Demographic Differences in Periodic Limb Movement Index and Apnea–Hypopnea Index in a Diverse Clinical Cohort
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Polysomnography
2.3. Statistics
3. Results
3.1. Demographics
3.2. Regression
3.3. Figures
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AASM | American Academy of Sleep Medicine |
| AHI | Apnea–Hypopnea Index |
| EEG | Electroencephalogram |
| EMG | Electromyogram |
| IRB | Institutional Review Board |
| OSA | Obstructive Sleep Apnea |
| PLMD | Periodic Limb Movement Disorder |
| PLMI | Periodic Limb Movement Index |
| SD | Standard Deviation |
References
- Park, M.; Senel, G.B.; Modi, H.; Jain, V.; DelRosso, L.M. Combined impact of obstructive sleep apnea and periodic limb movements on sleep parameters. Sleep Med. 2025, 129, 339–345. [Google Scholar] [CrossRef]
- Redline, S.; Azarbarzin, A.; Peker, Y. Obstructive sleep apnoea heterogeneity and cardiovascular disease. Nat. Rev. Cardiol. 2023, 20, 560–573. [Google Scholar] [CrossRef]
- Trzepizur, W.; Blanchard, M.; Ganem, T.; Balusson, F.; Feuilloy, M.; Girault, J.-M.; Meslier, N.; Oger, E.; Paris, A.; Pigeanne, T.; et al. Sleep Apnea–Specific Hypoxic Burden, Symptom Subtypes, and Risk of Cardiovascular Events and All-Cause Mortality. Am. J. Respir. Crit. Care Med. 2022, 205, 108–117. [Google Scholar] [CrossRef]
- Johnson, K.G. Obstructive Sleep Apnea. Continuum 2023, 29, 1071–1091. [Google Scholar] [CrossRef]
- Benjafield, A.V.; Ayas, N.T.; Eastwood, P.R.; Heinzer, R.; Ip, M.S.M.; Morrell, M.J.; Nunez, C.M.; Patel, S.R.; Penzel, T.; Pépin, J.-L.; et al. Estimation of the global prevalence and burden of obstructive sleep apnoea: A literature-based analysis. Lancet Respir. Med. 2019, 7, 687–698. [Google Scholar] [CrossRef]
- Ghavami, T.; Kazeminia, M.; Ahmadi, N.; Rajati, F. Global Prevalence of Obstructive Sleep Apnea in the Elderly and Related Factors: A Systematic Review and Meta-Analysis Study. J. PeriAnesthesia Nurs. 2023, 38, 865–875. [Google Scholar] [CrossRef]
- Hening, W.; Allen, R.; Earley, C.; Kushida, C.; Picchietti, D.; Silber, M. The treatment of restless legs syndrome and periodic limb movement disorder. Sleep 1999, 22, 970–999. [Google Scholar] [CrossRef][Green Version]
- Kang, Y.J.; An, J.S.; Park, J.M.; Park, C.-S. The accuracy and difference of scoring rules and methods to score respiratory event-related leg movements in obstructive sleep apnea patients. Sleep Med. 2023, 108, 71–78. [Google Scholar] [CrossRef]
