Accumulation Potential Cadmium and Lead by Sunflower (Helianthus annuus L.) under Citric and Glutaric Acid-Assisted Phytoextraction
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Preparation
2.2. Experimental Design
2.3. Analysis of Biomass and Heavy Metals
2.4. Quality Control and Statistical Analysis
3. Results
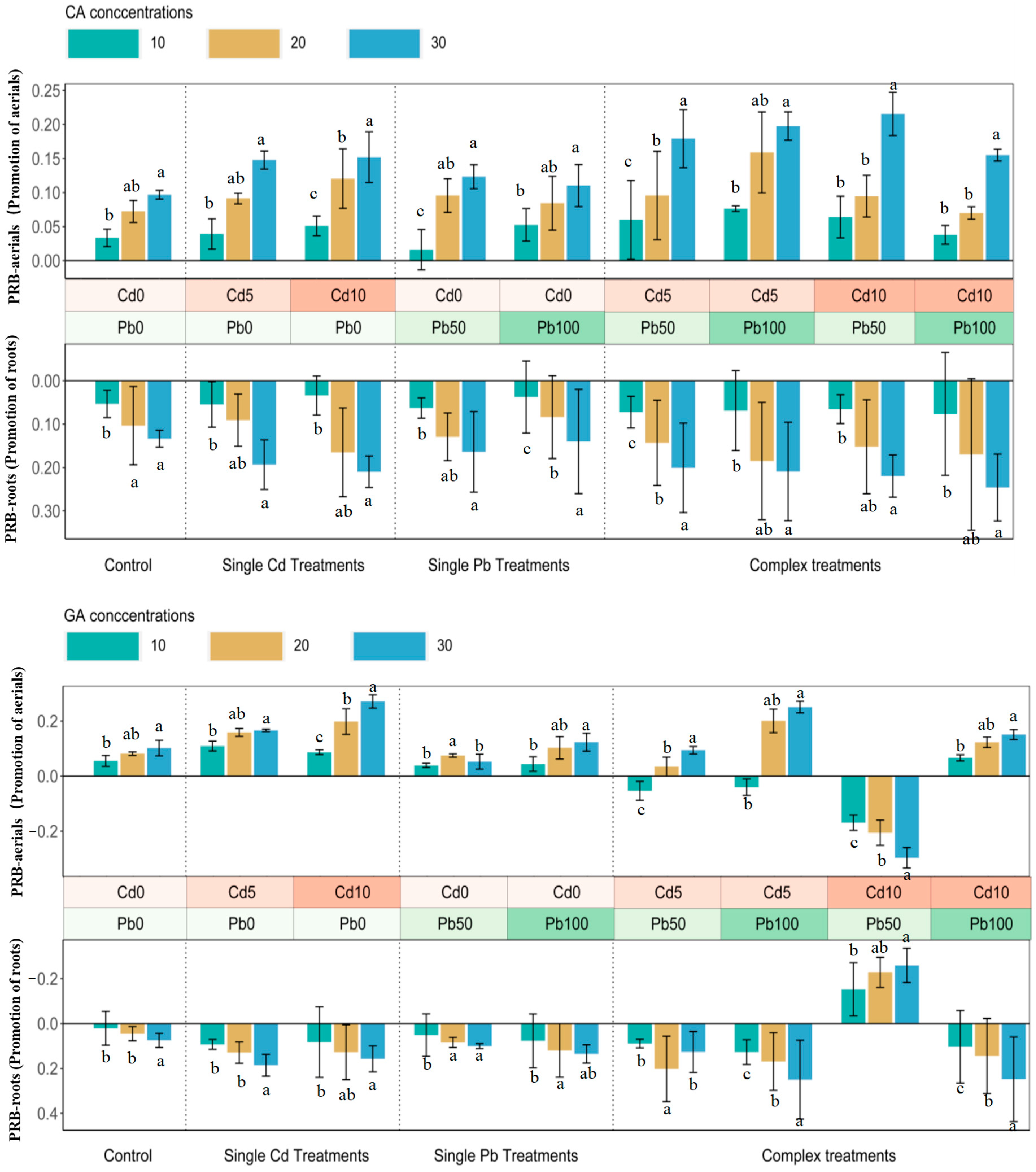
3.1. Biomass Promotion in Citric Acid and Glutaric Acid Treatments
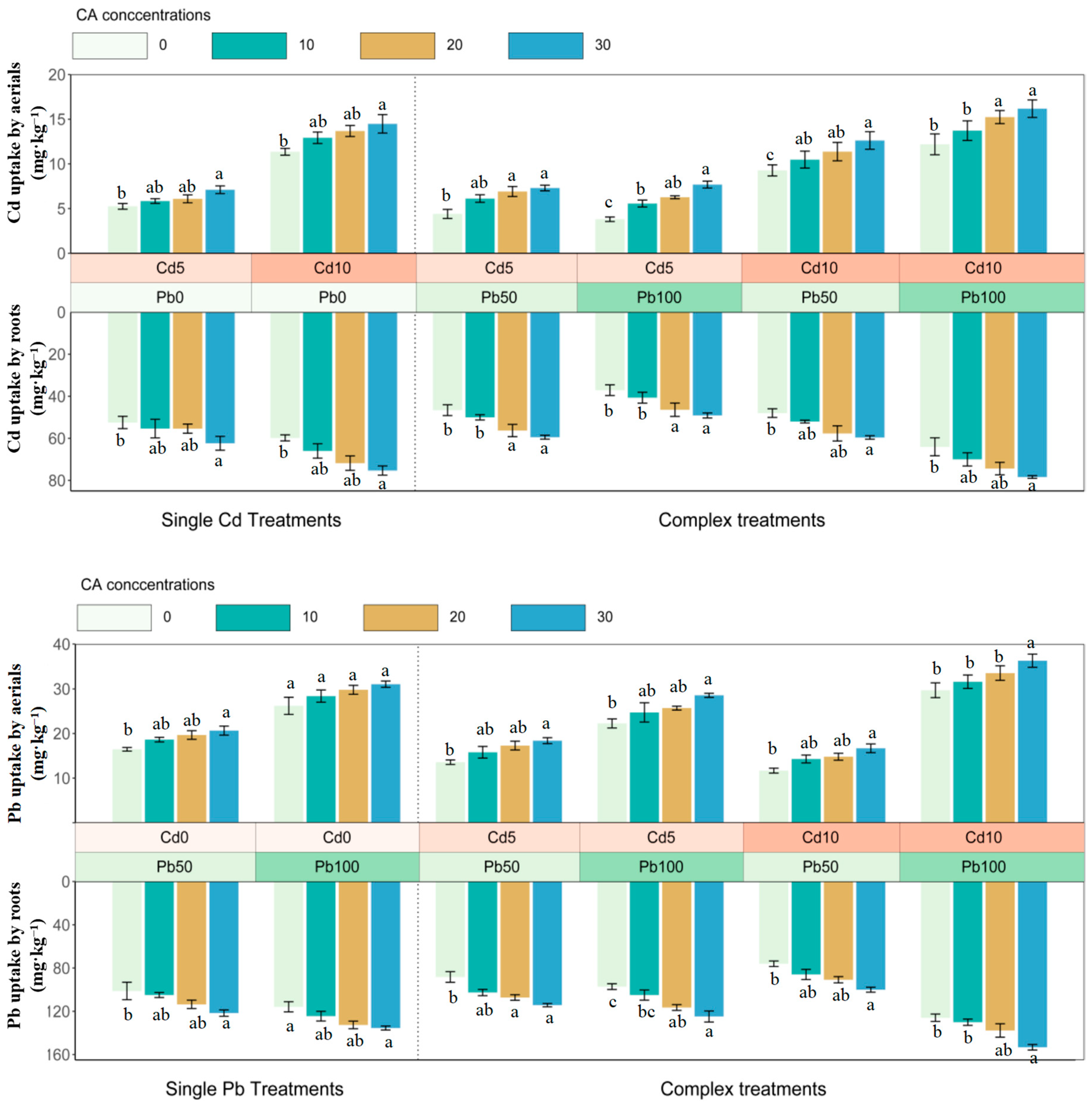
3.2. The Bioaccumulation of Cadmium and Lead
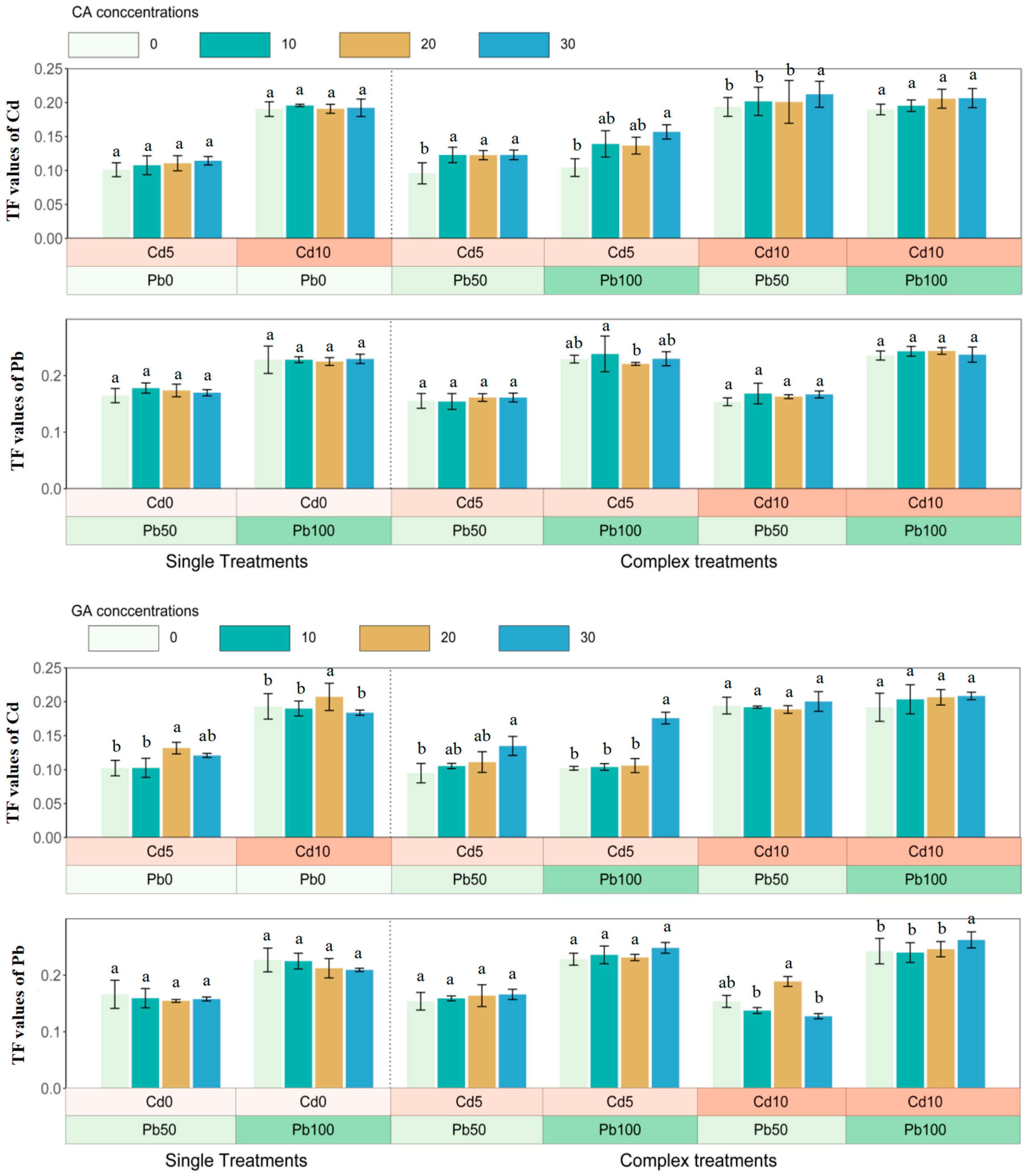
3.3. The Translocation of Cadmium and Lead in Sunflower
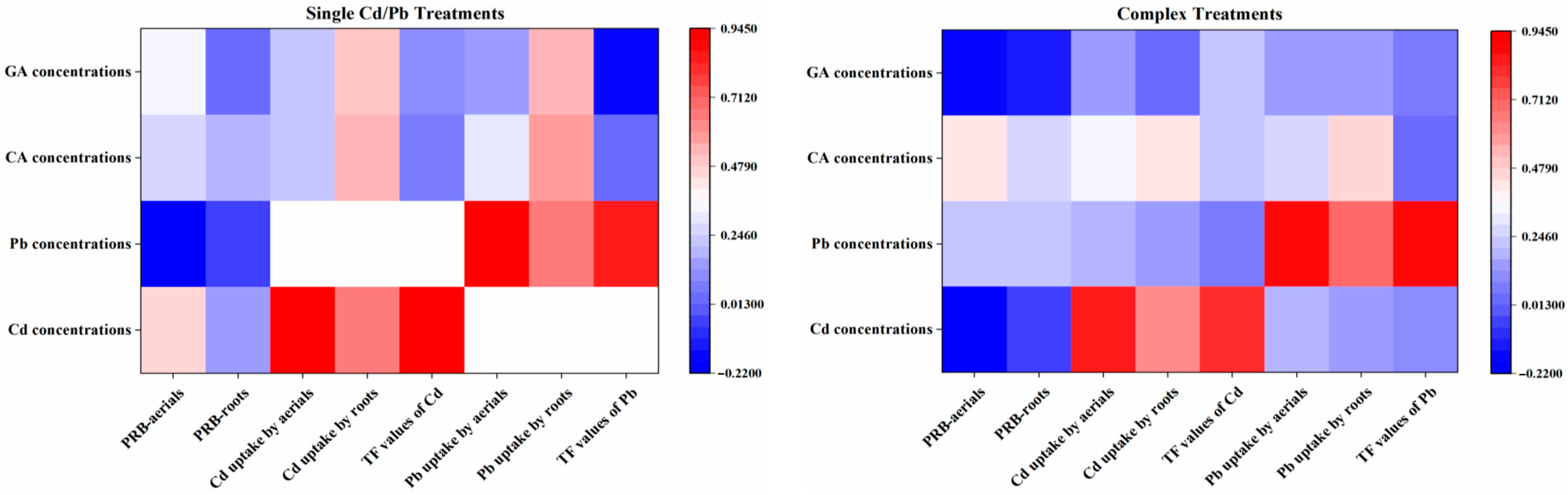
3.4. Relations between OAs Concentrations and PRB and Cd/Pb Uptake
4. Discussion
4.1. Biomass Promotion in Citric Acid and Glutaric Acid Treatments
4.2. The Bioaccumulation of Cadmium and Lead
4.3. The Translocation of Cadmium and Lead in Sunflower
4.4. Relations between OA Concentrations and PRB and Cd/Pb Uptake
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Khalid, A.; Farid, M.; Zubair, M.; Rizwan, M.; Iftikhar, U.; Ishaq, H.K.; Farid, S.; Latif, U.; Hina, K.; Ali, S. Efficacy of Alternanthera bettzickiana to remediate copper and cobalt contaminated soil physiological and biochemical alterations. Int. J. Environ. Res. 2020, 14, 243–255. [Google Scholar] [CrossRef]
- Farid, M.; Ali, S.; Saeed, R.; Rizwan, M.; Bukhari, S.A.H.; Abbasi, G.H.; Hussain, A.; Ali, B.; Zamir, M.S.I.; Ahmad, I. Combined application of citric acid and 5-aminolevulinic acid improved biomass; photosynthesis and gas exchange attributes of sunflower (Helianthus annuus L.) grown on chromium contaminated soil. Int. J. Phytoremediat. 2019, 21, 760–767. [Google Scholar] [CrossRef]
- Shakoor, N.; Nair, R.; Crasta, O.; Morris, G.; Feltus, A.; Kresovich, S. A Sorghum bicolorexpression atlas reveals dynamic genotype-specific expression profiles for vegetative tissues of grain; sweet and bioenergy sorghums. BMC Plant Biol. 2014, 14, 35. [Google Scholar] [CrossRef] [PubMed]
