Effects of Virtual Reality Exercise Program on Blood Glucose, Body Composition, and Exercise Immersion in Patients with Type 2 Diabetes
Abstract
1. Introduction
2. Materials and Methods
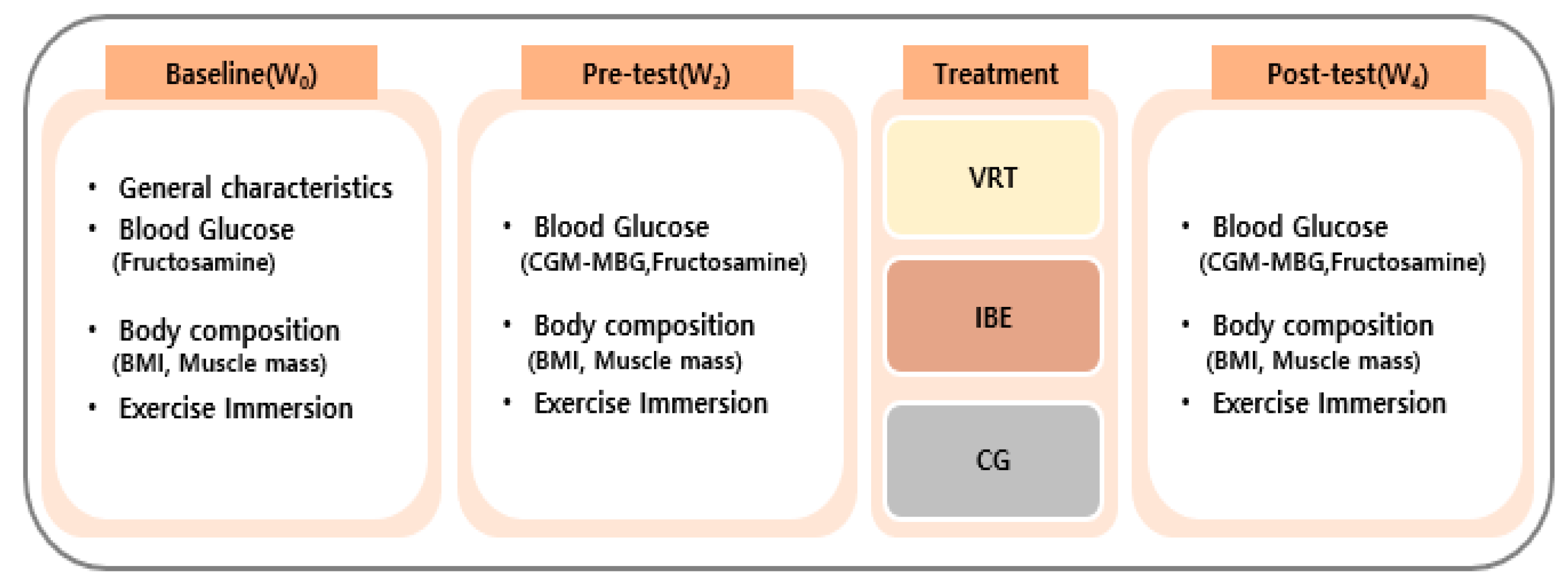
2.1. Study Design
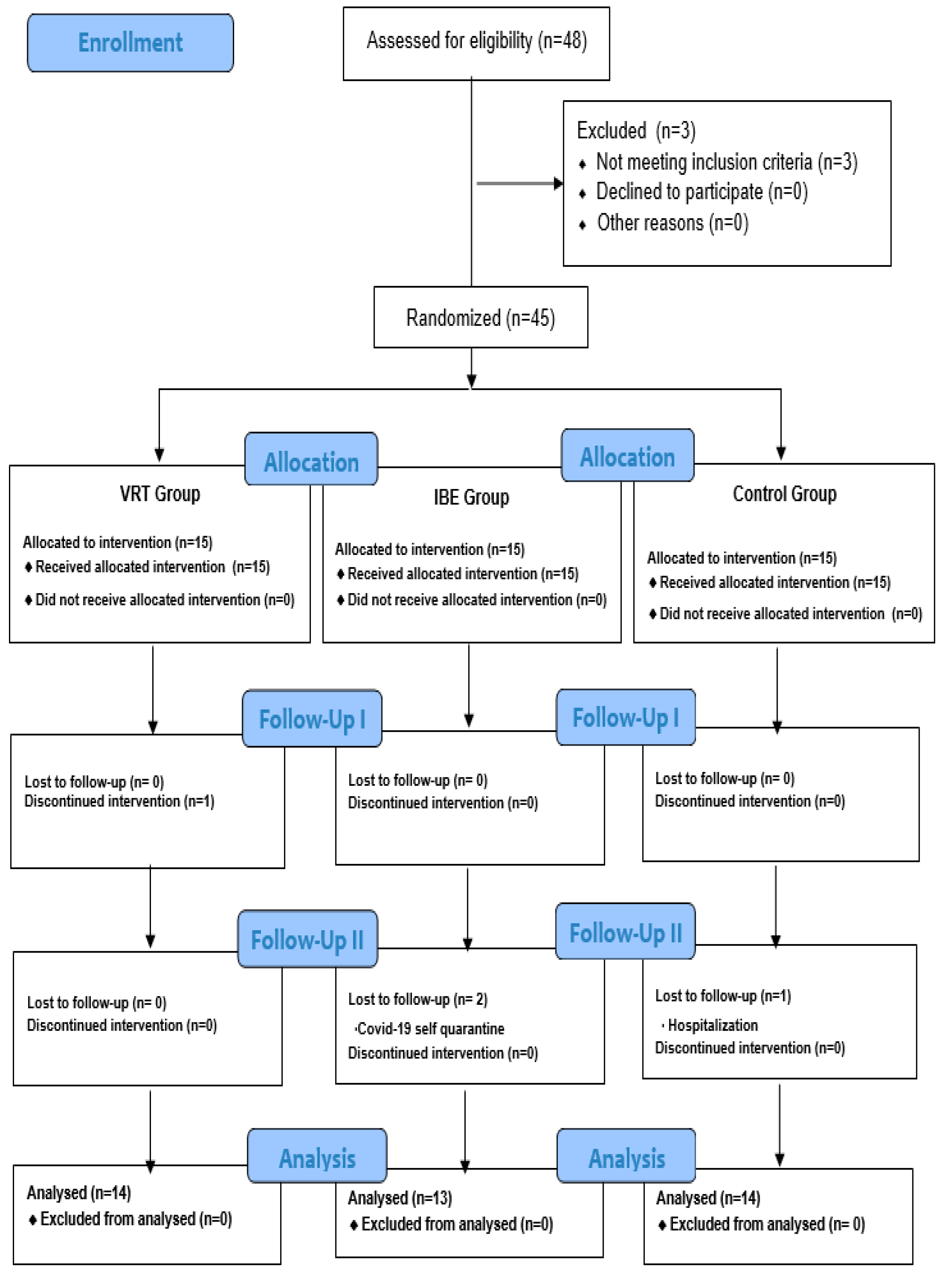
2.2. Participants
2.3. Experimental Intervention
2.4. Measurements
2.4.1. Mean Blood Glucose (MBG)
2.4.2. Serum Fructosamine
2.4.3. Body Composition
2.4.4. Exercise Immersion
2.5. Data Analysis
2.6. Ethical Considerations
3. Results
3.1. Homogeneity Test for the Participants’ General Characteristics and Previous Dependent Variables
3.2. Effects of VREP on the Blood Glucose, Body Composition, and Exercise Immersion
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Saeedi, P.; Petersohn, I.; Salpea, P.; Malanda, B.; Karuranga, S.; Unwin, N.; Colagiuri, S.; Guariguata, L.; Motala, A.A.; Ogurtsova, K.; et al. IDF Diabetes Atlas Committee. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas, 9th edition. Diabetes Res. Clin. Pract. 2019, 157, 107843. [Google Scholar] [CrossRef] [PubMed]
- Statistics Korea: Causes of Death Statistics in 2020. Available online: http://kostat.go.kr (accessed on 28 September 2021).
