Arctium lappa Lam. and Its Related Lignans Improve Hyperglycemia and Dyslipidemia in Diabetic Rodent Models: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Sources and Search Strategies
2.2. Inclusion and Exclusion Criteria
2.3. Data Extraction and Quality Assessment
2.4. Data Synthesis and Analysis
3. Results
3.1. Search Results
3.2. Study Characteristics and Quality Assessment
3.3. A. lappa Reduces BG Levels in DM Rodent Models
3.3.1. Forest Plot Analysis
3.3.2. Subgroup and Meta-Regression Analyses
3.3.3. Forest Plot, Subgroup, and Meta-Regression Analyses in Chemically Induced DM Rodents
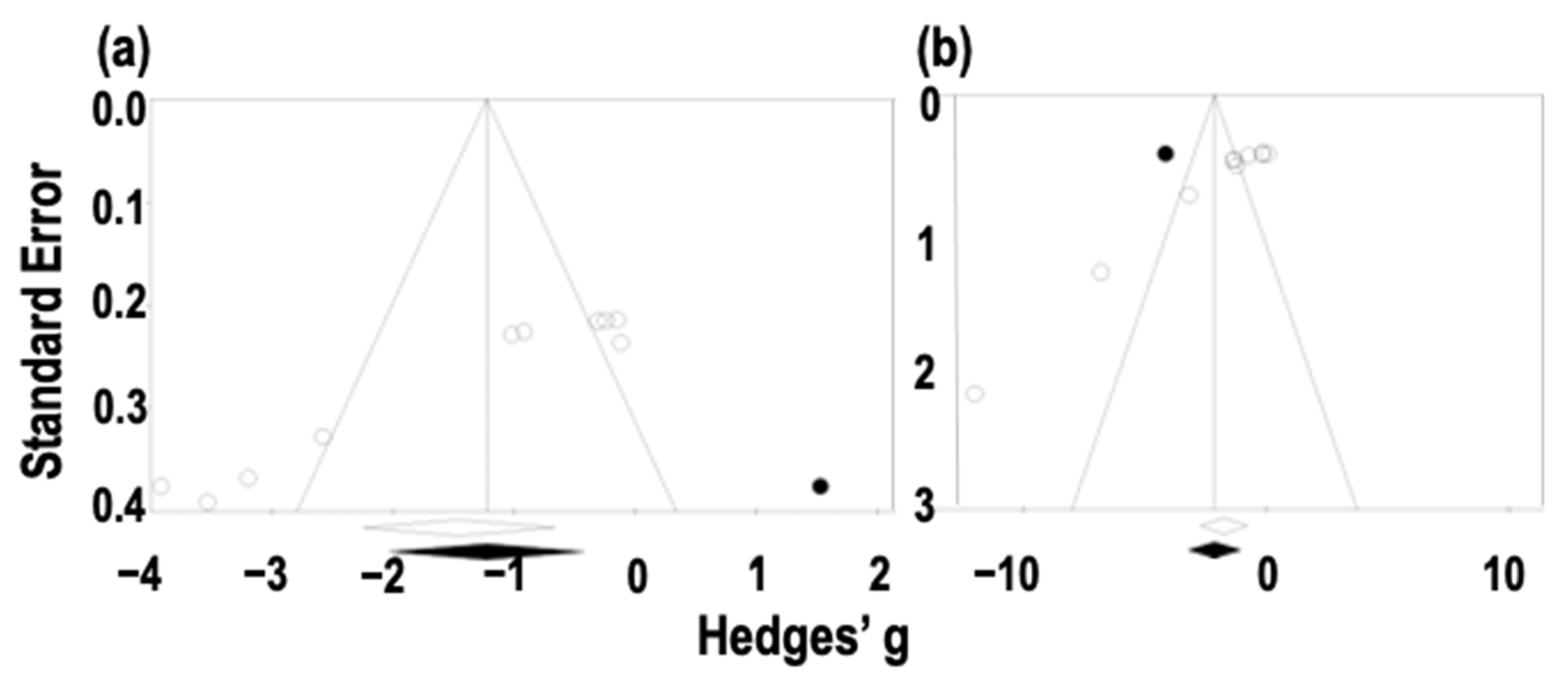
3.3.4. Assessment of Publication Bias
3.4. A. lappa Improved Serum/Plasma Lipid Levels in DM Rodent Models
3.4.1. Forest Plot Analysis
3.4.2. Subgroup and Meta-Regression Analyses
3.4.3. Forest Plot, Subgroup, and Meta-Regression Analyses in Chemically Induced DM Rodents
3.4.4. Assessment of Publication Bias
4. Discussion
4.1. Main Findings
4.2. Data Interpretation
4.2.1. Underlying Mechanisms by Which the Bioactive Compounds of A. lappa Combat DM
- (i)
- Inhibitory effects on α-glucosidase activities:
- (ii)
- Reduced glucagon expression and increased insulin secretion in pancreas:
- (iii)
- Enhanced glucose uptake in skeletal muscles:
- (iv)
- Suppression of gluconeogenesis and lipid synthesis in the liver:
- (v)
- Modulation of adiponectin levels:
- (vi)
- Reduced glucose absorption from intestine:
4.2.2. Strength and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Kalra, E.K. Nutraceutical—Definition and introduction. AAPS Pharm. Sci. 2003, 5, 27–28. [Google Scholar] [CrossRef] [PubMed]
- Brower, V. Nutraceuticals: Poised for a healthy slice of the healthcare market? Nat. Biotechnol. 1998, 16, 728–731. [Google Scholar] [CrossRef]
- Chan, Y.-S.; Cheng, L.-N.; Wu, J.-H.; Chan, E.; Kwan, Y.W.; Lee, S.M.-Y.; Leung, G.P.-H.; Yu, P.H.-F.; Chan, S.-W. A review of the pharmacological effects of Arctium lappa (burdock). Inflammopharmacology 2011, 19, 245–254. [Google Scholar] [CrossRef] [PubMed]
- Ferracane, R.; Graziani, G.; Gallo, M.; Fogliano, V.; Ritieni, A. Metabolic profile of the bioactive compounds of burdock (Arctium lappa) seeds, roots and leaves. J. Pharm. Biomed. Anal. 2010, 51, 399–404. [Google Scholar] [CrossRef] [PubMed]
- United States Department of Agriculture (USDA). Arctium lappa L. Available online: https://npgsweb.ars-grin.gov/gringlobal/taxon/taxonomydetail?id=3857 (accessed on 25 September 2022).