- Wipper, B.; Winkelman, J.W. The Long-Term Psychiatric and Cardiovascular Morbidity and Mortality of Restless Legs Syndrome and Periodic Limb Movements of Sleep. Sleep Med. Clin. 2021, 16, 279–288. [Google Scholar] [CrossRef]
- Drakatos, P.; Olaithe, M.; Verma, D.; Ilic, K.; Cash, D.; Fatima, Y.; Higgins, S.; Young, A.H.; Chaudhuri, K.R.; Steier, J.; et al. Periodic limb movements during sleep: A narrative review. J. Thorac. Dis. 2021, 13, 6476–6494. [Google Scholar] [CrossRef]
- Liu, S.; Pan, J.; Tang, K.; Lei, Q.; He, L.; Meng, Y.; Cai, X.; Li, Z. Sleep spindles, K-complexes, limb movements and sleep stage proportions may be biomarkers for amnestic mild cognitive impairment and Alzheimer’s disease. Sleep Breath. 2020, 24, 637–651. [Google Scholar] [CrossRef]
- Leng, Y.; Blackwell, T.; Stone, K.L.; Hoang, T.D.; Redline, S.; Yaffe, K. Periodic Limb Movements in Sleep are Associated with Greater Cognitive Decline in Older Men without Dementia. Sleep 2016, 39, 1807–1810. [Google Scholar] [CrossRef]
- Leng, Y.; Cavaillès, C.; Peltz, C.; O’bRyant, S.E.; Redline, S.; Yaffe, K. Racial and Ethnic and Sex Differences in At-Home Estimates of Obstructive Sleep Apnea Parameters among Diverse Adults. Ann. Am. Thorac. Soc. 2025, 22, 1401–1408. [Google Scholar] [CrossRef] [PubMed]
- Claman, D.M.; Redline, S.; Blackwell, T.; Ancoli-Israel, S.; Surovec, S.; Scott, N.; Cauley, J.A.; Ensrud, K.E.; Stone, K.L. Prevalence and correlates of periodic limb movements in older women. J. Clin. Sleep Med. 2006, 02, 438–445. [Google Scholar] [CrossRef]
- Young, T.; Palta, M.; Dempsey, J.; Skatrud, J.; Weber, S.; Badr, S. The occurrence of sleep-disordered breathing among middle-aged adults. N. Engl. J. Med. 1993, 328, 1230–1235. [Google Scholar] [CrossRef] [PubMed]
- Quan, S.F.; Howard, B.V.; Iber, C.; Kiley, J.P.; Nieto, F.J.; O’Connor, G.T.; Rapoport, D.M.; Redline, S.; Robbins, J.; Samet, J.M.; et al. The Sleep Heart Health Study: Design, rationale, and methods. Sleep 1997, 20, 1077–1085. [Google Scholar] [CrossRef]
- O’Connor, G.T.; Lind, B.K.; Lee, E.T.; Nieto, F.J.; Redline, S.; Samet, J.M.; Boland, L.L.; Walsleben, J.A.; Foster, G.L. Variation in symptoms of sleep-disordered breathing with race and ethnicity: The Sleep Heart Health Study. Sleep 2003, 26, 74–79. [Google Scholar] [CrossRef][Green Version]
