Technological, Ecological, and Energy-Economic Aspects of Using Solidified Carbon Dioxide for Aerobic Granular Sludge Pre-Treatment Prior to Anaerobic Digestion
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Materials
2.2.1. AGS and Inoculum of the Anaerobic Sludge (AS)
2.2.2. SCO2
2.3. Experimental Stations
2.3.1. Stage 1
2.3.2. Stage 2
2.4. Analytical Methods
2.5. Molecular Methods
2.6. Computation Methods
- PSCO2—power of SCO2 generator (W);
- MSCO2—mass of SCO2 (kg);
- YSCO2—yield of SCO2 generator (kg/h).
- YMethane—methane yield (dm3/kg fresh matter (FM));
- CVMethane—calorific value of methane (Wh/dm3);
- MAGS—mass of AGS (kg).
- Eout(Vx)—the energy output in n-variant (Wh);
- Eout(V1)—the energy output in V1 (Wh).
- Enout—the net energy output (Wh);
- Es—the specific energy input (Wh).
- Enet—the net energy gain (Wh);
- EP—energy price (EUR/Wh)—the energy price was adopted as the mean from 2020 to the first half of 2022 based on Eurostat data [29].
- MSCO2—mass of SCO2 (kg);
- CPP—price of EU Carbon Permits (EUR/kg)—the price of EU Carbon Permits was adopted as the mean from 2020 to the first half of 2022 based on Trading Economics data [30].
- EV—energy value (EUR);
- SCO2V—SCO2 value (EUR).
2.7. Statistical and Optimization Methods
3. Results and Discussion
3.1. Stage 1
3.2. Stage 2
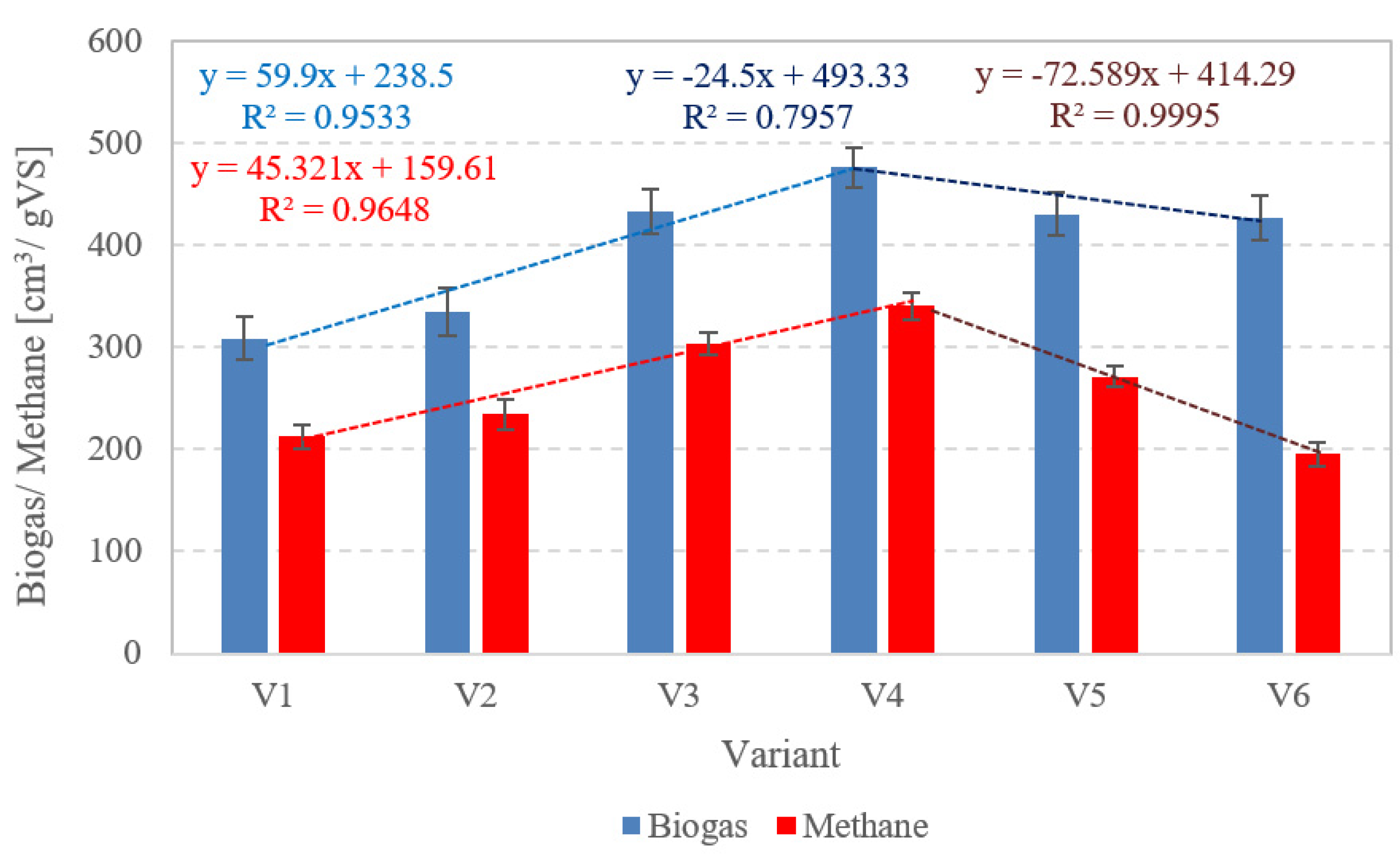
3.2.1. Biogas and Methane Production
3.2.2. pH and FOS/TAC
3.2.3. Bacterial Community Structure