- Hyam, S.R.; Lee, I.-A.; Gu, W.; Kim, K.-A.; Jeong, J.-J.; Jang, S.-E.; Han, M.J.; Kim, D.-H. Arctigenin ameliorates inflammation in vitro and in vivo by inhibiting the PI3K/AKT pathway and polarizing M1 macrophages to M2-like macrophages. Eur. J. Pharmacol. 2013, 708, 21–29. [Google Scholar] [CrossRef]
- Lee, Y.J.; Choi, D.H.; Cho, G.H.; Kim, J.S.; Kang, D.G.; Lee, H.S. Arctium lappa ameliorates endothelial dysfunction in rats fed with high fat/cholesterol diets. BMC Complement. Altern. Med. 2012, 12, 116. [Google Scholar] [CrossRef]
- Pereira, J.V.; Bergamo, D.C.B.; Pereira, J.O.; Franca, S.; Pietro, R.C.L.R.; Silva-Sousa, Y.T.C. Antimicrobial activity of Arctium lappa constituents against microorganisms commonly found in endodontic infections. Braz. Dent. J. 2005, 16, 192–196. [Google Scholar] [CrossRef]
- Moro, T.M.; Clerici, M.T. Burdock (Arctium lappa L.) roots as a source of inulin-type fructans and other bioactive compounds: Current knowledge and future perspectives for food and non-food applications. Food Res. Int. 2021, 141, 109889. [Google Scholar] [CrossRef]
- Li, L.; Su, C.; Chen, X.; Wang, Q.; Jiao, W.; Luo, H.; Tang, J.; Wang, W.; Li, S.; Guo, S. Chlorogenic Acids in Cardiovascular Disease: A Review of Dietary Consumption, Pharmacology, and Pharmacokinetics. J. Agric. Food Chem. 2020, 68, 6464–6484. [Google Scholar] [CrossRef]
- Faraji, H. Effect of decaffeinated coffee-enriched chlorogenic acid on blood glucose levels in healthy controls: A systematic review. Int. J. Prev. Med. 2018, 9, 112. [Google Scholar] [CrossRef]
- Cui, Z.; Zhao, X.; Amevor, F.K.; Du, X.; Wang, Y.; Li, D.; Shu, G.; Tian, Y.; Zhao, X. Therapeutic application of quercetin in aging-related diseases: SIRT1 as a potential mechanism. Front. Immunol. 2022, 13, 943321. [Google Scholar] [CrossRef] [PubMed]
- International Diabetes Federation. IDF Diabetes Atlas, 10th ed. 2021. Available online: https://www.diabetesatlas.org (accessed on 3 May 2021).
- Chen, M.; Xu, J.; Wang, Y.; Wang, Z.; Guo, L.; Li, X.; Huang, L. Arctium lappa L. polysaccharide can regulate lipid metabolism in type 2 diabetic rats through the SREBP-1/SCD-1 axis. Carbohydr. Res. 2020, 494, 108055. [Google Scholar] [CrossRef] [PubMed]
- Vengerovskii, A.I.; Yakimova, T.V.; Nasanova, O.N. Hypolipidemic Action of Medicinal Plant Extracts for Experimental Diabetes Mellitus. Pharm. Chem. J. 2019, 53, 239–242. [Google Scholar] [CrossRef]
- Li, X.; Zhao, Z.; Kuang, P.; Shi, X.; Wang, Z.; Guo, L. Regulation of lipid metabolism in diabetic rats by Arctium lappa L. polysaccharide through the PKC/NF-κB pathway. Int. J. Biol. Macromol. 2019, 136, 115–122. [Google Scholar] [CrossRef] [PubMed]
- Ahangarpour, A.; Heidari, H.; Oroojan, A.A.; Mirzavandi, F.; Esfehani, K.N.; Mohammadi, Z.D. Antidiabetic, hypolipidemic and hepatoprotective effects of Arctium lappa root’s hydro-alcoholic extract on nicotinamide-streptozotocin induced type 2 model of diabetes in male mice. Avicenna J. Phytomed. 2017, 7, 169–179. [Google Scholar] [CrossRef]