- Tan, E.; Polello, J.; Woodard, L.J. An Evaluation of the Current Type 2 Diabetes Guidelines: Where They Converge and Diverge. Clin. Diabetes 2014, 32, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Korean Diabetes Association: Diabetes Fact Sheet in Korea 2018. Available online: https://www.diabetes.or.kr/bbs/?code=fact_sheet&mode=view&number=1546&page=1&code=fact_sheet (accessed on 28 September 2021).
- Lin, C.H.; Ho, C.W.; Chen, L.C.; Chang, C.C.; Wang, Y.W.; Chiou, C.P.; Chiang, S.L. Effects of a 12-week exercise training on insulin sensitivity, quality of life, and depression status in patients with type 2 diabetes. J. Med. Sci. 2017, 37, 227–236. [Google Scholar]
- Warburton, D.E.; Nicol, C.W.; Bredin, S.S. Health benefits of physical activity: The evidence. CMAJ 2006, 174, 801–809. [Google Scholar] [CrossRef]
- Boulé, N.G.; Haddad, E.; Kenny, G.P.; Wells, G.A.; Sigal, R.J. Effects of Exercise on Glycemic Control and Body Mass in Type 2 Diabetes Mellitus: A Meta-analysis of Controlled Clinical Trials. JAMA 2001, 286, 1218–1227. [Google Scholar] [CrossRef]
- Kränkel, N.; Bahls, M.; Van Craenenbroeck, E.M.; Adams, V.; Serratosa, L.; Solberg, E.E.; Hansen, D.; Dörr, M.; Kemps, H. Exercise training to reduce cardiovascular risk in patients with metabolic syndrome and type 2 diabetes mellitus: How does it work? Eur. J. Prev. Cardiol. 2019, 26, 701–708. [Google Scholar] [CrossRef] [PubMed]
- Marçal, I.R.; Fernandes, B.; Viana, A.A.; Ciolac, E.G. The Urgent Need for Recommending Physical Activity for the Management of Diabetes During and Beyond COVID-19 Outbreak. Front. Endocrinol. 2020, 11, 584642. [Google Scholar] [CrossRef]
- Park, J.C.; Lee, J.H. Exercise Coaching System based on Indoor Bicycle for Diabetic Patients. J. Korea Contents Assoc. 2019, 19, 86–95. [Google Scholar]
- Song, H.W.; Ryu, H.K.; Seo, C.W.; Yoon, D.W.; Choi, E.S.; An, D.H. A study on the development of cycling machines based on the virtual reality. In Proceedings of the Korea Information Processing Society Conference; Korea Information Processing Society: Busan, Republic of Korea, 2017; pp. 1103–1104. [Google Scholar]
- Lee, T.E.; Lee, C.W. Virtual Reality Content Design for Improving Exercise Sustainability. J. Commun. Des. 2020, 71, 251–260. [Google Scholar]
- Weiss, P.L.; Rand, D.; Katz, N.; Kizony, R. Video capture virtual reality as a flexible and effective rehabilitation tool. J. Neuroeng. Rehabil. 2004, 1, 12. [Google Scholar] [CrossRef]
- Kim, Y.S.; Park, M.C. Comparison of Balance Ability according to the Immersion Level of Virtual Reality-based Training for the Balance Enhancement of the Elderly. PNF Mov. 2018, 16, 259–266. [Google Scholar]
