Determination and Quantification of Acetaldehyde, Acetone, and Methanol in Hand Sanitizers Using Headspace GC/MS: Effect of Storage Time and Temperature
Abstract
1. Introduction
| LOD | LOQ | Technique | Sample Types Studied | Reference | |
|---|---|---|---|---|---|
| Acetaldehyde | 0.5 μg/L | N/A | HS-GC/MS (NCI) | Drinking water | [28] |
| N/A | 104 μg/mL | HS-GC/MS | Hand sanitizers | [29] | |
| 0.1 μg/mL | 0.5 μg/mL | HS-GC/MS | Blood | [20] | |
| 0.209 μg/mL | 0.314 μg/mL | HS-GC/MS | Hand sanitizers | This study | |
| Methanol | 9 ppm | 31 ppm | HS-GC/MS | Hand sanitizers | [36] |
| N/A | 105 μg/mL | HS-GC/MS | Hand sanitizers | [29] | |
| 0.2 μg/mL | 1 μg/mL | HS-GC/MS | Blood | [20] | |
| 0.03 mg/mL | 0.05 mg/mL | HS-GC/MS | Blood | [32] | |
| 0.211 μg/mL | 0.316 μg/mL | HS-GC/MS | Hand sanitizers | This study | |
| Ethanol | 28 nM | N/A | HS-GC/MS | Aqueous environmental | [21] |
| 99 μg/mL | N/A | HS-GC-FID | Blood | [22] | |
| 0.4 μg/mL | 39.5 μg/mL | HS-GC/MS | Blood | [33] | |
| N/A | 104 μg/mL | HS-GC/MS | Hand sanitizers | [29] | |
| 0.1 μg/mL | 0.5 μg/mL | HS-GC/MS | Blood | [20] | |
| 0.158 μg/mL | 0.210 μg/mL | HS-GC/MS | Hand sanitizers | This study | |
| Isopropanol | N/A | 104 ug/mL | HS-GC/MS | Hand sanitizers | [29] |
| 11 μg/mL | 27 μg/mL | HS-GC-FID | Medicinal drugs | [37] | |
| 0.1 μg/mL | 2 μg/mL | HS-GC/MS | Warfarin derivatives | [38] | |
| 7 ppm | 21 ppm | HS-GC/MS | Terpenes | [39] | |
| 0.157 μg/mL | 0.209 μg/mL | HS-GC/MS | Hand sanitizers | This study | |
| Acetone | 0.1 μg/mL | 0.5 μg/mL | HS-GC/MS | Blood | [20] |
| 0.1 ppm | N/A | Sensor/IR laser | Breath | [40] | |
| 3 μg/mL | 7 μg/mL | HS-GC-FID | Medicinal drugs | [37] | |
| 5 ppm | 16 ppm | HS-GC/MS | Terpenes | [39] | |
| 0.157 μg/mL | 0.209 μg/mL | HS-GC/MS | Hand sanitizers | This study | |
| n-Propanol | 100 ppm | N/A | Sensor/CuO | CuO fiber | [41] |
| 100 ppb | N/A | Sensor/AgCrO2 | Nanoparticles | [42] | |
| 0.0100 μg/mL | 0.0201 μg/mL | HS-GC/MS | Hand sanitizers | This study |
2. Materials and Methods
2.1. Chemicals
2.2. Headspace Gas Chromatography/Mass Spectrometry (HS-GC/MS) Analysis
2.3. Hand Sanitizer Sample Preparation
3. Results and Discussion
3.1. GC/MS Analysis of Pure Alcohols, Acetone, and Acetaldehyde
3.2. Method Validation
3.3. GC/MS Analysis of Hand Sanitizers Kept in Common Storage Conditions and in Parked Cars
3.3.1. Analysis of Major Components in Hand Sanitizer