- Farid, M.; Farid, S.; Zubair, M.; Rizwan, M.; Ishaq, H.K.; Ali, S.; Ashraf, U.; Alhaithloul, H.A.S.; Gowayed, S.; Soliman, M.H. Efficacy of Zea mays L. for the management of marble effluent contaminated soil under citric acid amendment; morpho-physiological and biochemical response. Chemosphere 2020, 240, 124930. [Google Scholar] [CrossRef]
- Khalid, S.; Shahid, M.; Niazi, N.K.; Murtaza, B.; Bibi, I.; Dumat, C. A comparison of technologies for remediation of heavy metal contaminated soils. J. Geochem. Explor. 2017, 182, 247–268. [Google Scholar] [CrossRef]
- Prabha, J.; Kumar, M.R.; Tripathi, R. Phytoremediation of Environmental Pollutants; Chapter 17; Elsevier: Amsterdam, The Netherlands, 2021. [Google Scholar]
- Schwab, A.P.; Zhu, D.S.; Banks, M.K. Influence of organic acids on the transport of heavy metals in soil. Chemosphere 2008, 72, 986–994. [Google Scholar] [CrossRef]
- Allah, E.F.; Hashem, A.; Alqarawi, A.A.; Alwathnani, H.A. Alleviation of adverse impact of cadmium stress in sunflower (Helianthus annuus L.) by arbuscular mycorrhizal fungi. Pak. J. Bot. 2015, 47, 785–795. [Google Scholar]
- Piracha, M.A.; Ashraf, M.; Niaz, A. Arsenic fractionation and its impact on physiological behavior of sunflower (Helianthus annuus L.) in three texturally different soils under alkaline calcareous conditions. Environ. Sci. Pollut. Res. 2019, 26, 17438–17449. [Google Scholar] [CrossRef]
- Farid, M.; Ali, S.; Rizwan, M.; Ali, Q.; Saeed, R.; Nasir, T.; Abbasi, G.H.; Rehmani, M.I.A.; Ata-Ul-Karim, S.T.; Bukhari, S.A.H.; et al. Phyto-management of chromium contaminated soils through sunflower under exogenously applied 5-aminolevulinic acid. Ecotoxicol. Environ. Saf. 2018, 151, 255–265. [Google Scholar] [CrossRef] [PubMed]
- Farid, M.; Ali, S.; Zubair, M.; Saeed, R.; Rizwan, M.; Sallah-Ud-Din, R.; Azam, A.; Ashraf, R.; Ashraf, W. Glutamic acid assisted phyto-management of silver-contaminated soils through sunflower; physiological and biochemical response. Environ. Sci. Pollut. Res. 2018, 25, 25390–25400. [Google Scholar] [CrossRef]