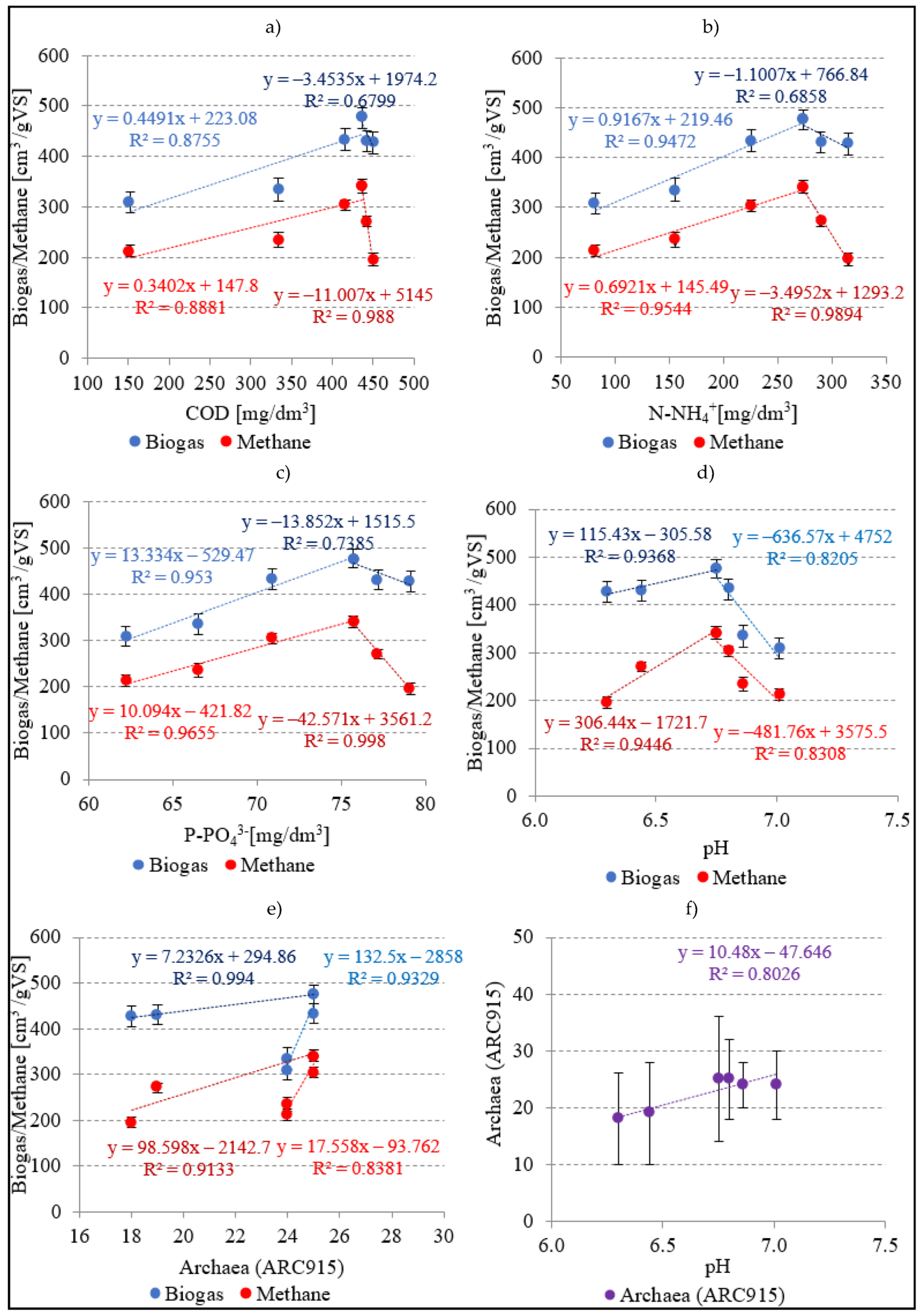
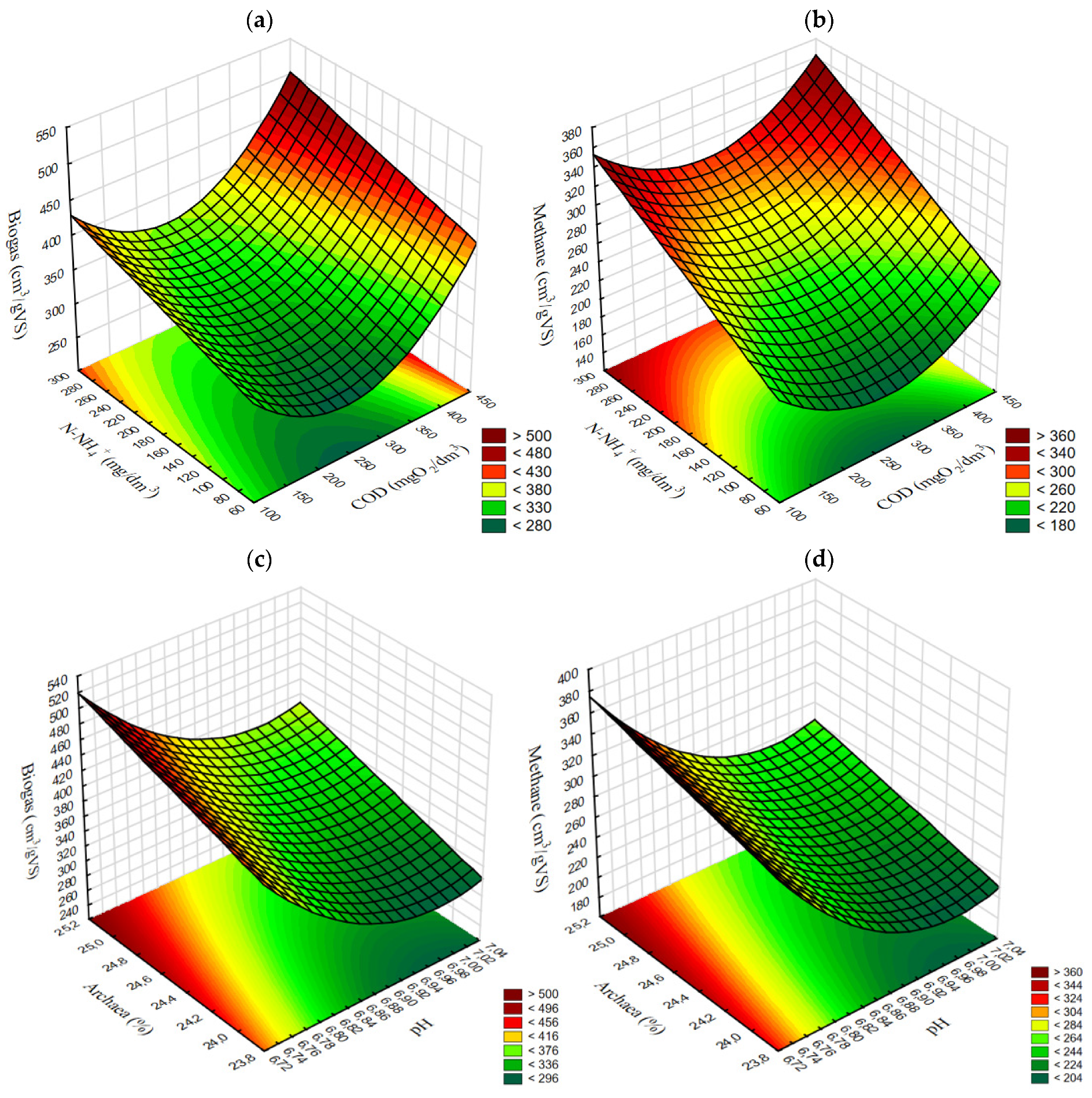
3.2.4. Empirical Models and Correlations
- BIOGAS—biogas yield, cm3/gVS;
- METHANE—methane yield, cm3/gVS;
- COD—COD concentration in the supernatant, mgO2/dm3;
- N-NH4+—N-NH4+ concentration in the supernatant, mg/dm3;
- SCO2/AGS—volume ratio of SCO2 to AGS.
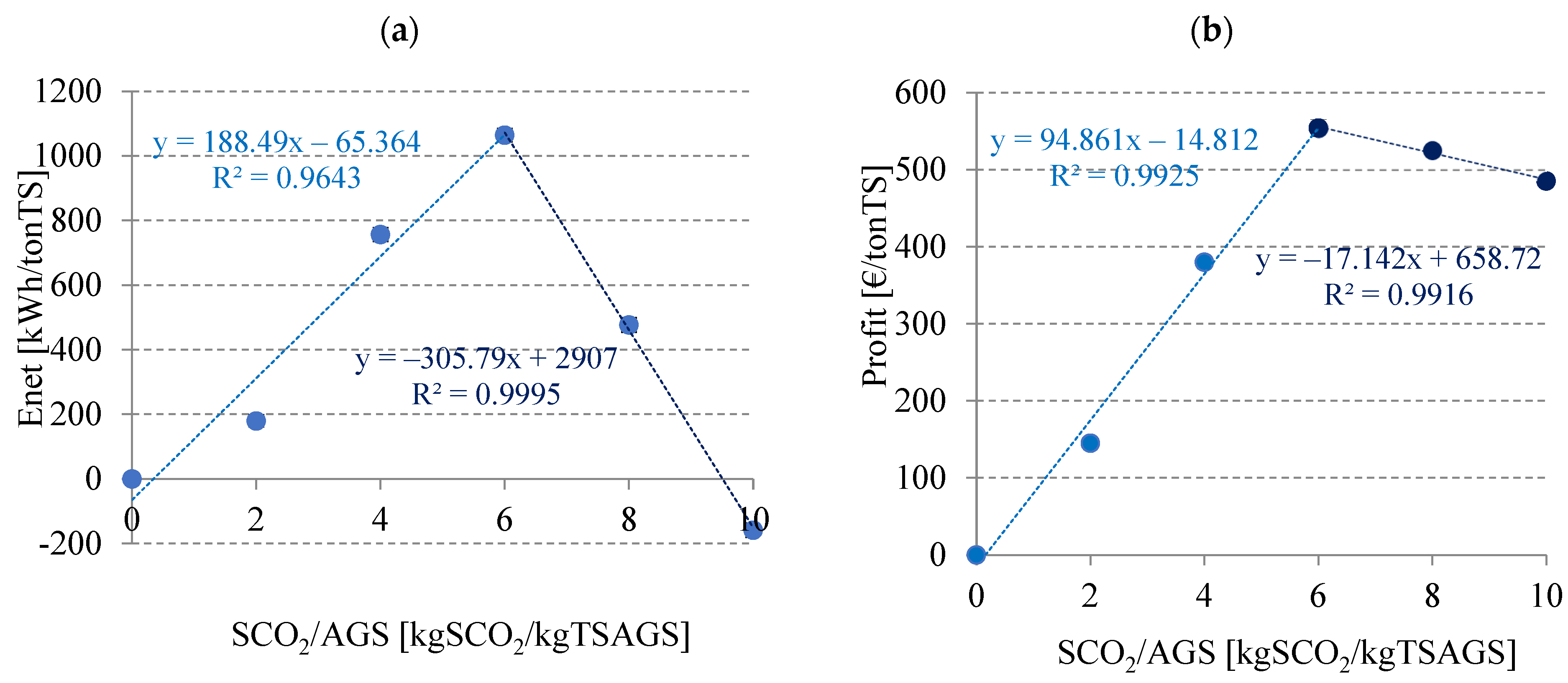
3.2.5. Energy and Economic Balance
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Silva Thomsen, L.B.; Carvalho, P.N.; dos Passos, J.S.; Anastasakis, K.; Bester, K.; Biller, P. Hydrothermal Liquefaction of Sewage Sludge; Energy Considerations and Fate of Micropollutants during Pilot Scale Processing. Water Res. 2020, 183, 116101. [Google Scholar] [CrossRef]
- Kosar, S.; Isik, O.; Cicekalan, B.; Gulhan, H.; Cingoz, S.; Yoruk, M.; Ozgun, H.; Koyuncu, I.; van Loosdrecht, M.C.M.; Ersahin, M.E. Coupling High-Rate Activated Sludge Process with Aerobic Granular Sludge Process for Sustainable Municipal Wastewater Treatment. J. Environ. Manag. 2023, 325, 116549. [Google Scholar] [CrossRef]
- Carrera, P.; Casero-Díaz, T.; Castro-Barros, C.M.; Méndez, R.; Val del Río, A.; Mosquera-Corral, A. Features of Aerobic Granular Sludge Formation Treating Fluctuating Industrial Saline Wastewater at Pilot Scale. J. Environ. Manag. 2021, 296, 113135. [Google Scholar] [CrossRef]