- Ha, C.W. The effects of virtual reality (VR) based game-type physical education lesson on the basal fitness and attention of students with autistic disorder. Spec. Educ. Res. 2019, 18, 5–28. [Google Scholar] [CrossRef]
- Weber, L.M.; Nilsen, D.M.; Gillen, G.; Yoon, J.; Stein, J. Immersive virtual reality mirror therapy for upper limb recovery after stroke: A pilot study. Am. J. Phys. Med. Rehabil. 2019, 98, 783–788. [Google Scholar] [CrossRef]
- Cjoi, K.W.; Baik, S.M.; Park, H.K. The effects of virtual reality exercise program with Wii on walking, balance and upper extremity function in stroke patients. Korea J. Neuromuscul. Rehabil. 2022, 12, 33–40. [Google Scholar]
- Kim, Y.G.; Kang, S.H. The effect of virtual reality-based exercise program on balance, gait, and falls efficacy in patients with Parkinson’s disease. J. Korean Soc. Phys. Med. 2019, 14, 103–113. [Google Scholar] [CrossRef]
- Hwang, H.S.; Yoo, D.H.; Kim, H.; Kim, S.K. Effects of virtual reality-based upper limb rehabilitation training on upper limb function, muscle activation, activities of daily living, and quality of life in stroke patients. Korea J. Occup. Ther. 2020, 28, 115–129. [Google Scholar] [CrossRef]
- Lee, G.H. Effects of a virtual reality exercise program (Wii) on cognitive function of elderly people with Alzheimer dementia. J. Korean Acad. Kinesiol. 2017, 19, 35–44. [Google Scholar]
- Faul, F.; Erdfelder, E.; Buchner, A.; Lang, A.-G. Statistical power analyses using G* Power 3.1: Tests for correlation and regression analyses. Behav. Res. Methods 2009, 41, 1149–1160. [Google Scholar] [CrossRef]
- Motahari-Tabari, N.; Shirvani, M.A.; Shirzad-E-Ahoodashty, M.; Yousefi-Abdolmaleki, E.; Teimourzadeh, M. The effect of 8 weeks aerobic exercise on insulin resistance in type 2 diabetes: A randomized clinical trial. Glob. J. Health Sci. 2015, 7, 115. [Google Scholar] [CrossRef]
- Swain, D.P.; Brawner, C.A.; Chambliss, H.O.; Nagelkirk, P.R.; Bayles, M.P.; Swank, A.M. ACSM’s Resource Manual for Guidelines for Exercise Testing and Prescription; Wolters Kluwer Health/Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2014. [Google Scholar]
- Colberg, S.R.; Sigal, R.J.; Yardley, J.E.; Riddel, M.C.; Dunstan, D.W.; Dempsey, P.C.; Horton, E.S.; Castorino, K.; Tate, D.F. Physical activity/exercise and diabetes: A position statement of the American Diabetes Association. Diabetes Care 2016, 39, 2065–2079. [Google Scholar] [CrossRef]
- Kim, H.S.; Shin, C.H.; Jin, J.K. Validity of RPE-13 as optimal exercise intensity. Exerc. Sci. 1997, 6, 33–44. [Google Scholar]
- American Heart Association. 2020. Available online: http://www.heart.org/en/healthy-living/fitness/fitness-basics/warm-up-cool-down (accessed on 28 September 2021).