- Blaylock, M.J.; Salt, D.E.; Dushenkov, S.; Zakharova, O.; Gussman, C.; Kapulnik, Y. Enhanced accumulation of Pb in Indian mustard by soil-applied chelating agents. Environ. Sci. Technol. 1997, 31, 860–865. [Google Scholar] [CrossRef]
- Willscher, S.; Jablonski, L.; Fona, Z.; Rahmi, R.; Wittig, J. Phytoremediation experiments with Helianthus tuberosus under different pH and heavy metal soil concentrations. Hydrometallurgy 2017, 168, 153–158. [Google Scholar] [CrossRef]
- Anwer, S.; Ashraf, M.Y.; Hussain, M.; Ashraf, M.; Jamil, A. Citric acid mediated phytoextraction of cadmium by maize (Zea mays L.). Pak. J. Bot. 2012, 44, 1831–1836. [Google Scholar]
- Zaheer, I.E.; Ali, S.; Rizwan, M.; Farid, M.; Shakoor, M.B.; Gill, R.A.; Ullah, N.; Iqbal, N.; Ahmad, R. Citric acid assisted phytoremediation of copper by Brassica napus L. Ecotoxicol. Environ. Saf. 2015, 120, 310–317. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Zhang, L.; Gu, J.; Zhao, J.; Fu, J. Citric acid and EDTA on the growth; photosynthetic properties and heavy metal accumulation of Iris halophila Pall. cultivated in Pb mine tailings. Int. Biodeter. Biodeger. 2018, 128, 15–21. [Google Scholar] [CrossRef]
- Wu, J.; Tang, Y.; Li, Z.; Yang, G. Tolerance of cordate houttuynia to lead and Zinc. J. Basic Sci. Eng. 2011, 19, 179–187. [Google Scholar]
- Mühlbachová, G. Microbial biomass dynamics after addition of EDTA into heavy metal contaminated soils. Plant Soil Environ. 2009, 55, 544–550. [Google Scholar] [CrossRef]
- Afshan, S.; Ali, S.; Bharwana, S.A.; Rizwan, M.; Farid, M.; Abbas, F.; Ibrahim, M.; Mehmood, M.A.; Abbasi, G.H. Citric acid enhances the phytoextraction of chromium; plant growth; and photosynthesis by alleviating the oxidative damages in Brassica napus L. Environ. Sci. Pollut. Res. 2015, 22, 11679–11689. [Google Scholar] [CrossRef]