- Ahangarpour, A.; Ali-Akbari, F.R.; Mohaghegh, S.M.; Asadinia, E. Effects of Arctium lappa aqueous extract on lipid profile and hepatic enzyme levels of sucrose-induced metabolic syndrome in female rats. Braz. J. Pharm. Sci. 2016, 52, 425–431. [Google Scholar] [CrossRef]
- Xu, Z.; Ju, J.; Wang, K.; Gu, C.; Feng, Y. Evaluation of hypoglycemic activity of total lignans from Fructus Arctii in the spontaneously diabetic Goto-Kakizaki rats. J. Ethnopharmacol. 2014, 151, 548–555. [Google Scholar] [CrossRef]
- Annunziata, G.; Barrea, L.; Ciampaglia, R.; Cicala, C.; Arnone, A.; Savastano, S.; Nabavi, S.M.; Tenore, G.C.; Novellino, E. Arctium lappa contributes to the management of type 2 diabetes mellitus by regulating glucose homeostasis and improving oxidative stress: A critical review of in vitro and in vivo animal-based studies. Phytother. Res. 2019, 33, 2213–2220. [Google Scholar] [CrossRef]
- Zhang, L.; Li, P.; Zhang, X.; Wu, J.; Bao, M. Clinical observation on treatment of diabetic nephropathy with fructus arctii powders. Sichuan Yixue 2011, 32, 656–658. [Google Scholar]
- Ma, S.; Liu, D.; Niu, R.; Liu, R.; Ji, Q.; Zhan, J. Double-blind, randomized, placebo-controlled multi-center phase III clinical trial of Arctiin granule in the treatment of diabetic nephropathy. Chin. J. Clin. Pharmacol. 2011, 27, 15–18. [Google Scholar]
- Wang, H.-Y.; Chen, Y.-P. Clinical observation on treatment of diabetic nephropathy with compound fructus arctii mixture. Zhongguo Zhong Xi Yi Jie He Za Zhi 2004, 24, 589–592. [Google Scholar] [PubMed]
- Chen, Z.H. Fructus Arctii is hypolycemic and good for back and joints. Fujian J. TCM 1999, 30, 17. [Google Scholar]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Shamseer, L.; Clarke, M.; Ghersi, D.; Liberati, A.; Petticrew, M.; Shekelle, P.; Stewart, L.A. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst. Rev. 2015, 4, 1. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.; Welch, V.A. Cochrane Handbook for Systematic Reviews of Interventions version 6.2; John Wiley & Sons: Hoboken, NJ, USA, 2021. [Google Scholar]
- Watanabe, S.; Okoshi, H.; Yamabe, S.; Shimada, M. Moringa oleifera Lam. in Diabetes Mellitus: A Systematic Review and Meta-Analysis. Molecules 2021, 26, 3513. [Google Scholar] [CrossRef]
- Borenstein, M.; Hedges, L.V.; Higgins, J.P.; Rothstein, H.R. Introduction to Meta-Analysis, 2nd ed.; John Wiley & Sons: Hoboken, NJ, USA, 2021; ISBN 978-111-955-835-4. [Google Scholar]
- Zhang, H.; Gao, Y.; Zhang, J.; Wang, K.; Jin, T.; Wang, H.; Ruan, K.; Wu, F.; Xu, Z. The effect of total lignans from Fructus Arctii on Streptozotocin-induced diabetic retinopathy in Wistar rats. J. Ethnopharmacol. 2020, 255, 112773. [Google Scholar] [CrossRef]