3.3.2. Quantification of Minor Components in Hand Sanitizer
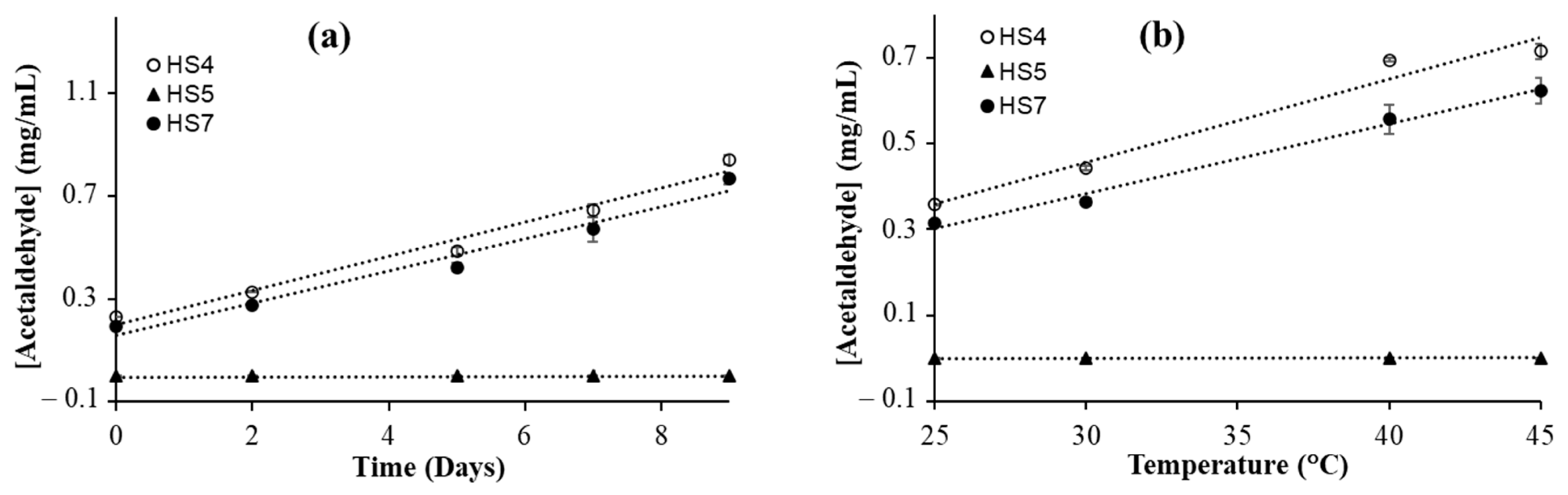
3.4. Effect of Storage Time on Acetaldehyde Formation in Ethanol-Based Hand Sanitizers
3.5. Effect of Temperature on Acetaldehyde Formation in Ethanol-Based Hand Sanitizers
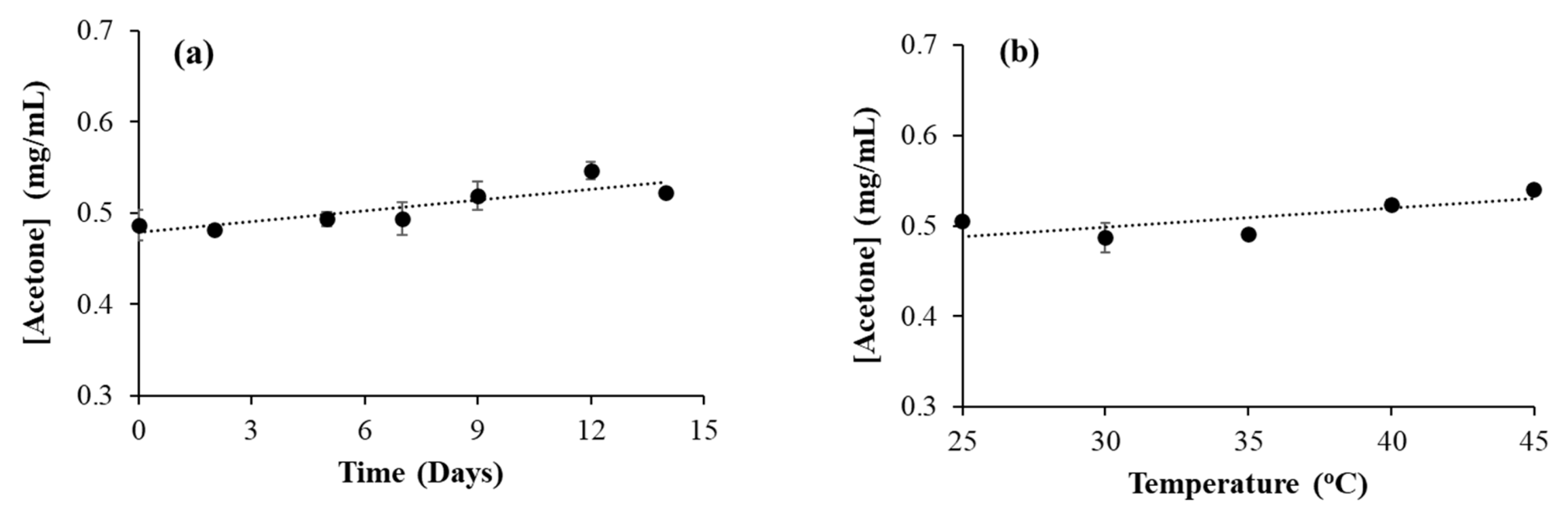
3.6. Effect of Time and Temperature on Acetone Formation in Isopropanol-Based Hand Sanitizer
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Correction Statement
References
- Filipe, H.A.L.; Fiuza, S.M.; Henriques, C.A.; Antunes, F.E. Antiviral and Antibacterial Activity of Hand Sanitizer and Surface Disinfectant Formulations. Int. J. Pharm. 2021, 609, 121139. [Google Scholar] [PubMed]
- Tilley, F.W.; Schaffer, J.M. Relation between the chemical constitution and germicidal activity of the monohydric alcohols and phenols. J. Bacteriol. 1926, 12, 303–309. [Google Scholar] [CrossRef] [PubMed]
- Golin, A.P.; Choi, D.; Ghahary, A. Hand sanitizers: A review of ingredients, mechanisms of action, modes of delivery, and efficacy against coronaviruses. Am. J. Infect. Control 2020, 48, 1062–1067. [Google Scholar] [CrossRef] [PubMed]
- Jing, J.L.J.; Pei Yi, T.; Bose, R.J.C.; McCarthy, J.R.; Tharmalingam, N.; Madheswaran, T. Hand Sanitizers: A Review on Formulation Aspects, Adverse Effects, and Regulations. Int. J. Environ. Res. Public Health 2020, 17, 3326. [Google Scholar] [CrossRef]
- WHO. WHO Guidelines on Hand Hygiene in Health Care: First Global Patient Safety Challenge: Clean Care Is Safer Care; World Health Organization, Patient Safety: Geneva, Switzerland, 2009.
- Pereira, B.M.P.; Tagkopoulos, I. Benzalkonium Chlorides: Uses, Regulatory Status, and Microbial Resistance. Appl. Environ. Microbiol. 2019, 85, e00377-19. [Google Scholar]
- Mohapatra, S.; Yutao, L.; Goh, S.G.; Ng, C.; Luhua, Y.; Tran, N.H.; Gin, K.Y.-H. Quaternary Ammonium Compounds of Emerging Concern: Classification, Occurrence, Fate, Toxicity and Antimicrobial Resistance. J. Hazard. Mater. 2022, 445, 130393. [Google Scholar] [PubMed]
- Lunn, R.M.; Mehta, S.S.; Jahnke, G.D.; Wang, A.; Wolfe, M.S.; Berridge, B.R. Cancer hazard evaluations for contemporary needs: Highlights from new national toxicology program evaluations and methodological advancements. J. Natl. Cancer Inst. 2022, 114, 1441–1448. [Google Scholar] [CrossRef]