- Han, Y.; Wu, X.; Gu, J.; Zhao, J.; Huang, S.; Yuan, H. Effects of organic acids on the photosynthetic and antioxidant properties and accumulations of heavy metals of Melilotus officinalis grown in Cu tailing. Environ. Sci. Pollut. Res. 2016, 23, 1–9. [Google Scholar] [CrossRef]
- Guo, D.; Ali, A.; Ren, C.; Du, J.; Li, R.; Lahori, A.H.; Xiao, R.; Zhang, Z.Y.; Zhang, Z.Q. EDTA and organic acids assisted phytoextraction of Cd and Zn from a smelter contaminated soil by potherb mustard (Brassica juncea; Coss) and evaluation of its bioindicators. Ecotoxicol. Environ. Saf. 2019, 167, 396–403. [Google Scholar] [CrossRef]
- Gill, S.S.; Anjum, N.A.; Gill, R.; Yadav, S.; Hasanuzzaman, M.; Fujita, M.; Mishra, P.; Sabat, S.C.; Tuteja, N. Superoxide dismutasedmentor of abiotic stress tolerance in crop plants. Environ. Sci. Pollut. Res. 2015, 22, 10375–10394. [Google Scholar] [CrossRef]
- Sallah-Ud-Din, R.; Farid, M.; Saeed, R.; Ali, S.; Rizwan, M.; Tauqeer, H.M.; Bukhari, S.A.H. Citric acid enhanced the antioxidant defense system and chromium uptake by Lemna minor L. grown in hydroponics under Cr stress. Environ. Sci. Pollut. Res. 2017, 24, 17669–17678. [Google Scholar] [CrossRef] [PubMed]
- Ryan, P.R.; Delhaize, E.; Jones, D.L. Function and mechanism of organic an ion exudation from plant roots. Ann. Rev. Plant Biol. 2001, 52, 527–560. [Google Scholar] [CrossRef] [PubMed]
- Najeeb, U.; Jilani, G.; Ali, S.; Sarwar, M.; Xu, L.; Zhou, W. Insights into cadmium induced physiological and ultra-structural disorders in Juncus effusus L. and its remediation through exogenous citric acid. J. Hazard Mater. 2011, 186, 565–574. [Google Scholar] [CrossRef] [PubMed]
- Qiao, K.; Liang, S.; Wang., F.; Wang, H.; Hu, Z.; Chai, T. Effects of cadmium toxicity on diploid wheat (Triticum Urartu L.) and the molecular mechanism of the cadmium response. J. Hazard Mater. 2019, 374, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Ash, C.; Tejnecký, V.; Boruvka, L.; Drabek, O. Different low-molecular-mass organic acids specifically control leaching of arsenic and lead from contaminated soil. J. Contam. Hydrol. 2016, 187, 18–30. [Google Scholar] [CrossRef]