- Chen, X.; Wang, R.; Zee, P.; Lutsey, P.L.; Javaheri, S.; Alcántara, C.; Jackson, C.L.; Williams, M.A.; Redline, S. Racial/Ethnic Differences in Sleep Disturbances: The Multi-Ethnic Study of Atherosclerosis (MESA). Sleep 2015, 38, 877–888. [Google Scholar] [CrossRef]
- Redline, S.; Sotres-Alvarez, D.; Loredo, J.; Hall, M.; Patel, S.R.; Ramos, A.; Shah, N.; Ries, A.; Arens, R.; Barnhart, J.; et al. Sleep-disordered breathing in Hispanic/Latino individuals of diverse backgrounds. The Hispanic Community Health Study/Study of Latinos. Am. J. Respir. Crit. Care Med. 2014, 189, 335–344. [Google Scholar] [CrossRef]
- Senaratna, C.V.; Perret, J.L.; Lodge, C.J.; Lowe, A.J.; Campbell, B.E.; Matheson, M.C.; Hamilton, G.S.; Dharmage, S.C. Prevalence of obstructive sleep apnea in the general population: A systematic review. Sleep Med. Rev. 2017, 34, 70–81. [Google Scholar] [CrossRef]
- Hnin, K.; Mukherjee, S.; Antic, N.A.; Catcheside, P.; Chai-Coetzer, C.L.; McEvoy, D.; Vakulin, A. The impact of ethnicity on the prevalence and severity of obstructive sleep apnea. Sleep Med. Rev. 2018, 41, 78–86. [Google Scholar] [CrossRef]
- Ben Sason, Y.; Oksenberg, A.; Sobel, J.A.; Behar, J.A. Characteristics of patients with positional OSA according to ethnicity and the identification of a novel phenotype—Lateral positional patients: A Multi-Ethnic Study of Atherosclerosis (MESA) study. J. Clin. Sleep Med. 2023, 19, 529–538. [Google Scholar] [CrossRef]
- Berry, R.B.; Brooks, R.; Gamaldo, C.; Harding, S.M.; Lloyd, R.M.; Quan, S.F. AASM Scoring Manual Version 2.6. 2019. J. Clin. Sleep Med. 2017, 13, 665–666. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Marcus, C.L.; Moore, R.H.; Rosen, C.L.; Giordani, B.; Garetz, S.L.; Taylor, H.G.; Mitchell, R.B.; Amin, R.; Katz, E.S.; Arens, R.; et al. A randomized trial of adenotonsillectomy for childhood sleep apnea. N. Engl. J. Med. 2013, 368, 2366–2376. [Google Scholar] [CrossRef] [PubMed]
- Hornyak, M.; Feige, B.; Riemann, D.; Voderholzer, U. Periodic leg movements in sleep and periodic limb movement disorder: Prevalence, clinical significance and treatment. Sleep Med. Rev. 2006, 10, 169–177. [Google Scholar] [CrossRef]