- Ramchandani, V.A.; Bosron, W.F.; Li, T.K. Research advances in ethanol metabolism. Pathol. Biol. 2001, 49, 676–682. [Google Scholar] [CrossRef]
- Mello, T.; Ceni, E.; Surrenti, C.; Galli, A. Alcohol induced hepatic fibrosis: Role of acetaldehyde. Mol. Asp. Med. 2008, 29, 17–21. [Google Scholar] [CrossRef]
- Lieber, C.S. Ethanol metabolism, cirrhosis and alcoholism. Clin. Chim. Acta 1997, 257, 59–84. [Google Scholar] [CrossRef]
- Nordmann, R.; Ribiere, C.; Rouach, H.; Beauge, F.; Giudicelli, Y.; Nordmann, J. Metabolic pathways involved in the oxidation of isopropanol into acetone by the intact rat. Life Sci. 1973, 13, 919–932. [Google Scholar] [CrossRef]
- Kalapos, M.P. On the mammalian acetone metabolism: From chemistry to clinical implications. Biochim. Biophys. Acta (BBA)-Gen. Subj. 2003, 1621, 122–139. [Google Scholar] [CrossRef]
- Ross, J.A.; Borek, H.A.; Holstege, C.P.; King, J.D. Toxic Alcohol Poisoning. Emerg. Med. Clin. N. Am. 2022, 40, 327–341. [Google Scholar] [CrossRef]
- Daniel, D.R.; McAnalley, B.H.; Garriott, J.C. Isopropyl Alcohol Metabolism After Acute Intoxication in Humans. J. Anal. Toxicol. 1981, 5, 110–112. [Google Scholar] [CrossRef] [PubMed]
- Jones, A.W. Elimination Half-Life of Acetone in Humans: Case Reports and Review of the Literature. J. Anal. Toxicol. 2000, 24, 8–10. [Google Scholar] [CrossRef] [PubMed]
- Tomsia, M.; Głaz, M.; Nowicka, J.; Cieśla, J.; Sosnowski, M.; Chełmecka, E. Fatal Methanol Poisoning Caused by Drinking Industrial Alcohol: Silesia Region, Poland, April–June 2022. Toxics 2022, 10, 800. [Google Scholar] [CrossRef] [PubMed]
- Hovda, K.E.; Hunderi, O.H.; Tafjord, A.B.; Dunlop, O.; Rudberg, N.; Jacobsen, D. Methanol outbreak in Norway 2002-2004: Epidemiology, clinical features and prognostic signs. J. Intern. Med. 2005, 258, 181–190. [Google Scholar] [CrossRef] [PubMed]
- Liberski, S.; Kaluzny, B.J.; Kocięcki, J. Methanol-induced optic neuropathy: A still-present problem. Arch. Toxicol. 2022, 96, 431–451. [Google Scholar] [CrossRef] [PubMed]
- Cordell, R.L.; Pandya, H.; Hubbard, M.; Turner, M.A.; Monks, P.S. GC-MS analysis of ethanol and other volatile compounds in micro-volume blood samples—Quantifying neonatal exposure. Anal. Bioanal. Chem. 2013, 405, 4139–4147. [Google Scholar] [CrossRef]
- Mead, R.N.; Cala, J.M.; Felix, J.D.; Shimizu, M.S.; Casas, M.S.; Lathrope, T.; Avery, G.B.; Kieber, R.J.; Willey, J.D. A static headspace GC-MS/MS method for the determination of ethanol, iso-butanol, and n -butanol at nanomolar concentrations in aqueous environmental samples. Limnol. Oceanogr. 2017, 15, 1007–1014. [Google Scholar] [CrossRef]