- Bao, Y.; Guo, A.; Ma, J.; Pan, C.; Hu, L. Citric acid and glycine reduce the uptake and accumulation of Fe2O3 nanoparticles and oxytetracycline in rice seedlings upon individual and combined exposure. Sci. Total Environ. 2019, 695, 133859. [Google Scholar] [CrossRef]
- Farid, M.; Ali, S.; Rizwan, M.; Yasmeen, T.; Arif, M.S.; Riaz, M.; Saqib, M.; Rehman, M.Z.U.; Ayub, M.A. Combined effects of citric acid and 5-aminolevulinic acid in mitigating chromium toxicity in sunflower (Helianthus annuus L.) grown in Cr spiked soil. Pak. J. Agric. Sci. 2020, 57, 477–488. [Google Scholar]
- Ranieri, E.; Moustakas, K.; Barbafieri, M.; Ranieri, A.C.; Herrera-Melian, J.A.; Petrella, A.; Tommasi, F. Phytoextraction technologies for mercury- and chromium-contaminated soil: A review. J. Chem. Technol. Biotechnol. 2020, 95, 317–327. [Google Scholar] [CrossRef]
- Xu, X.; Chen, Q.; Mo, S.; Qian, Y.; Wu, X.; Jin, Y.; Ding, H. Transcriptome-wide modulation combined with morpho-physiological analyses of Typha orientalis roots in response to lead challenge. J. Hazard Mater. 2020, 384, 121405. [Google Scholar] [CrossRef]
- Wang, G.; Zhang, S.; Xu, X.; Zhong, Q.; Zhang, C.; Jia, Y.; Li, T.; Deng, O.; Li, Y. Heavy metal removal by GLDA washing: Optimization; redistribution; recycling; and changes in soil fertility. Sci. Total Environ. 2016, 11, 557–568. [Google Scholar] [CrossRef] [PubMed]
- Attinti, R.; Barrett, K.R.; Datta, R.; Sarkar, D. Ethylenediaminedisuccinic acid (EDDS) enhances phytoextraction of lead by vetiver grass from contaminated residential soils in a panel study in the field. Environ. Pollut. 2017, 225, 524–533. [Google Scholar] [CrossRef] [PubMed]
- Kurtyka, R.; Burdach, Z.; Siemieniuk, A.; Karcz, W. Single and combined effects of Cd and Pb on the growth; medium pH; membrane potential and metal contents in maize (Zea mays L.) coleoptile segments. Ecotoxicol. Environ. Saf. 2018, 161, 8–16. [Google Scholar] [CrossRef] [PubMed]