- Zhang, J.; Cao, P.; Gui, J.; Wang, X.; Han, J.; Wang, Y.; Wang, G. Arctigenin ameliorates renal impairment and inhibits endoplasmic reticulum stress in diabetic db/db mice. Life Sci. 2019, 223, 194–201. [Google Scholar] [CrossRef]
- Gao, Y.; Gu, C.; Wang, K.; Wang, H.; Ruan, K.; Xu, Z.; Feng, Y. The effects of hypoglycemia and weight loss of total lignans from Fructus Arctii in KKAy mice and its mechanisms of the activity. Phytother. Res. 2018, 32, 631–642. [Google Scholar] [CrossRef]
- Bok, S.-H.; Cho, S.S.; Bae, C.-S.; Park, D.-H.; Park, K.-M. Safety of 8-weeks oral administration of Arctium lappa L. Lab. Anim. Res. 2017, 33, 251–255. [Google Scholar] [CrossRef]
- Naeimeh, D.; Sheyda, A.; Hamed, D.; Nazanin, S.J.; Farzad, P. Antidiabetic Effect of Burdock Tuber (Arctiumlappa L.) Extract on Aloxan Diabetic. Biosci. Biotechnol. Res. Asia 2015, 12, 1251–1254. [Google Scholar] [CrossRef]
- Xu, Z.; Gu, C.; Wang, K.; Ju, J.; Wang, H.; Ruan, K.; Feng, Y. Arctigenic acid, the key substance responsible for the hypoglycemic activity of Fructus Arctii. Phytomedicine 2015, 22, 128–137. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.-T.; Liu, D.-L.; Deng, J.-J.; Niu, R.; Liu, R.-B. Effect of Arctiin on Glomerular Filtration Barrier Damage in STZ-Induced Diabetic Nephropathy Rats. Phytother. Res. 2013, 27, 1474–1480. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.-C.; Zhou, W.; Li, Z.-H.; Yu, C.-P.; Li, C.-W.; Luo, M.-H.; Xie, H. Effects of Arctiin on Streptozotocin-Induced Diabetic Retinopathy in Sprague-Dawley Rats. Planta Medica 2012, 78, 1317–1323. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.-L.; Yu, R.-T.; Gong, J.; Feng, Y.; Dai, Y.-L.; Hu, F.; Hu, Y.-H.; Tao, Y.-D.; Leng, Y. Arctigenin, a natural compound, activates AMP-activated protein kinase via inhibition of mitochondria complex I and ameliorates metabolic disorders in ob/ob mice. Diabetologia 2012, 55, 1469–1481. [Google Scholar] [CrossRef]
- Xu, Z.; Wang, X.; Zhou, M.; Ma, L.; Deng, Y.; Zhang, H.; Zhao, A.; Zhang, Y.; Jia, W. The antidiabetic activity of total lignan from Fructus Arctii against alloxan-induced diabetes in mice and rats. Phytother. Res. 2008, 22, 97–101. [Google Scholar] [CrossRef]
- Wang, D.; Bădărau, A.S.; Swamy, M.K.; Shaw, S.; Maggi, F.; Da Silva, L.E.; López, V.; Yeung, A.W.K.; Mocan, A.; Atanasov, A.G. Arctium Species Secondary Metabolites Chemodiversity and Bioactivities. Front. Plant Sci. 2019, 10, 834. [Google Scholar] [CrossRef]
- Franco, R.R.; Carvalho, D.D.S.; de Moura, F.B.R.; Justino, A.B.; Silva, H.C.G.; Peixoto, L.G.; Espindola, F.S. Antioxidant and anti-glycation capacities of some medicinal plants and their potential inhibitory against digestive enzymes related to type 2 diabetes mellitus. J. Ethnopharmacol. 2018, 215, 140–146. [Google Scholar] [CrossRef]
- Oboh, G.; Agunloye, O.; Adefegha, S.A.; Akinyemi, A.; Ademiluyi, A.O. Caffeic and chlorogenic acids inhibit key enzymes linked to type 2 diabetes (in vitro): A comparative study. J. Basic Clin. Physiol. Pharmacol. 2015, 26, 165–170. [Google Scholar] [CrossRef]