- Jung, Y.G. Validity Verification of Sport Commitment Behavior Scale. Korean J. Sport Psychol. 2004, 15, 1–21. [Google Scholar]
- Kim, J.G. The Effect of a 24-Week Aerobic Exercise on Vascular Inflammation in Elderly Women with Type 2 Diabetes Mellitus. Int. J. Coach. Sci. 2018, 20, 70–76. [Google Scholar]
- Choi, J.H.; Shin, W.S.; Rho, K.T.; Yeon, P.S. Effects of acute forest walking exercise on blood glucose of IGT, NIDDM in the elderly. J. Korean Soc. For. Sci. 2010, 99, 47–51. [Google Scholar]
- Kang, S.J.; Ko, K.J.; Baek, U.H. Effects of 12 weeks combined aerobic and resistance exercise on heart rate variability in type 2 diabetes mellitus patients. J. Phys. Ther. Sci. 2016, 28, 2088–2093. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.W. Effects of 16-week Walking and Tubing Exercise on Type 2 Diabetes, Insulin Resistance and Cardiovascular Function in Elderly Women. Korea Sport Soc. 2020, 18, 423–429. [Google Scholar] [CrossRef]
- Moura, B.P.; Amorom, P.R.; Silva, B.P.; Franceschini, S.C.; Reis, J.C.; Marins, J.C. Effect of a short-term exercise program on glycemic control measured by fructosamine test in type 2 diabetes patients. Diabetol. Metab. Syndr. 2014, 6, 16. [Google Scholar] [CrossRef]
- Cho, Y.H.; Hwang, Y.S.; Oh, S.I. The effects of aerobic exercise for 8 weeks on the body composition, blood lipid and liver enzyme of obese women. J. Sport Leis Stud. 2009, 38, 755–764. [Google Scholar] [CrossRef]
- Jeon, J.H.; Kim, S.B.; Yoon, J.H. Effects of Walking Exercise Program on Insulin Resistance and Peripheral Artery in Type 2 Diabetic Patients. Korean Soc. Wellness 2013, 8, 177–188. [Google Scholar]
- Kim, Y.J.; Lee, J.Y. Effect of Pilates and Resistance Exercises on Vascular Compliance, Body Composition in obese middle-aged Women. Off. J. Koeran Soc. Danc. Sci. 2015, 32, 115–126. [Google Scholar]
- Kim, S.H. Effects of elastic-band exercise on physical fitness for activities of daily living, muscle mass and pain in elderly women. J. Coach. Dev. 2012, 14, 67–77. [Google Scholar]
- Ha, S.M.; Kim, J.S.; Ha, M.S.; Kim, B.S.; Kim, D.Y. Effects of combined exercise on irisin, body composition and glucose metabolism in obese elderly women with type 2 diabetes mellitus. J. Korean Appl. Sci. Technol. 2019, 36, 1268–1280. [Google Scholar]