- Karalija, N.; Johansson, J.; Papenberg, G.; Wåhlin, A.; Salami, A.; Köhncke, Y.; Brandmaier, A.M.; Andersson, M.; Axelsson, J.; Riklund, K.; et al. Longitudinal Dopamine D2 Receptor Changes and Cerebrovascular Health in Aging. Neurology 2022, 99, e1278–e1289. [Google Scholar] [CrossRef]
- Winkelman, J.W.; Blackwell, T.; Stone, K.; Ancoli-Israel, S.; Redline, S. Associations of Incident Cardiovascular Events With Restless Legs Syndrome and Periodic Leg Movements of Sleep in Older Men, for the Outcomes of Sleep Disorders in Older Men Study (MrOS Sleep Study). Sleep 2017, 40, zsx023. [Google Scholar] [CrossRef]
- Redline, S.; Kump, K.; Tishler, P.V.; Browner, I.; Ferrette, V. Gender differences in sleep disordered breathing in a community-based sample. Am. J. Respir. Crit. Care Med. 1994, 149 Pt 1, 722–726. [Google Scholar] [CrossRef]
- Punjabi, N.M. The epidemiology of adult obstructive sleep apnea. Proc. Am. Thorac. Soc. 2008, 5, 136–143. [Google Scholar] [CrossRef]
- Šnobrová, B.; Burdová, K.; Weiss, V.; Šonka, K.; Weiss, P. Screening for sleep apnoea risk in testosterone-treated transgender men. Front. Neurol. 2023, 14, 1289429. [Google Scholar] [CrossRef]
- Valipour, A.; Lothaller, H.; Rauscher, H.; Zwick, H.; Burghuber, O.C.; Lavie, P. Gender-related differences in symptoms of patients with suspected breathing disorders in sleep: A clinical population study using the sleep disorders questionnaire. Sleep 2007, 30, 312–319. [Google Scholar] [CrossRef]
- Dy-Hollins, M.E.; Mendizabal, A.; Tsai, A.C. Challenges in studying disparities in neuropsychiatric hyperkinetic movement disorders. Curr. Opin. Neurol. 2025, 38, 343–348. [Google Scholar] [CrossRef]
- Alkhazna, A.; Saeed, A.; Rashidzada, W.; Romaker, A.M. Racial differences in the prevalence of restless legs syndrome in a primary care setting. Hosp. Pract. 2014, 42, 131–137. [Google Scholar] [CrossRef]
- Kutner, N.G.; Zhang, R.; Huang, Y.; Bliwise, D.L. Racial differences in restless legs symptoms and serum ferritin in an incident dialysis patient cohort. Int. Urol. Nephrol. 2012, 44, 1825–1831. [Google Scholar] [CrossRef]
- Lee, H.B.; Hening, W.A.; Allen, R.P.; Earley, C.J.; Eaton, W.W.; Lyketsos, C.G. Race and restless legs syndrome symptoms in an adult community sample in east Baltimore. Sleep Med. 2006, 7, 642–645. [Google Scholar] [CrossRef] [PubMed]