- Ondrasek, G.; Rengel, Z.; Romic, D. Humic acids decrease uptake and distribution of trace metals, but not the growth of radish exposed to cadmium toxicity. Ecotoxicol. Environ. Saf. 2018, 151, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Li, Z.; Li, S.; Deng, N.; Mei, P. Effects of root exudates on the activation and remediation of cadmium ion in contaminated soils. Environ. Sci. Pollut. Res. 2020, 27, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Fu, H.J.; Yu, H.Y.; Li, T.X.; Zhang, X.Z. Influence of cadmium stress on root exudates of high cadmium accumulating rice line (Oryza sativa L.). Ecotoxicol. Environ. Saf. 2018, 150, 168–175. [Google Scholar] [CrossRef] [PubMed]
- Tatár, E.; Mihucz, V.G.; Varga, A.; Cseh, E.; Záray, G. Effect of lead, nickel and vanadium contamination on organic acid transport in xylem sap of cucumber. J. Inorg. Bioch. 1999, 75, 219–223. [Google Scholar] [CrossRef]
- Niu, Z.X.; Li, X.D.; Sun, L.N.; Sun, T.H. Changes of three organic acids in the process of Cd and Pb phytoextraction by Helianthus annuus L. Plant Soil Environ. 2012, 58, 487–494. [Google Scholar] [CrossRef]
- Chen, H.C.; Zhang, S.L.; Wu, K.J.; Li, R.; He, X.R.; Wei, H. The effects of exogenous organic acids on the growth; photosynthesis and cellular ultrastructure of Salix variegata Franch. Under Cd stress. Ecotoxicol. Environ. Saf. 2019, 187, 109790. [Google Scholar] [CrossRef]
- Tatár, E.; Mihucz, V.G.; Kmethy, B.; Záray, G.; Fodor, F. Determination of organic acids and their role in nickel transport within cucumber plants. Microchem. J. 2000, 67, 73–81. [Google Scholar] [CrossRef]
- Wang, S.T.; Dong, Q.; Wang, Z.L. Differential effects of citric acid on cadmium uptake and accumulation between tall fescue and Kentucky bluegrass. Ecotoxicol. Environ. Saf. 2017, 145, 200–206. [Google Scholar] [CrossRef] [PubMed]
- Ashraf, M.; Aziz, A.; Kausar, R.; Sahazad, S.M.; Akhtar, N. Efficiency of Different Amendments for the Mitigation of Cadmium Toxicity in Sunflower (Helianthus annuus L.). J. Soil Plant Biol. Cit. 2020, 1, 73–86. [Google Scholar]