- Kim, W.J.; Hur, M.H. Effect of resistance exercise program for middle-aged women with myofascial pain syndrome on shoulder pain, angle of shoulder range of motion, and body composition randomized controlled trial, RCT. J Korean Acad. Nurs. 2020, 50, 286–297. [Google Scholar] [CrossRef] [PubMed]
- Ko, D.S.; Jung, D.I.; Lee, S.H. Physical functions of industrial workers with chronic low back pain and changes in health-related quality of life according to virtual reality exercise program. J. Korea Acad.-Ind. Coop. Soc. 2012, 13, 4564–4571. [Google Scholar]
- Choi, H.J.; Cha, H.J.; Choi, S.M. VR skywalking: A VR fitness system using a skywalker equipment. Proc. HCI Korea 2018, 157, 280–283. [Google Scholar]
- Jung, E.K.; Choi, S.S.; Jung, J.Y. Comparison of educational interest, satisfaction, and achievements of educational virtual reality and videos education before simulation training. Korean J. Emerg. Med. Serv. 2018, 22, 93–102. [Google Scholar]
- Kim, N.R.; Seo, J.R. The Relationship Between Motivation, Satisfaction with Exercise, Immersion in Exercise, And Continuous Behaviors in Cycling Based on Gamification Content. Korean J. Sport. Sci. 2021, 30, 339–350. [Google Scholar] [CrossRef]
- Yao, S.; Kim, G. The effects of immersion in a virtual reality game: Presence and physical activity. In HCI in Games; Fang, X.W., Ed.; Springer: Berlin/Heidelberg, Germany, 2019; pp. 234–242. [Google Scholar]
| Variables | Category | VRT (n = 14) | IBE (n = 13) | CG (n = 14) | χ² or F | p Value |
|---|---|---|---|---|---|---|
| Mean ± SD or N (%) | Mean ± SD or N (%) | Mean ± SD or N (%) | ||||
| Age (y) | 52.93 ± 7.40 | 49.15 ± 5.15 | 53.14 ± 7.06 | 1.518 | 0.232 | |
| Length of illness since onset (m) | 57.79 ± 28.25 | 52.46 ± 34.12 | 52.71 ± 26.73 | 0.141 | 0.869 | |
| Gender | Female | 7 (50.0) | 6 (46.2) | 7 (50.0) | ||
| Male | 7 (50.0) | 7 (53.8) | 7 (50.0) | 0.053 | 0.974 | |
| Education | High school | 7 (50.0) | 4 (30.8) | 8 (57.1) | ||
| More than college | 7 (50.0) | 9 (69.2) | 6 (42.9) | 2.000 | 0.368 | |
| Smoking | Yes | 1 (7.1) | 0 | 1 (7.1) | ||
| No | 13 (92.9) | 13 (100%) | 13 (92.9) | 0.976 | 0.614 | |
| Admission by DM | Yes | 1 (7.1) | 0 | 0 | ||
| No | 13 (92.9) | 13 (100) | 14 (100) | 1.977 | 0.372 | |