- Hamza, R.; Rabii, A.; Ezzahraoui, F.Z.; Morgan, G.; Iorhemen, O.T. A Review of the State of Development of Aerobic Granular Sludge Technology over the Last 20 Years: Full-Scale Applications and Resource Recovery. Case Stud. Chem. Environ. Eng. 2022, 5, 100173. [Google Scholar] [CrossRef]
- Liu, X.; Lee, D.-J. Aerobic Granular Sludge Processes. In Current Developments in Biotechnology and Bioengineering; Elsevier: Amsterdam, The Netherlands, 2022; pp. 193–225. [Google Scholar] [CrossRef]
- Miyake, M.; Hasebe, Y.; Furusawa, K.; Shiomi, H.; Inoue, D.; Ike, M. Pilot-Scale Demonstration of Aerobic Granular Sludge Augmentation Applied to Continuous-Flow Activated Sludge Process for the Treatment of Low-Strength Municipal Wastewater. J. Water Process Eng. 2023, 51, 103392. [Google Scholar] [CrossRef]
- Kazimierowicz, J.; Dębowski, M. Aerobic Granular Sludge as a Substrate in Anaerobic Digestion—Current Status and Perspectives. Sustainability 2022, 14, 10904. [Google Scholar] [CrossRef]
- Wan, C.; Fu, L.; Li, Z.; Liu, X.; Lin, L.; Wu, C. Formation, Application, and Storage-Reactivation of Aerobic Granular Sludge: A Review. J. Environ. Manag. 2022, 323, 116302. [Google Scholar] [CrossRef] [PubMed]
- Nancharaiah, Y.V.; Sarvajith, M. Aerobic Granular Sludge Process: A Fast Growing Biological Treatment for Sustainable Wastewater Treatment. Curr. Opin. Environ. Sci. Health 2019, 12, 57–65. [Google Scholar] [CrossRef]
- Kumar, A.; Samadder, S.R. Performance Evaluation of Anaerobic Digestion Technology for Energy Recovery from Organic Fraction of Municipal Solid Waste: A Review. Energy 2020, 197, 117253. [Google Scholar] [CrossRef]