- Shi, X.; Zhang, C.; Wang, H.; Zhang, F. Effect of Si on the distribution of Cd in rice seedlings. Plant Soil 2005, 272, 53–60. [Google Scholar] [CrossRef]
- Leung, M. Can we possibly derive environmental quality benchmarks for chemical mixtures? In Proceedings of the 6th SETAC World Congress and 22nd Annual Meeting of the Society of SETAC, Berlin, Germany, 20–24 May 2012. [Google Scholar]
- Asati, A.; Pichhode, M.; Nikhil, K. Effect of heavy metals on plants: An overview. Int. J. Appl. Innov. Eng. Manag. 2016, 5, 2319–4847. [Google Scholar]
- Niu, Z.X.; Sun, L.N.; Sun, T.H. Characteristics of Cadmium and Lead Phytoextraction by Sunflower (Helianthus annuus L.) in Sand Culture. Adv. Mater. Res. 2011, 185, 1496–1504. [Google Scholar] [CrossRef]
- Jagetiya, B.; Sharma, A. Optimization of chelators to enhance uranium uptake from tailings for phytoremediation. Chemosphere 2013, 91, 692–696. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Liu, Y.H.; Song, Z.G.; Wang, D.; Qiu, W.W. Chelator complexes enhanced Amaranthus hypochondriacus L. phytoremediation efficiency in Cd-contaminated soils. Chemosphere 2019, 237, 124480. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Zhang, S.; Zhong, Q.; Peijnenburg, W.J.G.M.; Vijver, M.G. Feasibility of Chinese cabbage (Brassica bara) and lettuce (Lactuca sativa) cultivation in heavily metals contaminated soil after washing with biodegradable chelators. J. Clean. Prod. 2018, 197, 479–490. [Google Scholar] [CrossRef]
- Wang, G.Y.; Zhang, S.; Zhong, Q.; Xu, X.; Li, T.; Jia, Y.; Zhang, Y.; Peijnenburg, W.J.G.M.; Vijver, M.G. Effect of soil washing with biodegradable chelators on the toxicity of residual metals and soil biological properties. Sci. Total Environ. 2018, 625, 1021–1029. [Google Scholar] [CrossRef]
- Wang, J.; Xing, Y.; Li, P.; Xia, J.; Liu, T.; Feng, X. Chemically-assisted phytoextraction from metal(loid)s-polluted soil at a typical carlin-type gold mining area in southwest China. J. Clean Prod. 2018, 189, 612–619. [Google Scholar] [CrossRef]