- Mihretu, L.D.; Gebru, A.G.; Mekonnen, K.N.; Asgedom, A.G.; Desta, Y.H. Determination of ethanol in blood using headspace gas chromatography with flameionization detector (HS-GC-FID): Validation of a method. Cogent Chem. 2020, 6, 1760187. [Google Scholar] [CrossRef]
- Swift, R. Direct measurement of alcohol and its metabolites. Addiction 2003, 98, 73–80. [Google Scholar] [CrossRef]
- Ellis, D.I.; Muhamadali, H.; Xu, Y.; Eccles, R.; Goodall, I.; Goodacre, R. Rapid through-container detection of fake spirits and methanol quantification with handheld Raman spectroscopy. Analyst 2019, 144, 324–330. [Google Scholar] [CrossRef] [PubMed]
- Enrico, P.; Diana, M. On the Accuracy of In Vivo Ethanol and Acetaldehyde Monitoring, a Key Tile in the Puzzle of Acetaldehyde as a Neuroactive Agent. Front. Behav. Neurosci. 2017, 11, 97. [Google Scholar] [CrossRef] [PubMed]
- Kristoffersen, L.; Smith-Kielland, A. An Automated Alcohol Dehydrogenase Method for Ethanol Quantification in Urine and Whole Blood. J. Anal. Toxicol. 2005, 29, 387–389. [Google Scholar] [CrossRef] [PubMed]
- Church, A.S.; Witting, M.D. Laboratory testing in ethanol, methanol, ethylene glycol, and isopropanol toxicities. J. Emerg. Med. 1997, 15, 687–692. [Google Scholar] [CrossRef] [PubMed]
- Sugaya, N.; Nakagawa, T.; Sakurai, K.; Morita, M.; Onodera, S. Analysis of Aldehydes in Water by Head Space-GC/MS. J. Health Sci. 2001, 47, 21–27. [Google Scholar] [CrossRef][Green Version]
- Abrigo, N.; Ruzicka, C.; Faustino, P.; Stiber, N.; NguyenPho, A.; O’Connor, T.; Shakleya, D. Development and validation of a headspace GC-MS method to evaluate the interconversion of impurities and the product quality of liquid hand sanitizers. AAPS Open 2022, 8, 1. [Google Scholar] [CrossRef]
- Tiscione, N.B.; Alford, I.; Yeatman, D.T.; Shan, X. Ethanol Analysis by Headspace Gas Chromatography with Simultaneous Flame-Ionization and Mass Spectrometry Detection. J. Anal. Toxicol. 2011, 35, 501–511. [Google Scholar] [CrossRef]
- Chun, H.-J.; Poklis, J.L.; Poklis, A.; Wolf, C.E. Development and Validation of a Method for Alcohol Analysis in Brain Tissue by Headspace Gas Chromatography with Flame Ionization Detector. J. Anal. Toxicol. 2016, 40, 653–658. [Google Scholar] [CrossRef]