- Chojnacka, K.; Skrzypczak, D.; Szopa, D.; Izydorczyk, G.; Moustakas, K.; Witek-Krowiak, A. Management of Biological Sewage Sludge: Fertilizer Nitrogen Recovery as the Solution to Fertilizer Crisis. J. Environ. Manag. 2023, 326, 116602. [Google Scholar] [CrossRef]
- Liu, Y.; Nilsen, P.J.; Maulidiany, N.D. Thermal Pretreatment to Enhance Biogas Production of Waste Aerobic Granular Sludge with and without Calcium Phosphate Precipitates. Chemosphere 2019, 234, 725–732. [Google Scholar] [CrossRef] [PubMed]
- Volschan Junior, I.; de Almeida, R.; Cammarota, M.C. A Review of Sludge Pretreatment Methods and Co-Digestion to Boost Biogas Production and Energy Self-Sufficiency in Wastewater Treatment Plants. J. Water Process Eng. 2021, 40, 101857. [Google Scholar] [CrossRef]
- Siami, S.; Aminzadeh, B.; Karimi, R.; Hallaji, S.M. Process Optimization and Effect of Thermal, Alkaline, H 2 O 2 Oxidation and Combination Pretreatment of Sewage Sludge on Solubilization and Anaerobic Digestion. BMC Biotechnol. 2020, 20, 21. [Google Scholar] [CrossRef]
- Zieliński, M.; Dębowski, M.; Kazimierowicz, J. The Effect of Static Magnetic Field on Methanogenesis in the Anaerobic Digestion of Municipal Sewage Sludge. Energies 2021, 14, 590. [Google Scholar] [CrossRef]