- Ferri, R.; Picchietti, D.L.; Sharon, D.; Spruyt, K.; Owens, J.A.; Walters, A.S.; DelRosso, L.M. Rethinking pediatric “Periodic Limb Movement Disorder” (PLMD): A clinical review of pediatric PLMD and consensus criteria for an updated pediatric diagnostic category “Sleep Leg Movement Disorder of Childhood”. Sleep Med. 2025, 131, 106498. [Google Scholar] [CrossRef] [PubMed]
- Ancoli-Israel, S.; Kripke, D.F.; Klauber, M.R.; Mason, W.J.; Fell, R.; Kaplan, O. Sleep-disordered breathing in community-dwelling elderly. Sleep 1991, 14, 486–495. [Google Scholar] [CrossRef] [PubMed]
- Jordan, A.S.; McSharry, D.G.; Malhotra, A. Adult obstructive sleep apnoea. Lancet 2014, 383, 736–747. [Google Scholar] [CrossRef]
- Fabio, R.A.; Suriano, R.; Gangemi, A. Effects of Transcranial Direct Current Stimulation on Potential P300-Related Events and Alpha and Beta EEG Band Rhythms in Parkinson’s Disease. J. Integr. Neurosci. 2024, 23, 25. [Google Scholar] [CrossRef]
- Gangemi, A.; Suriano, R.; Fabio, R.A. Longitudinal Exploration of Cortical Brain Activity in Cognitive Fog: An EEG Study in Patients with and without Anosmia. J. Integr. Neurosci. 2024, 23, 105. [Google Scholar] [CrossRef]
| Variable | Mean | Std Dev | IQR |
|---|---|---|---|
| Age (years) | 57.17 | 17.93 | (44, 71) |
| Sleep Latency (min) | 30.06 | 35.66 | (8.5, 36.5) |
| REM Latency (min) | 169.22 | 95.99 | (90, 231.05) |
| Wake after Sleep onset (min) | 89.08 | 84.25 | (38.8, 116.5) |
| Total Sleep Time (min) | 298.73 | 91.70 | (248.5, 363.5) |
| Minimum Oxygen saturation (%) | 82.27 | 6.98 | (79, 87) |
| Mean Oxygen Saturation (%) | 93.12 | 2.32 | (92, 95) |
| Awake percent (%) | 28.91 | 20.16 | (13.7, 39) |
| N1 percent | 5.86 | 4.25 | (3.3, 7.2) |
| N2 percent | 44.87 | 15.76 | (35.6, 55.5) |
| N3 percent | 9.35 | 7.47 | (4, 13.2) |
| REM percent | 10.99 | 7.74 | (4.7, 16.2) |
| Central apnea index (events/h) | 1.12 | 3.65 | (0, 0.6) |
| Total Apnea–Hypopnea index (events/h) | 19.29 | 25.79 | (4.5, 22.55) |
| Periodic Limb Movement Index (PLMS/h) | 21.85 | 31.02 | (1.1, 30.2) |
| Count | % | |
|---|---|---|
| Sex | ||
| Female | 395 | 55.56% |
| Male | 316 | 44.44% |
| Ethnicity | ||
| White | 380 | 53.67% |
| Black | 43 | 6.07% |
| Hispanic | 224 | 31.64% |
| Asian | 48 | 6.78% |
| Other | 13 | 1.84% |
| PLMI | AHI | |||||
|---|---|---|---|---|---|---|
| N | M (SD) | p-Value | N | M (SD) | p-Value | |
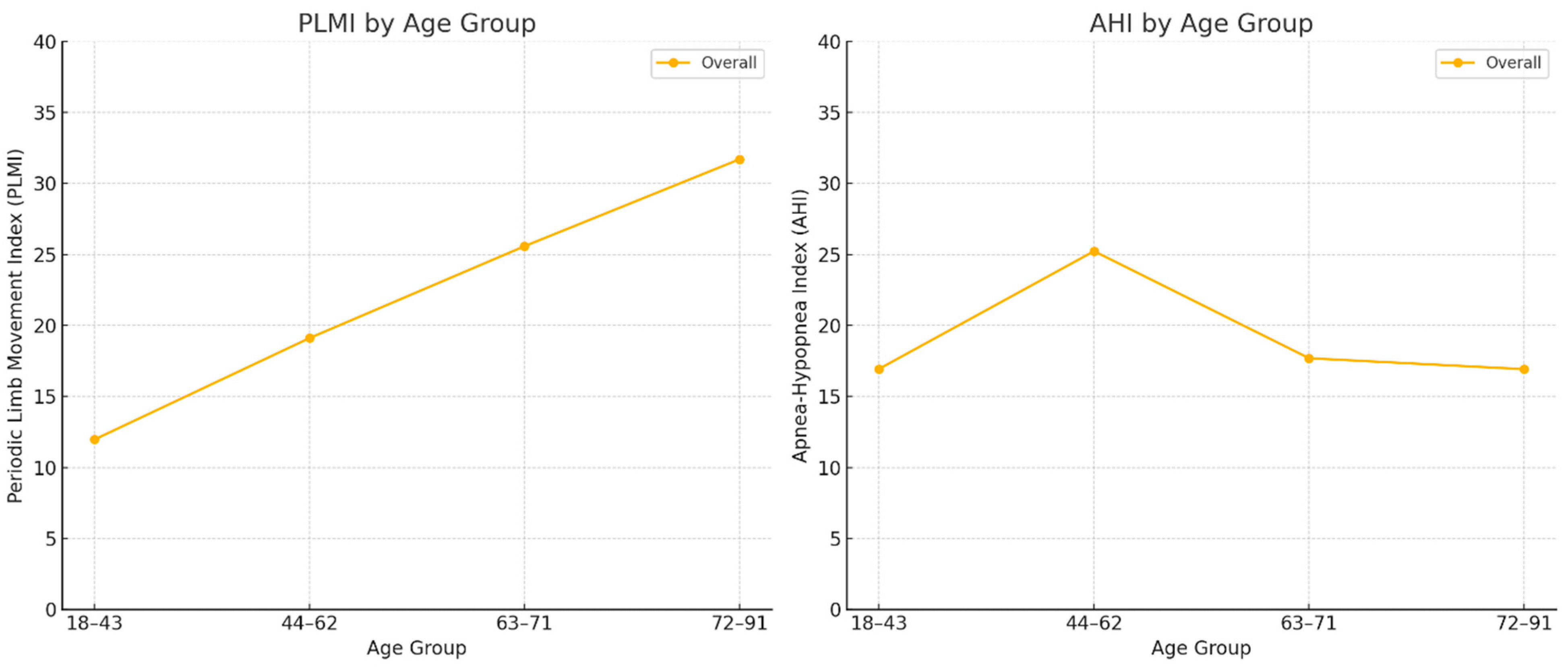
| Age | ||||||
| 18–43 | 178 | 11.97 (19.08) | <0.001 | 178 | 16.94 (22.02) | 0.004 |