- Quartacci, M.F.; Irtelli, B.; Baker, A.J.M.; Navari-Izzo, F. The use of NTA and EDDS for enhanced phytoextraction of metals from a multiply contaminated soil by Brassica carinata. Chemosphere 2007, 68, 1920–1928. [Google Scholar] [CrossRef] [PubMed]
- Lozano, J.C.; Rodríguez, P.B.; Tom’e, F.V.; Calvo, C.P. Enhancing uranium solubilization in soils by citrate; EDTA; and EDDS chelating amendments. J. Hazard. Mater. 2011, 198, 224–231. [Google Scholar] [CrossRef] [PubMed]
- Yanai, J.; Zhao, F.J.; McGrath, S.P.; Kosaki, T. Effect of soil characteristics on Cd uptake by the hyperaccumulator Thlaspi caerulescens. Environ. Pollut. 2006, 139, 167–175. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Niu, Z.; Li, X.; Mahamood, M. Accumulation Potential Cadmium and Lead by Sunflower (Helianthus annuus L.) under Citric and Glutaric Acid-Assisted Phytoextraction. Int. J. Environ. Res. Public Health 2023, 20, 4107. https://doi.org/10.3390/ijerph20054107
Niu Z, Li X, Mahamood M. Accumulation Potential Cadmium and Lead by Sunflower (Helianthus annuus L.) under Citric and Glutaric Acid-Assisted Phytoextraction. International Journal of Environmental Research and Public Health. 2023; 20(5):4107. https://doi.org/10.3390/ijerph20054107
Chicago/Turabian StyleNiu, Zhixin, Xiaojun Li, and Mohammad Mahamood. 2023. "Accumulation Potential Cadmium and Lead by Sunflower (Helianthus annuus L.) under Citric and Glutaric Acid-Assisted Phytoextraction" International Journal of Environmental Research and Public Health 20, no. 5: 4107. https://doi.org/10.3390/ijerph20054107
APA StyleNiu, Z., Li, X., & Mahamood, M. (2023). Accumulation Potential Cadmium and Lead by Sunflower (Helianthus annuus L.) under Citric and Glutaric Acid-Assisted Phytoextraction. International Journal of Environmental Research and Public Health, 20(5), 4107. https://doi.org/10.3390/ijerph20054107







