, H.; Kaplan, A.; Yaskolka Meir, A.; Tsaban, G.; Rinott, E.; Shelef, I.; Tirosh, A.; Brikner, D.; Pupkin, E.; Qi, L.; et al. The Effect of Wolffia globosa Mankai, a Green Aquatic Plant, on Postprandial Glycemic Response: A Randomized Crossover Controlled Trial. Diabetes Care 2019, 42, 1162–1169. [Google Scholar] [CrossRef]
- Yaskolka Meir, A.; Rinott, E.; Tsaban, G.; Zelicha, H.; Kaplan, A.; Rosen, P.; Shelef, I.; Youngster, I.; Shalev, A.; Blüher, M.; et al. Effect of Green-Mediterranean Diet on Intrahepatic Fat: The DIRECT PLUS Randomised Controlled Trial. Gut 2021, 70, 2085–2095. [Google Scholar] [CrossRef]
- Taulavuori, K.; Pyysalo, A.; Taulavuori, E.; Julkunen-Tiitto, R. Responses of Phenolic Acid and Flavonoid Synthesis to Blue and Blue-Violet Light Depends on Plant Species. Environ. Exp. Bot. 2018, 150, 183–187. [Google Scholar] [CrossRef]
- Samuolienė, G.; Viršilė, A.; Brazaitytė, A.; Jankauskienė, J.; Sakalauskienė, S.; Vaštakaitė, V.; Novičkovas, A.; Viškelienė, A.; Sasnauskas, A.; Duchovskis, P. Blue Light Dosage Affects Carotenoids and Tocopherols in Microgreens. Food Chem. 2017, 228, 50–56. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).