- Tadera, K.; Minami, Y.; Takamatsu, K.; Matsuoka, T. Inhibition of alpha-glucosidase and alpha-amylase by flavonoids. J. Nutr. Sci. Vitaminol. 2006, 52, 149–153. [Google Scholar] [CrossRef]
- Miyazawa, M.; Yagi, N.; Taguchi, K. Inhibitory Compounds of alpha Glucosidase Activity from Arctium lappa L. J. Oleo Sci. 2005, 54, 589–594. [Google Scholar] [CrossRef]
- MacDonald, P.E.; El-Kholy, W.; Riedel, M.J.; Salapatek, A.M.F.; Light, P.E.; Wheeler, M.B. The Multiple Actions of GLP-1 on the Process of Glucose-Stimulated Insulin Secretion. Diabetes 2002, 51, S434–S442. [Google Scholar] [CrossRef] [PubMed]
- Tousch, D.; Bidel, L.P.R.; Cazals, G.; Ferrare, K.; Leroy, J.; Faucanié, M.; Chevassus, H.; Tournier, M.; Lajoix, A.-D.; Azay-Milhau, J. Chemical Analysis and Antihyperglycemic Activity of an Original Extract from Burdock Root (Arctium lappa). J. Agric. Food Chem. 2014, 62, 7738–7745. [Google Scholar] [CrossRef] [PubMed]
- Tousch, D.; Ferrare, K.; Leroy, J.; Faucanié, M.; Chevassus, H.; Tournier, M.; Gross, R.; Petit, P.; Azay-Milhau, J. Evidence for antihyperglycemic properties of burdock root hydro-alcoholic extract. Fundam. Clin. Pharmacol. 2011, 25, 26. [Google Scholar] [CrossRef]
- Yamauchi, T.; Kamon, J.; Waki, H.; Terauchi, Y.; Kubota, N.; Hara, K.; Mori, Y.; Ide, T.; Murakami, K.; Tsuboyama-Kasaoka, N.; et al. The fat-derived hormone adiponectin reverses insulin resistance associated with both lipoatrophy and obesity. Nat. Med. 2001, 7, 941–946. [Google Scholar] [CrossRef]
- Díez, J.J.; Iglesias, P. The role of the novel adipocyte-derived hormone adiponectin in human disease. Eur. J. Endocrinol. 2003, 148, 293–300. [Google Scholar] [CrossRef]
- Okada, Y.; Okada, M.; Sagesaka, Y. Screening of Dried Plant Seed Extracts for Adiponectin Production Activity and Tumor Necrosis Factor-Alpha Inhibitory Activity on 3T3-L1 Adipocytes. Mater. Veg. 2010, 65, 225–232. [Google Scholar] [CrossRef]
- Shao, T.; Yu, Q.; Zhu, T.; Liu, A.; Gao, X.; Long, X.; Liu, Z. Inulin from Jerusalem artichoke tubers alleviates hyperglycaemia in high-fat-diet-induced diabetes mice through the intestinal microflora improvement. Br. J. Nutr. 2020, 123, 308–318. [Google Scholar] [CrossRef]
- Kaze, A.D.; Santhanam, P.; Musani, S.K.; Ahima, R.; Echouffo-Tcheugui, J.B. Metabolic Dyslipidemia and Cardiovascular Outcomes in Type 2 Diabetes Mellitus: Findings From the Look AHEAD Study. J. Am. Heart Assoc. 2021, 10, e016947. [Google Scholar] [CrossRef]
- Cao, J.; Li, C.; Zhang, P.; Cao, X.; Huang, T.; Bai, Y.; Chen, K. Antidiabetic effect of burdock (Arctium lappa L.) root ethanolic extract on streptozotocin-induced diabetic rats. Afr. J. Biotechnol. 2012, 11, 9079–9085. [Google Scholar]
- Diab, R.A.H.; Fares, M.; Abedi-Valugerdi, M.; Kumagai-Braesch, M.; Holgersson, J.; Hassan, M. Immunotoxicological effects of streptozotocin and alloxan: In vitro and in vivo studies. Immunol. Lett. 2015, 163, 193–198. [Google Scholar] [CrossRef]