| 44–62 | 188 | 19.11 (29.18) | 187 | 25.23 (33.46) | ||
| 63–71 | 177 | 25.58 (33.41) | 177 | 17.69 (23.79) | ||
| 72–91 | 166 | 31.7 (36.88) | 165 | 16.92 (20.24) | ||
| Sex | ||||||
| Female | 395 | 18.92 (26.7) | 0.005 | 393 | 15.41 (24.04) | <0.001 |
| Male | 315 | 25.53 (35.41) | 315 | 24.12 (27.09) | ||
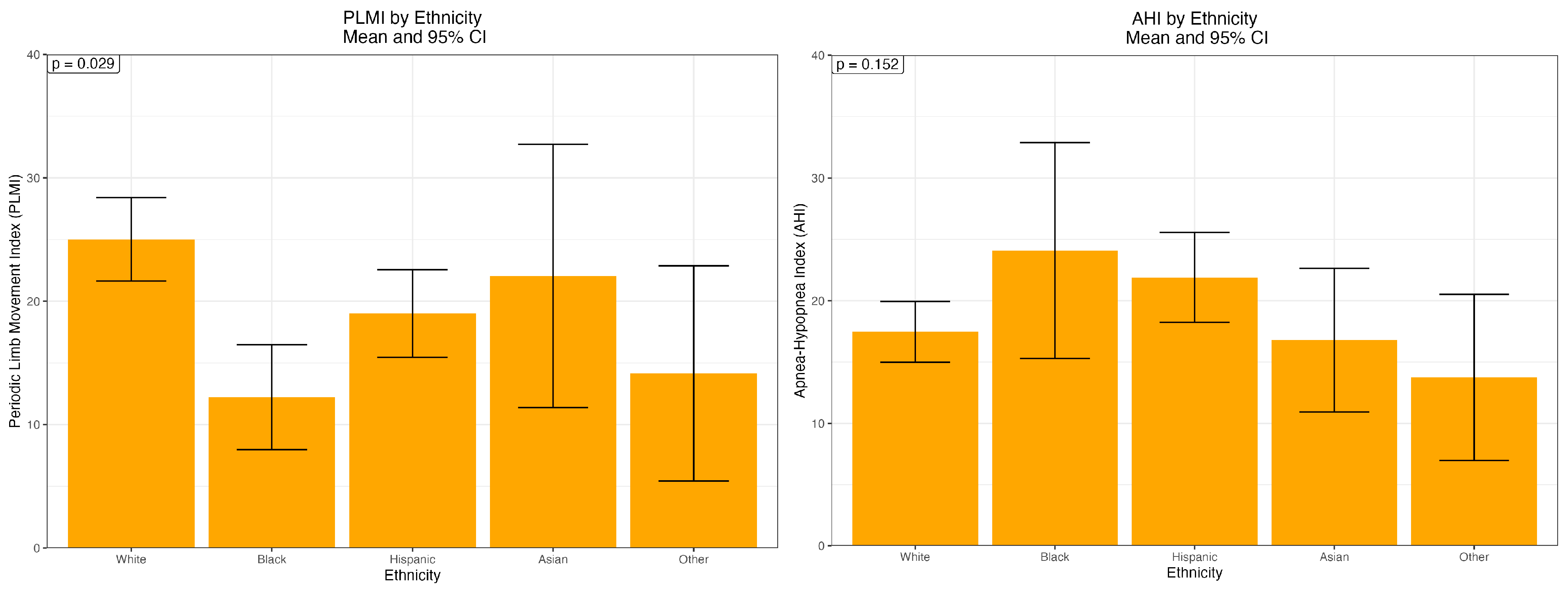
| Ethnicity | ||||||
| White | 379 | 25.02 (33.66) | 0.029 | 379 | 17.47 (24.6) | 0.152 |
| Black | 43 | 12.23 (14.23) | 43 | 24.09 (29.39) | ||
| Hispanic | 224 | 19.01 (27.11) | 223 | 21.9 (27.93) | ||
| Asian | 48 | 22.05 (37.74) | 48 | 16.79 (20.66) | ||
| Other | 13 | 14.15 (16.04) | 12 | 13.76 (12.44) | ||
| PLMI | AHI | |
|---|---|---|
| B (SD) | B (SD) | |
| Intercept | −0.02 (4.26) | 12.25 (3.59) *** |
| Age | 0.37 (0.06) *** | 0.02 (0.05) |
| Sex (reference Woman) | 6.02 (2.29) ** | 8.55 (1.93) *** |
| Ethnicity (reference white) | ||
| Black | −12.71 (4.9) * | 7.26 (4.12) |
| Hispanic | −3.04 (2.58) | 4.97 (2.17) * |
| Asian | −0.44 (4.63) | −0.67 (3.9) |
| Other | −11.28 (8.53) | −1.28 (7.45) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
DelRosso, L.M.; Modi, H.; Chan-Golston, A.M.; Sandhu, P.; Jain, V.; Park, M. Demographic Differences in Periodic Limb Movement Index and Apnea–Hypopnea Index in a Diverse Clinical Cohort. Int. J. Environ. Res. Public Health 2025, 22, 1476. https://doi.org/10.3390/ijerph22101476
DelRosso LM, Modi H, Chan-Golston AM, Sandhu P, Jain V, Park M. Demographic Differences in Periodic Limb Movement Index and Apnea–Hypopnea Index in a Diverse Clinical Cohort. International Journal of Environmental Research and Public Health. 2025; 22(10):1476. https://doi.org/10.3390/ijerph22101476
Chicago/Turabian StyleDelRosso, Lourdes M., Harshil Modi, Alec M. Chan-Golston, Prabhvir Sandhu, Viraj Jain, and Moon Park. 2025. "Demographic Differences in Periodic Limb Movement Index and Apnea–Hypopnea Index in a Diverse Clinical Cohort" International Journal of Environmental Research and Public Health 22, no. 10: 1476. https://doi.org/10.3390/ijerph22101476
APA StyleDelRosso, L. M., Modi, H., Chan-Golston, A. M., Sandhu, P., Jain, V., & Park, M. (2025). Demographic Differences in Periodic Limb Movement Index and Apnea–Hypopnea Index in a Diverse Clinical Cohort. International Journal of Environmental Research and Public Health, 22(10), 1476. https://doi.org/10.3390/ijerph22101476