| Treatment | Oral drug | 13 (92.9) | 13 (100) | 14 (100) | ||
| Oral drug + insulin injection | 1 (7.1) | 0 | 0 | 1.977 | 0.372 | |
| CGM-MBG (mg/dL) | 137.86 ± 31.11 | 132.62 ± 24.57 | 133.36 ± 21.84 | 0.161 | 0.851 | |
| Fructosamine (µmol/L) | 320.07 ± 21.72 | 314.54 ± 41.72 | 311.93 ± 28.07 | 0.246 | 0.783 | |
| BMI (kg/m2) | 25.24 ± 3.14 | 25.22 ± 2.03 | 25.07 ± 2.26 | 0.018 | 0.982 | |
| Muscle mass (kg) | 26.18 ± 5.25 | 28.12 ± 4.70 | 25.10 ± 4.82 | 1.284 | 0.289 | |
| Exerciseimmersion | 26.43 ± 3.86 | 27.00 ± 5.49 | 25.71 ± 9.15 | 0.130 | 0.879 |
| Variables | VRT (n = 14) | IBE (n = 13) | CG (n = 14) | F (p) | Post Hoc Test | Sources | F (p) | |
|---|---|---|---|---|---|---|---|---|
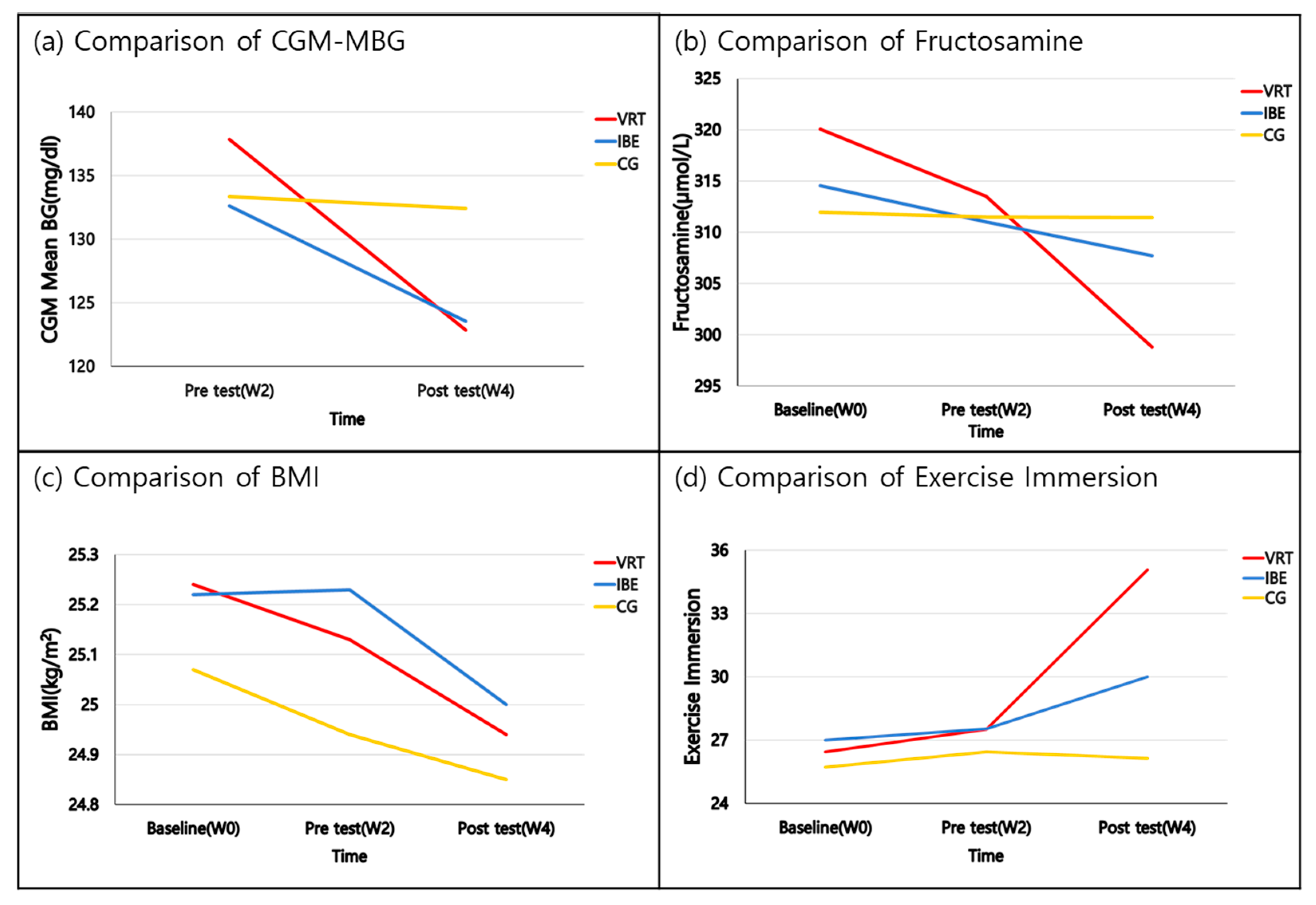
| CGM-MBG (mg/dL) | Baseline (W0) | - | - | - | - | Group Time G*T | 0.135 (0.874) 48.878 (<0.001) 12.001 (<0.001) | |
| Pre-test (W2) | 137.86 ± 31.11 | 132.62 ± 24.57 | 133.36 ± 21.84 | 0.161 (0.851) | ||||
| Post-test (W4) | 122.86 ± 24.77 | 123.54 ± 22.85 | 132.43 ± 19.50 | 0.783 (0.464) | ||||
| Difference (W4-W2) | 15.00 ± 10.26 a | −9.08 ± 6.45 b | −0.93 ± 5.15 c | 12.001 (<0.001) | a, b > c | |||
| Fructosamine (µmol/L) | Baseline (W0) | 320.07 ± 21.72 | 314.54 ± 41.72 | 311.93 ± 28.07 | 0.246 (0.783) | Group Time G*T | 0.003 (0.997) 7.375 (0.001) 3.274 (0.016) | |
| Pre-test (W2) | 313.50 ± 22.10 | 311.00 ± 28.99 | 311.50 ± 33.00 | 0.03 (0.971) | ||||
| Post-test (W4) | 298.79 ± 27.31 | 307.69 ± 38.07 | 311.43 ± 33.15 | 0.541 (0.587) | ||||
| Difference (W2-W0) | −6.57 ± 7.40 | −3.54 ± 24.32 | −0.43 ± 14.73 | 0.472 (0.627) | ||||
| Difference (W4-W2) | −14.71 ± 15.35 a | −3.31 ± 13.63 | −0.07 ± 16.88 c | 3.481 (0.041) | a > c (p = 0.053) ** | |||
| BMI (kg/m2) | Baseline (W0) | 25.24 ± 3.14 | 25.22 ± 2.03 | 25.07 ± 2.26 | 0.018 (0.982) | Group Time G*T | 0.024 (0.977) 6.906 (0.003) 0.835 (0.508) | |
| Pre-test (W2) | 25.13 ± 3.02 | 25.23 ± 2.11 | 24.94 ± 2.23 | 0.049 (0.952) | ||||
| Post-test (W4) | 24.94 ± 2.96 | 25.00 ± 2.04 | 24.85 ± 2.09 | 0.013 (0.987) | ||||
| Difference (W2-W0) | −0.11 ± 0.24 | 0.02 ± 0.25 | −0.14 ± 0.37 | 1.037 (0.365) | ||||
| Difference (W4-W2) | −0.19 ± 0.36 | −0.23 ± 0.29 | −0.09 ± 0.33 | 0.725 (0.491) | ||||