- Szkudelski, T. The mechanism of alloxan and streptozotocin action in B cells of the rat pancreas. Physiol. Res. 2001, 50, 537–546. [Google Scholar] [PubMed]
- Kleinert, M.; Clemmensen, C.; Hofmann, S.; Moore, M.C.; Renner, S.; Woods, S.C.; Huypens, P.; Beckers, J.; de Angelis, M.H.; Schürmann, A.; et al. Animal models of obesity and diabetes mellitus. Nat. Rev. Endocrinol. 2018, 14, 140–162. [Google Scholar] [CrossRef]
- Paulsen, E. Contact sensitization from Compositae-containing herbal remedies and cosmetics. Contact Dermat. 2002, 47, 189–198. [Google Scholar] [CrossRef]
- Rodriguez, P.; Blanco, J.; Juste, S.; Garcés, M.; Pérez, R.; Alonso, L.; Marcos, M. Allergic contact dermatitis due to burdock (Arctium lappa). Contact Dermat. 1995, 33, 134–135. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, Y.; Kimura, Y.; Tsunoda, T.; Tagami, H. Anaphylaxis due to burdock. Int. J. Dermatol. 2003, 42, 472–473. [Google Scholar] [CrossRef] [PubMed]
- Gordon, S.M.; Li, H.; Zhu, X.; Shah, A.S.; Lu, L.J.; Davidson, W.S. A Comparison of the Mouse and Human Lipoproteome: Suitability of the Mouse Model for Studies of Human Lipoproteins. J. Proteome Res. 2015, 14, 2686–2695. [Google Scholar] [CrossRef]
- Drouin, G.; Godin, J.-R.; Page, B. The Genetics of Vitamin C Loss in Vertebrates. Curr. Genom. 2011, 12, 371–378. [Google Scholar] [CrossRef]
- Nishikimi, M.; Fukuyama, R.; Minoshima, S.; Shimizu, N.; Yagi, K. Cloning and chromosomal mapping of the human nonfunctional gene for L-gulono-gamma-lactone oxidase, the enzyme for L-ascorbic acid biosynthesis missing in man. J. Biol. Chem. 1994, 269, 13685–13688. [Google Scholar] [CrossRef]
- Blais, E.M.; Rawls, K.D.; Dougherty, B.V.; Li, Z.I.; Kolling, G.L.; Ye, P.; Wallqvist, A.; Papin, J.A. Reconciled rat and human metabolic networks for comparative toxicogenomics and biomarker predictions. Nat. Commun. 2017, 8, 14250. [Google Scholar] [CrossRef]
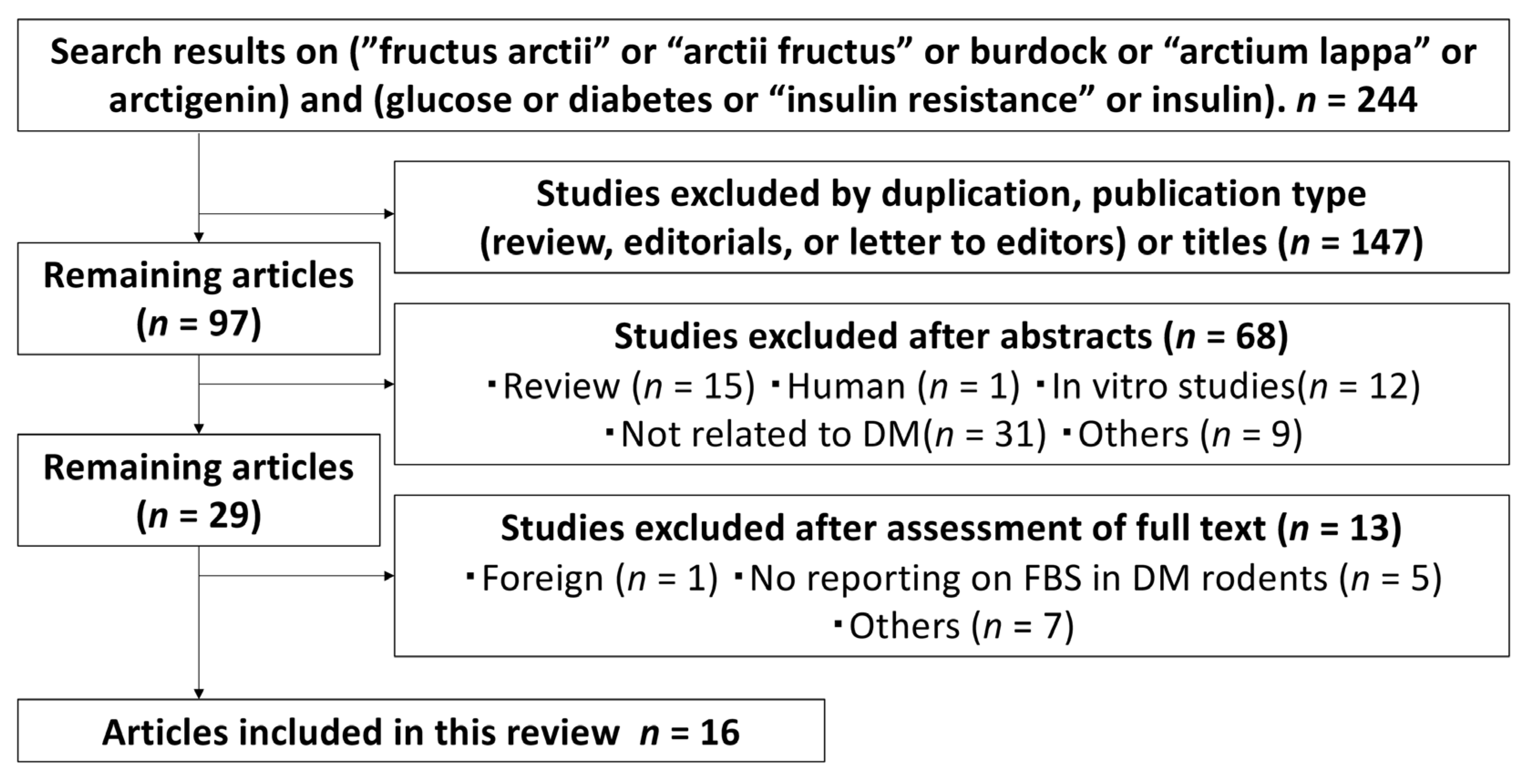
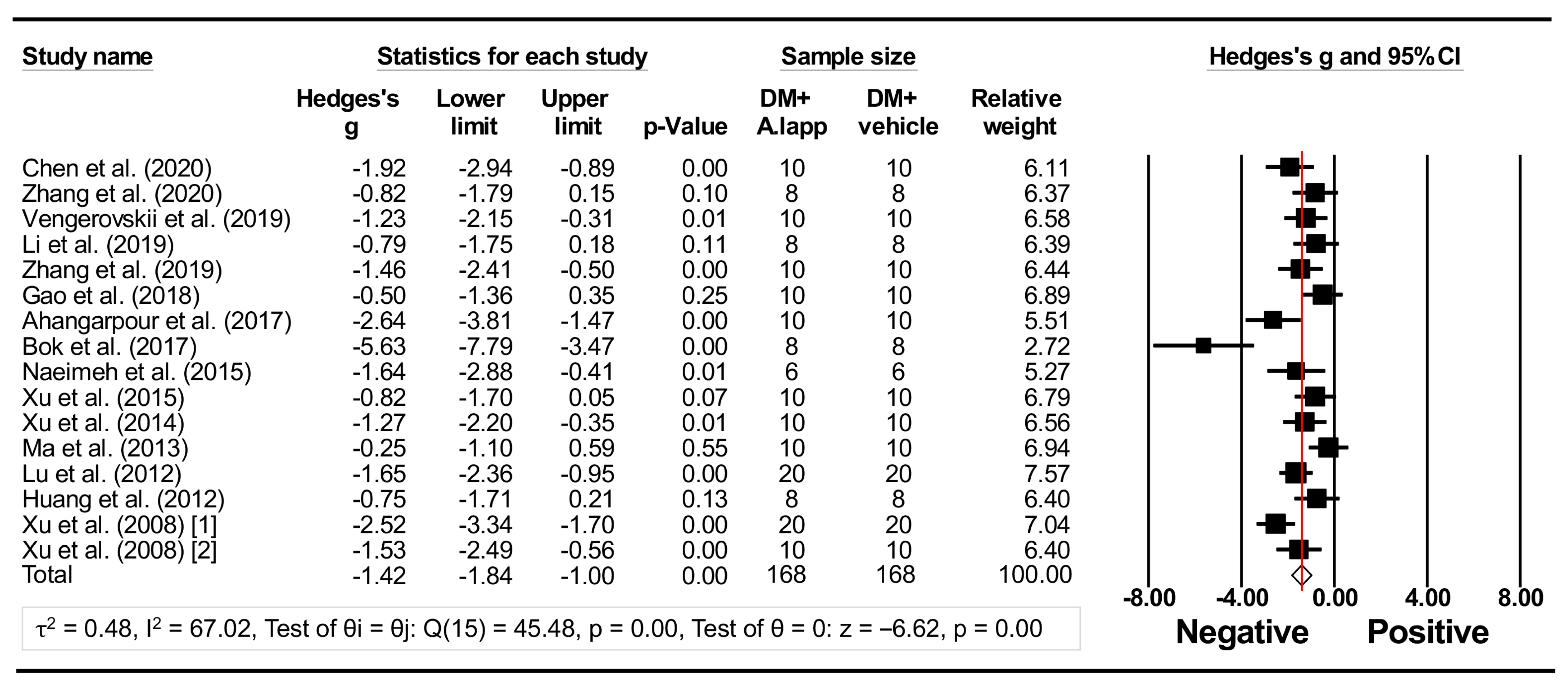
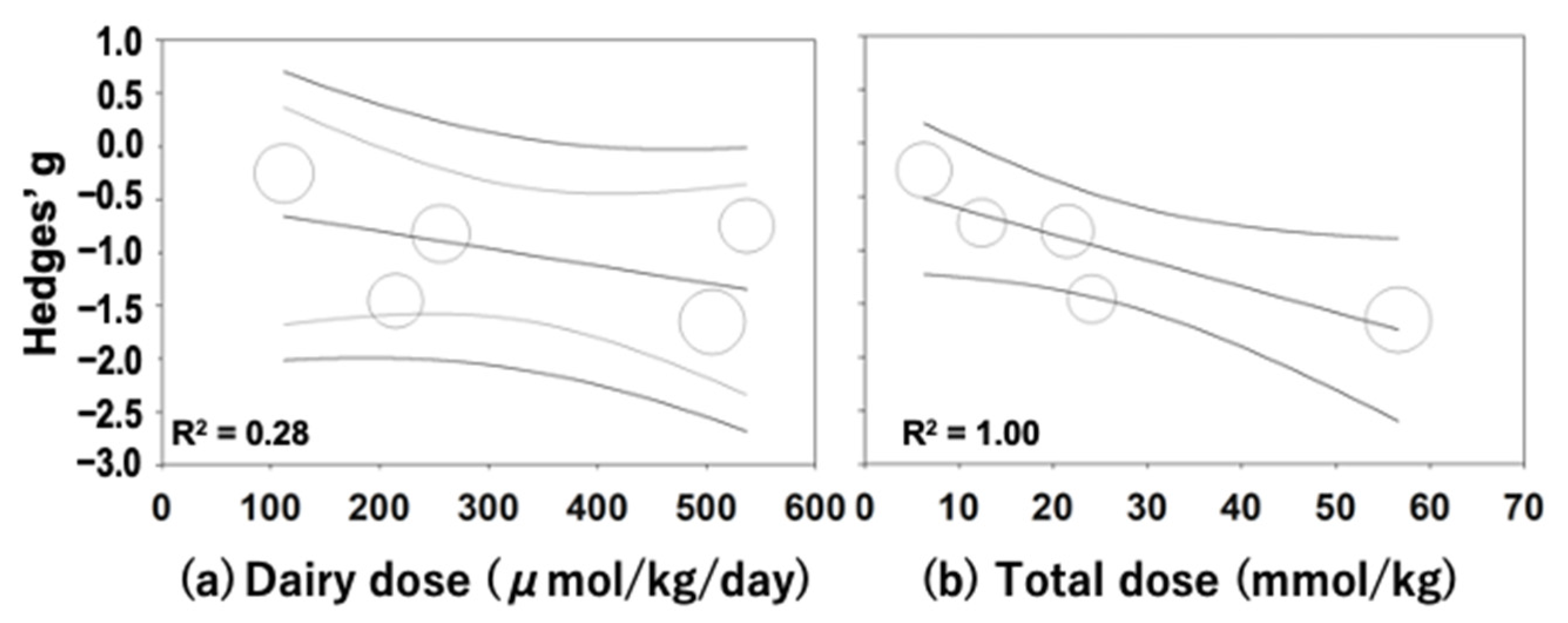
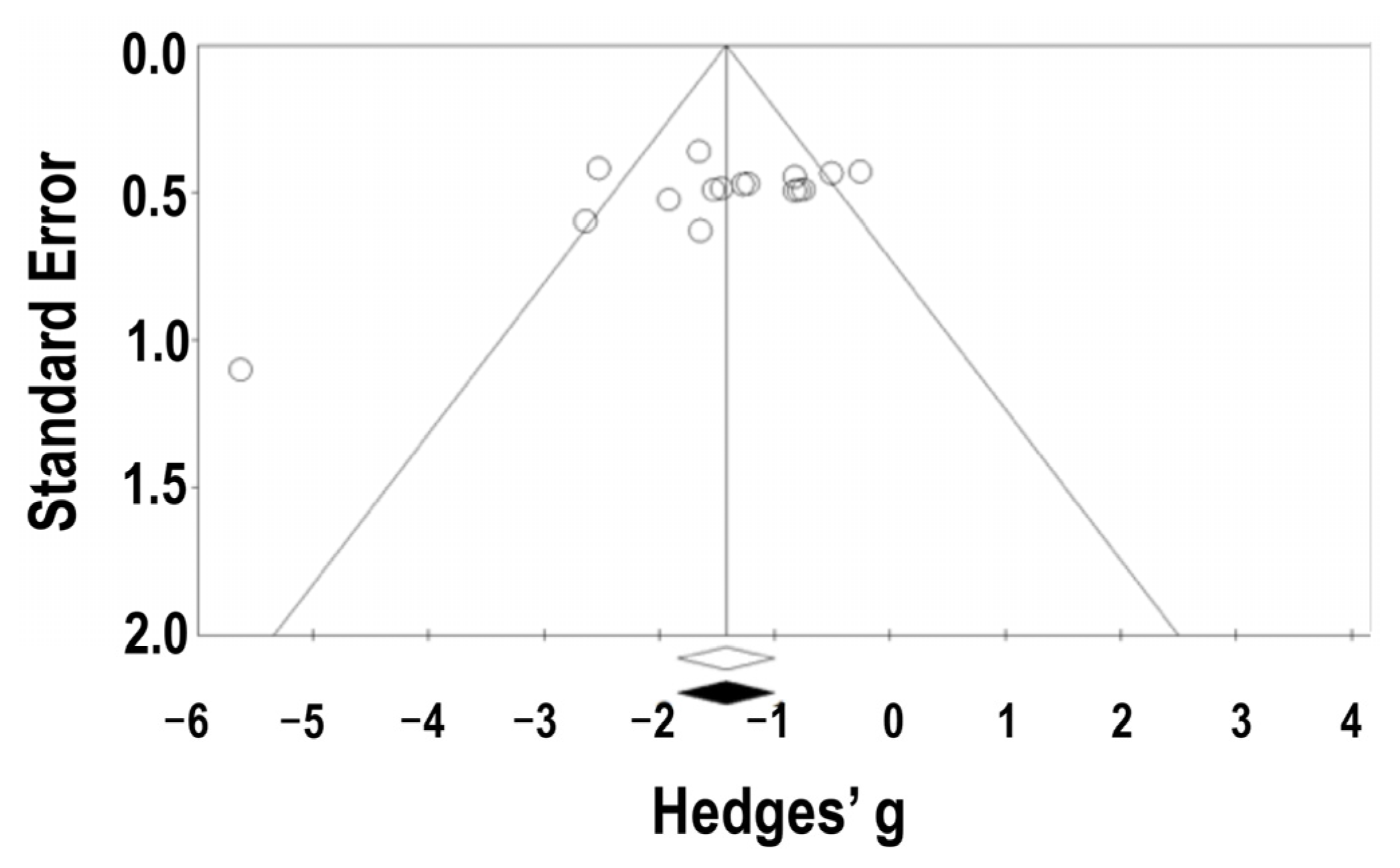


| Authors (Year) | Parts or Compounds | Animal Models (Doses of STZ or Alloxan) | Study No. (+/−) | Weight or Age at a Baseline | Sex | Diet | Sample Type for BG/Lipids | Measured Data |
|---|---|---|---|---|---|---|---|---|
| Chen et al. (2020) [14] | roots | Rats, STZ (120 mg/kgBW) | 10/10 | NS | Males | HFSD | Whole blood/serum | BG, TG, TC, and HDL-C |
| Zhang et al. (2020) [30] | Fructus Arctii | Rats, STZ (60 mg/kgBW) | 8/8 | 100~120 g | Males | HFSD | Whole blood/ND | BG |
| Vengerovskii et al. (2019) [15] | roots | Rats, STZ (30 mg/kgBW) | 10/10 | 200~220 g | Males | HFD | Whole blood/serum | BG, TG, TC, and HDL-C |
| Li et al. (2019) [16] | roots | Rats, STZ (120 mg/kgBW) | 8/8 | NS | Males | HFSD | Whole blood/plasma | BG, TG, TC, and HDL-C |