- Khanh Nguyen, V.; Kumar Chaudhary, D.; Hari Dahal, R.; Hoang Trinh, N.; Kim, J.; Chang, S.W.; Hong, Y.; Duc La, D.; Nguyen, X.C.; Hao Ngo, H.; et al. Review on Pretreatment Techniques to Improve Anaerobic Digestion of Sewage Sludge. Fuel 2021, 285, 119105. [Google Scholar] [CrossRef]
- Liu, X.; Wang, Q.; Tang, Y.; Pavlostathis, S.G. Hydrothermal Pretreatment of Sewage Sludge for Enhanced Anaerobic Digestion: Resource Transformation and Energy Balance. Chem. Eng. J. 2021, 410, 127430. [Google Scholar] [CrossRef]
- Sahu, A.K.; Mitra, I.; Kleiven, H.; Holte, H.R.; Svensson, K. Cambi Thermal Hydrolysis Process (CambiTHP) for Sewage Sludge Treatment. Clean Energy Resour. Recover. Wastewater Treat. Plants Biorefineries 2022, 2, 405–422. [Google Scholar] [CrossRef]
- Moretti, C. Reflecting on the Environmental Impact of the Captured Carbon Feedstock. Sci. Total Environ. 2023, 854, 158694. [Google Scholar] [CrossRef]
- Roosa, S.A.; Jhaveri, A.G. Carbon Reduction Technologies. In Energy Management Handbook; River Publishers: New York, NY, USA, 2020; pp. 561–581. [Google Scholar] [CrossRef]
- Machnicka, A.; Grübel, K.; Wacławek, S.; Sikora, K. Waste-Activated Sludge Disruption by Dry Ice: Bench Scale Study and Evaluation of Heat Phase Transformations. Environ. Sci. Pollut. Res. 2019, 26, 26488–26499. [Google Scholar] [CrossRef]
- Sun, F.; Xiao, K.K.; Zhu, W.; Withanage, N.; Zhou, Y. Enhanced Sludge Solubilization and Dewaterability by Synergistic Effects of Nitrite and Freezing. Water Res. 2018, 130, 208–214. [Google Scholar] [CrossRef]