- Islek, D.; Ramadanoglu, S. Headspace-gas chromatography/mass spectrometry analysis of methanol in blood. Med. Sci. Int. Med. J. 2017, 6, 372–374. [Google Scholar] [CrossRef]
- Xiao, H.-T.; He, L.; Tong, R.-S.; Yu, J.-Y.; Chen, L.; Zou, J.; Li, J.-Q.; Bian, Y.; Zhang, Y. Rapid and Sensitive Headspace Gas Chromatography-Mass Spectrometry Method for the Analysis of Ethanol in the Whole Blood. J. Clin. Lab. Anal. 2014, 28, 386–390. [Google Scholar] [CrossRef] [PubMed]
- Wunder, C.; Pogoda, W.; Paulke, A.; Toennes, S.W. Assay of ethanol and congener alcohols in serum and beverages by headspace gas chromatography/mass spectrometry. MethodsX 2021, 8, 101563. [Google Scholar] [CrossRef] [PubMed]
- Patel, J.P.; Sowers, M.L.; Herring, J.L.; Theruvathu, J.A.; Emmett, M.R.; Hawkins, B.E.; Zhang, K.; DeWitt, D.S.; Prough, D.S.; Sowers, L.C. Measurement of Postreplicative DNA Metabolism and Damage in the Rodent Brain. Chem. Res. Toxicol. 2015, 28, 2352–2363. [Google Scholar] [CrossRef] [PubMed]
- Jang, M.; Yang, H.; Shin, G.; Koo, J.M.; Hwang, S.Y.; Park, J.X.; Oh, D. Determination of Methanol in Commercialized Alcohol-based Hand Sanitizing and Other Similar Products using Headspace GC-MS. Curr. Anal. Chem. 2022, 18, 774–780. [Google Scholar] [CrossRef]
- Valavala, S.; Seelam, N.; Tondepu, S.; Jagarlapudi, V.S.K.; Sundarmurthy, V. Analytical Method Development and Validation for the Quantification of Acetone and Isopropyl Alcohol in the Tartaric Acid Base Pellets of Dipyridamole Modified Release Capsules by Using Headspace Gas Chromatographic Technique. J. Anal. Methods Chem. 2018, 2018, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Rahman, Z.; Akhtar, S.; Siddiqui, A.; Ciavarella, A.B.; Nguyenpho, A.; Faustino, P.J.; Khan, M.A. A headspace-gas chromatography method for isopropanol determination in warfarin sodium products as a measure of drug crystallinity. Acta Pharm. 2018, 68, 31–46. [Google Scholar] [CrossRef]
- Elzinga, S.; Dominguez-Alonzo, J.; Keledjian, R.; Douglass, B.; Raber, J.C. Acetone as Artifact of Analysis in Terpene Samples by HS-GC/MS. Molecules 2022, 27, 6037. [Google Scholar] [CrossRef]
- Massick, S. Portable Breath Acetone Measurements Combine Chemistry and Spectroscopy. SPIE Newsroom 2007. Available online: https://spie.org/news/0948-portable-breath-acetone-measurements-combine-chemistry-and-spectroscopy?highlight=x2416&SSO=1 (accessed on 6 January 2024).