| Zhang et al. (2019) [31] | arctigenin | db/db mice | 10/10 | 6 wks | Males | Control | Whole blood/ND | BG |
| Gao et al. (2018) [32] | Fructus Arctii | KKAy mice | 10/10 | 9 wks | Males | HFD | Whole blood/plasma | BG, TG, TC, and HDL-C |
| Ahangarpour et al. (2017) [17] | roots | Mice, STZ (50 mg/kgBW) | 10/10 | 30~35 g | Males | Control | Whole blood/serum | BG, TG, TC, and HDL-C |
| Bok et al. (2017) [33] | roots | Mice, diet-induced | 8/8 | NS | Both | HFD | Serum/ND | BG |
| Ahangarpour et al. (2016) [18] | roots | Rats, diet-induced | 8/8 | 150~250 g | Females | Control + sucrose in water | ND/serum | TG, TC, and HDL-C |
| Naeimeh et al. (2015) [34] | roots | Rats, alloxan (160 mg/kgBW) | 6/6 | NS | Males | NS | Whole blood/ND | BG |
| Xu et al. (2015) [35] | arctigenin acid | GK rats | 10/10 | 9 wks | Males | HFD | Whole blood/serum | BG, TG, TC, and HDL-C |
| Xu et al. (2014) [19] | Fructus Arctii | GK rats | 10/10 | 9 wks | Males | HFD | Whole blood/serum | BG, TG, TC, and HDL-C |
| Ma et al. (2013) [36] | arctiin | Rats, STZ (65 mg/kgBW) | 10/10 | 180~200 g | Males | Control | Whole blood/ND | BG |
| Lu et al. (2012) [37] | arctiin | Rats, STZ (30 mg/kgBW) | 20/20 | 160~180 g | Males | HFSD | Serum/ND | BG |
| Huang et al. (2012) [38] | arctigenin | ob/ob mice | 8/8 | 6~7 wks | Females | NS | Whole blood/serum | BG, TG, and TC |
| Xu et al. (2008) (1) [39] | Fructus Arctii | Mice, alloxan (90 mg/kgBW) | 20/20 | 6 wks or 20 ± 2 g | Both | Control | Whole blood/NS | BG |
| Xu et al. (2008) (2) [39] | Fructus Arctii | Rats, alloxan (50 mg/kgBW) | 10/10 | 6 wks or 120 ± 10 g | Both | Control + fat emulsion | Whole blood/serum | BG, TG, TC, and HDL-C |
| Subgroups | Effect Size | Heterogeneity (I2) | Test of Group Difference (p) | ||||
|---|---|---|---|---|---|---|---|
| Study No. | g | 95% CI | p-Value | ||||
| DM rodent models | |||||||
| chemical | 10 | −1.48 | −1.89 | −1.06 | <0.001 | 60.98 | 0.001 |
| diet | 1 | −5.63 | −7.97 | −3.28 | <0.001 | 0.00 | |
| genetic | 5 | −0.95 | −1.53 | −0.37 | 0.001 | 0.00 | |
| Rodent type | |||||||
| mice | 6 | −1.86 | −2.57 | −1.16 | <0.001 | 83.64 | 0.13 |
| rats | 10 | −1.18 | −1.70 | −0.66 | <0.001 | 19.54 | |
| Sample type | |||||||
| whole blood | 14 | −1.27 | −1.71 | −0.83 | <0.001 | 55.14 | 0.04 |
| serum | 2 | −2.70 | −4.00 | −1.40 | <0.001 | 91.49 | |
| Sex | |||||||
| male | 12 | −1.21 | −1.64 | −0.79 | <0.001 | 43.22 | 0.02 |
| females | 1 | −0.75 | −2.23 | 0.74 | 0.32 | 0.00 | |
| both | 3 | −2.59 | −3.52 | −1.65 | <0.001 | 83.09 | |
| Parts | |||||||
| extracts | 11 | −1.64 | −2.15 | −1.12 | <0.001 | 70.61 | 0.16 |
| arctigenin-related compounds | 5 | −1.00 | −1.71 | −0.28 | 0.006 | 47.01 | |
| TG Levels | |||||||
| Subgroups | Effect Size | Heterogeneity (I2) | Test of Group Difference (p) | ||||
| Study No. | g | 95% CI | p-Value | ||||
| DM rodent models | |||||||