- Ofman, P.; Skoczko, I.; Włodarczyk-Makuła, M. Biosorption of LMW PAHs on Activated Sludge Aerobic Granules under Varying BOD Loading Rate Conditions. J. Hazard. Mater. 2021, 418, 126332. [Google Scholar] [CrossRef] [PubMed]
- Jamil, M.; Iqbal, A.; He, N.; Cheok, Q. Thermophysical Properties and Heat Transfer Performance of Novel Dry-Ice-Based Sustainable Hybrid Lubri-Coolant. Sustainability 2022, 14, 2430. [Google Scholar] [CrossRef]
- Kazimierowicz, J.; Bartkowska, I.; Walery, M. Effect of Low-Temperature Conditioning of Excess Dairy Sewage Sludge with the Use of Solidified Carbon Dioxide on the Efficiency of Methane Fermentation. Energies 2020, 14, 150. [Google Scholar] [CrossRef]
- PN-EN 15935:2022-01; Soil, Waste, Treated Bio-Waste and Sewage Sludge—Determination of Losses on Ignition. Health, Environment and Medicine Sector. Technical Body of Soil Chemistry: Warsaw, Poland, 2022.
- ImageJ. Available online: https://imagej.net/software/imagej/ (accessed on 17 December 2022).
- Cold Jet. P3000—Fully Automatic Dry Ice Pelletizer. Available online: https://www.coldjet.com/our-equipment/dry-ice-production-equipment/p3000/ (accessed on 17 December 2022).
- Eurostat. Electricity Price Statistics. 2022. Available online: https://ec.europa.eu/eurostat/statistics-explained/index.php?title=Electricity_price_statistics (accessed on 18 December 2022).
- Trading Economics. EU Carbon Permits—2022 Data—2005-2021 Historical—2023 Forecast—Price—Quote. 2022. Available online: https://tradingeconomics.com/commodity/carbon (accessed on 28 December 2022).