- Dong, C.; Xing, X.; Chen, N.; Liu, X.; Wang, Y. Synthesis of Hollow CuO Fibers for Low-Ppm-Level n-Propanol Detection via a Facile Solution Combustion Method. Sens Actuators B. 2016, 230, 1–8. [Google Scholar] [CrossRef]
- Li, W.; Li, X.; Zong, Y.; Kong, L.; Zhu, W.; Xu, M.; Liu, H. Detection of n-Propanol Down to the Sub-ppm Level Using p-Type Delafossite AgCrO2 Nanoparticles. ACS Sens. 2023, 8, 289–296. [Google Scholar] [CrossRef]
- Manuel, C.S.; Yeomans, D.J.; Williams, J.A.; Fricker, C.; Kucera, K.; Light, D.; Arbogast, J.W. Presence of unsafe chemical impurities, accelerated evaporation of alcohol, and lack of key labeling requirements are risks and concerns for some alcohol-based hand sanitizers and dispenser practices during the COVID-19 pandemic. PLoS ONE 2022, 17, e0265519. [Google Scholar] [CrossRef] [PubMed]
- Gloekler, L.E.; de Gandiaga, E.J.; Binczewski, N.R.; Steimel, K.G.; Massarsky, A.; Kozal, J.; Vincent, M.; Zisook, R.; LaGuardia, M.J.; Dotson, S.; et al. Evaluation of the Safety and Efficacy of Hand Sanitizer Products Marketed to Children Available during the COVID-19 Pandemic. Int. J. Environ. Res. Public Health 2022, 19, 14424. [Google Scholar] [CrossRef] [PubMed]
- Nisbar, N.D.; Jamal Khair, S.K.; Bujang, N.B.; Mohd Yusop, A.Y. Determination of ethanol, isopropyl alcohol and methanol in alcohol-based hand sanitiser to ensure product quality, safety and efficacy. Sci. Rep. 2023, 13, 9478. [Google Scholar] [CrossRef] [PubMed]
- Moreira, M.T.G.; Pereira, P.R.; Aquino, A.; Conte-Junior, C.A.; Paschoalin, V.M.F. Aldehyde Accumulation in Aged Alcoholic Beer: Addressing Acetaldehyde Impacts on Upper Aerodigestive Tract Cancer Risks. Int. J. Mol. Sci. 2022, 23, 14147. [Google Scholar] [CrossRef] [PubMed]
- Shin, K.S.; Lee, J.H. Acetaldehyde contents and quality characteristics of commercial alcoholic beverages. Food Sci. Biotechnol. 2019, 28, 1027–1036. [Google Scholar] [CrossRef] [PubMed]
- Han, G.; Webb, M.R.; Waterhouse, A.L. Acetaldehyde reactions during wine bottle storage. Food Chem. 2019, 290, 208–215. [Google Scholar] [CrossRef]
- Heitler, C.; Scaife, D.B.; Thompson, B.W. The oxidation of ethanol by hydrogen peroxide. Part I. Catalysis by ferric ion. J. Chem. Soc. A 1967, 1967, 1409–1413. [Google Scholar] [CrossRef]
- Martinsson, A.; Hasani, M.; Theliander, H. The influence of transition metal ions on the oxidation of kraft pulp using hydrogen peroxide under mildly acidic conditions. Holzforschung 2023, 77, 318–325. [Google Scholar] [CrossRef]
- Snopek, L.; Mlcek, J.; Sochorova, L.; Baron, M.; Hlavacova, I.; Jurikova, T.; Kizek, R.; Sedlackova, E.; Sochor, J. Contribution of Red Wine Consumption to Human Health Protection. Molecules 2018, 23, 1684. [Google Scholar] [CrossRef]