| chemical | 5 | −2.10 | −2.89 | −1.30 | <0.001 | 79.64 | <0.001 |
| diet | 1 | −3.52 | −5.56 | −1.49 | 0.001 | 0.00 | |
| genetic | 4 | −0.20 | −1.00 | 0.60 | 0.62 | 0.00 | |
| Rodent type | |||||||
| mice | 3 | −0.44 | −1.84 | 0.95 | 0.53 | 0.00 | 0.09 |
| rats | 7 | −1.93 | −2.89 | −0.97 | <0.001 | 86.42 | |
| Sample type | |||||||
| plasma | 2 | −1.36 | −3.25 | 0.53 | 0.16 | 88.03 | 0.90 |
| serum | 8 | −1.50 | −2.45 | −0.54 | 0.002 | 84.42 | |
| Sex | |||||||
| male | 7 | −1.10 | −1.96 | −0.23 | 0.03 | 74.45 | 0.12 |
| female | 2 | −1.60 | −3.29 | 0.09 | 0.06 | 92.82 | |
| both | 1 | −3.91 | −6.44 | −1.38 | 0.002 | 0.00 | |
| Parts | |||||||
| extracts | 8 | −1.80 | −2.68 | −0.92 | <0.001 | 84.17 | 0.09 |
| arctigenin-related compounds | 2 | −0.18 | −1.86 | 1.50 | 0.84 | 0.00 | |
| TC Levels | |||||||
| Subgroups | Effect Size | Heterogeneity (I2) | Test of Group Difference (p) | ||||
| Study No. | g | 95% CI | p-Value | ||||
| DM rodent models | |||||||
| chemical | 5 | −2.10 | −3.22 | −0.99 | <0.001 | 87.55 | <0.001 |
| diet | 1 | −6.76 | −10.01 | −3.52 | <0.001 | 0.00 | |
| genetic | 4 | −0.31 | −1.42 | 0.81 | 0.59 | 26.35 | |
| Rodent type | |||||||
| mice | 3 | −0.65 | −2.41 | 1.11 | 0.47 | 19.45 | 0.13 |
| rats | 7 | −2.34 | −3.60 | −1.09 | <0.001 | 90.90 | |
| Sample type | |||||||
| plasma | 2 | −1.53 | −3.77 | 0.71 | 0.18 | 92.12 | 0.80 |
| serum | 8 | −1.85 | −3.02 | −0.68 | 0.002 | 87.87 | |
| Sex | |||||||
| male | 7 | −1.46 | −2.71 | −0.21 | 0.02 | 87.20 | 0.38 |
| female | 2 | −3.38 | −5.83 | −0.92 | 0.01 | 93.87 | |
| both | 1 | −1.31 | −4.45 | 1.82 | 0.41 | 0.00 | |
| Parts | |||||||
| extracts | 8 | −2.11 | −3.27 | −0.94 | <0.001 | 89.47 | 0.24 |
| arctigenin-related compounds | 2 | −0.63 | −2.80 | 1.54 | 0.57 | 59.91 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Watanabe, S.; Yamabe, S.; Shimada, M. Arctium lappa Lam. and Its Related Lignans Improve Hyperglycemia and Dyslipidemia in Diabetic Rodent Models: A Systematic Review and Meta-Analysis. Nutraceuticals 2022, 2, 335-349. https://doi.org/10.3390/nutraceuticals2040026
Watanabe S, Yamabe S, Shimada M. Arctium lappa Lam. and Its Related Lignans Improve Hyperglycemia and Dyslipidemia in Diabetic Rodent Models: A Systematic Review and Meta-Analysis. Nutraceuticals. 2022; 2(4):335-349. https://doi.org/10.3390/nutraceuticals2040026
Chicago/Turabian StyleWatanabe, Shihori, Shizuko Yamabe, and Masako Shimada. 2022. "Arctium lappa Lam. and Its Related Lignans Improve Hyperglycemia and Dyslipidemia in Diabetic Rodent Models: A Systematic Review and Meta-Analysis" Nutraceuticals 2, no. 4: 335-349. https://doi.org/10.3390/nutraceuticals2040026
APA StyleWatanabe, S., Yamabe, S., & Shimada, M. (2022). Arctium lappa Lam. and Its Related Lignans Improve Hyperglycemia and Dyslipidemia in Diabetic Rodent Models: A Systematic Review and Meta-Analysis. Nutraceuticals, 2(4), 335-349. https://doi.org/10.3390/nutraceuticals2040026