| Muscle mass (kg) | Baseline (W0) | 26.18 ± 5.25 | 28.12 ± 4.70 | 25.10 ± 4.82 | 1.284 (0.289) | Group Time G*T | 1.408 (0.257) 1.779 (0.176) 4.445 (0.003) | |
| Pre-test (W2) | 26.16 ± 5.15 | 28.05 ± 4.56 | 25.09 ± 4.80 | 1.273 (0.292) | ||||
| Post-test (W4) | 26.48 ± 5.16 | 28.31 ± 4.58 | 24.87 ± 4.76 | 1.695 (0.197) | ||||
| Difference (W2-W0) | −0.01 ± 0.28 | −0.07 ± 0.54 | −0.01 ± 0.41 | 0.087 (0.817) | ||||
| Difference (W4-W2) | 0.31 ± 0.32 a | 0.26 ± 0.35 b | −0.22 ± 0.37 c | 9.970 (<0.001) | a, b > c | |||
| Exercise immersion | Baseline (W0) | 26.43 ± 3.86 | 27.00 ± 5.50 | 25.71 ± 9.15 | 0.130 (0.879) | Group Time G*T | 3.574 (0.038) 9.741 (<0.001) 4.418 (0.004) | |
| Pre-test (W2) | 27.50 ± 3.18 | 27.54 ± 3.33 | 26.43 ± 3.86 | 0.455 (0.638) | ||||
| Post-test (W4) | 35.07 ± 4.53 | 30.00 ± 6.23 | 26.14 ± 4.07 | 11.242 (<0.001) | ||||
| Difference (W2-W0) | 1.07 ± 5.11 | 0.54 ± 3.78 | 0.71 ± 9.75 | 0.022 (0.978) | ||||
| Difference (W4-W2) | 7.57 ± 6.19 a | 2.46 ± 5.84 b | −0.29 ± 1.90 c | 8.858 (0.001) | a > b, c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, Y.-j.; Hong, J.-h.; Hur, M.-h.; Seo, E.-y. Effects of Virtual Reality Exercise Program on Blood Glucose, Body Composition, and Exercise Immersion in Patients with Type 2 Diabetes. Int. J. Environ. Res. Public Health 2023, 20, 4178. https://doi.org/10.3390/ijerph20054178
Lee Y-j, Hong J-h, Hur M-h, Seo E-y. Effects of Virtual Reality Exercise Program on Blood Glucose, Body Composition, and Exercise Immersion in Patients with Type 2 Diabetes. International Journal of Environmental Research and Public Health. 2023; 20(5):4178. https://doi.org/10.3390/ijerph20054178
Chicago/Turabian StyleLee, Yu-jin, Jun-hwa Hong, Myung-haeng Hur, and Eun-young Seo. 2023. "Effects of Virtual Reality Exercise Program on Blood Glucose, Body Composition, and Exercise Immersion in Patients with Type 2 Diabetes" International Journal of Environmental Research and Public Health 20, no. 5: 4178. https://doi.org/10.3390/ijerph20054178
APA StyleLee, Y.-j., Hong, J.-h., Hur, M.-h., & Seo, E.-y. (2023). Effects of Virtual Reality Exercise Program on Blood Glucose, Body Composition, and Exercise Immersion in Patients with Type 2 Diabetes. International Journal of Environmental Research and Public Health, 20(5), 4178. https://doi.org/10.3390/ijerph20054178