- da Silva, G.; Bozzelli, J.W.; Liang, L.; Farrell, J.T. Ethanol oxidation: Kinetics of the alpha-hydroxyethyl radical + O2 reaction. J. Phys. Chem. A 2009, 113, 8923–8933. [Google Scholar] [CrossRef]
- Molom-Ochir, S.; Davis, K.M. Effects of Temperature on Hand Sanitizer Efficiency. J. Emerg. Investig. 2022, 5, 1. [Google Scholar] [CrossRef] [PubMed]
- Dalziel, K.; Dickinson, F.M. The kinetics and mechanism of liver alcohol dehydrogenase with primary and secondary alcohols as substrates. Biochem. J. 1966, 100, 34–46. [Google Scholar] [CrossRef] [PubMed]

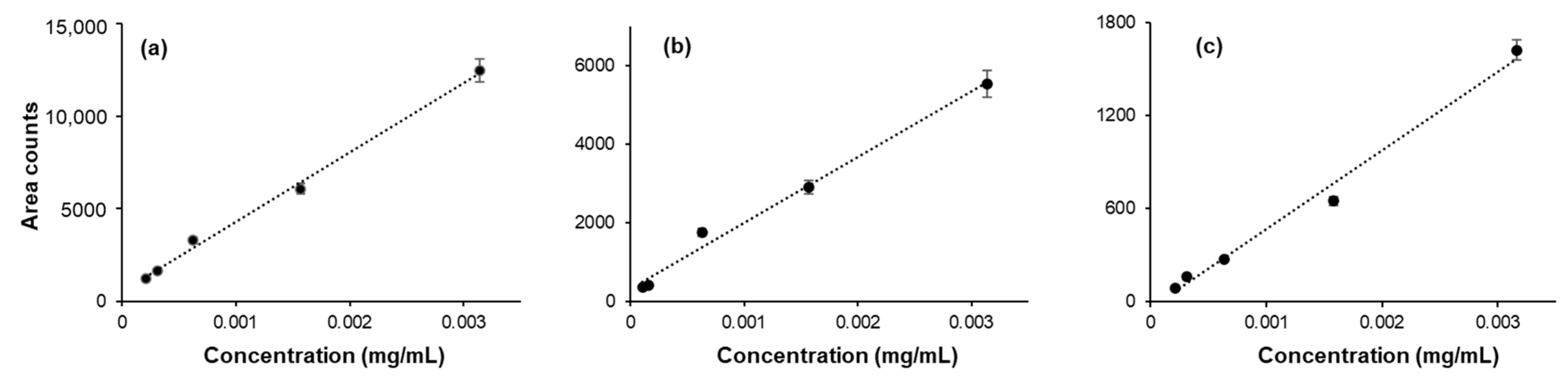
| Sample | Major Component | Minor Components (mg/mL) | ||||
|---|---|---|---|---|---|---|
| Methanol | Isopropanol | n-Propanol | Acetaldehyde | Acetone | ||
| HS1 | Ethanol | 0.000921 | 0.4539 | n.d | 0.00585 | n.d |
| HS2 | Ethanol | 0.000312 | 13.840 | 0.00267 | 0.00402 | n.d |
| HS3 | Ethanol | 0.00139 | 0.4967 | 0.000907 | 0.05206 | n.d |
| HS4 | Ethanol | 0.00389 | 0.4969 | 0.000474 | 0.23120 | n.d |
| HS5 | BenzalkCl | 0.000143 | n.d | n.d | 0.00062 | n.d |
| HS6 | Ethanol | 0.00457 | 0.5035 | 0.00143 | 0.00228 | n.d |
| HS7 | Ethanol | 0.01519 | 0.6050 | 0.1520 | 0.19420 | n.d |
| HS8 | Ethanol | 0.00204 | 0.5059 | 0.00829 | 0.00158 | n.d |
| HS9 | Isopropanol | 0.00156 | N/A | 0.02492 | 0.02043 | 0.6966 |
| FDA limit | NMT 0.63 | NR | NMT 1.00 | NMT 0.05 | NMT 4.40 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
To, N.D.K.; Theruvathu, J.A. Determination and Quantification of Acetaldehyde, Acetone, and Methanol in Hand Sanitizers Using Headspace GC/MS: Effect of Storage Time and Temperature. Int. J. Environ. Res. Public Health 2024, 21, 74. https://doi.org/10.3390/ijerph21010074
To NDK, Theruvathu JA. Determination and Quantification of Acetaldehyde, Acetone, and Methanol in Hand Sanitizers Using Headspace GC/MS: Effect of Storage Time and Temperature. International Journal of Environmental Research and Public Health. 2024; 21(1):74. https://doi.org/10.3390/ijerph21010074
Chicago/Turabian StyleTo, Ngoc Diem Kieu, and Jacob A. Theruvathu. 2024. "Determination and Quantification of Acetaldehyde, Acetone, and Methanol in Hand Sanitizers Using Headspace GC/MS: Effect of Storage Time and Temperature" International Journal of Environmental Research and Public Health 21, no. 1: 74. https://doi.org/10.3390/ijerph21010074
APA StyleTo, N. D. K., & Theruvathu, J. A. (2024). Determination and Quantification of Acetaldehyde, Acetone, and Methanol in Hand Sanitizers Using Headspace GC/MS: Effect of Storage Time and Temperature. International Journal of Environmental Research and Public Health, 21(1), 74. https://doi.org/10.3390/ijerph21010074