- Jean, D.S.; Lee, D.J.; Chang, C.Y. Direct Sludge Freezing Using Dry Ice. Adv. Environ. Res. 2001, 5, 145–150. [Google Scholar] [CrossRef]
- Machnicka, A.; Nowicka, E.; Grübel, K. Disintegration as a Key-Step in Pre-Treatment of Surplus Activated Sludge. J. Water Chem. Technol. 2017, 39, 47–55. [Google Scholar] [CrossRef]
- Zawieja, I. Effect of Dry Ice Modification of Excess Sludge on the Methane Fermentation Process. Annu. Set Environ. Prot. 2018, 20, 558–573. [Google Scholar]
- Stabnikova, O.; Liu, X.Y.; Wang, J.Y. Digestion of Frozen/Thawed Food Waste in the Hybrid Anaerobic Solid–Liquid System. Waste Manag. 2008, 28, 1654–1659. [Google Scholar] [CrossRef]
- Hu, K.; Jiang, J.Q.; Zhao, Q.L.; Lee, D.J.; Wang, K.; Qiu, W. Conditioning of Wastewater Sludge Using Freezing and Thawing: Role of Curing. Water Res. 2011, 45, 5969–5976. [Google Scholar] [CrossRef]
- Nowicka, E.; Machnicka, A. Confirmation of Effectiveness Surplus Activated Sludge Dry Ice Disruption by Infrared Wave Analysis. In Proceedings of the 42nd International Conference of Slovak Society of Chemical Engineering, Tatranské Matliare, Slovakia, 25–29 May 2015. [Google Scholar]
- Zawieja, I.E. The Course of the Methane Fermentation Process of Dry Ice Modified Excess Sludge. Arch. Environ. Prot. 2019, 45, 50–58. [Google Scholar] [CrossRef]
- Montusiewicz, A.; Lebiocka, M.; Rozej, A.; Zacharska, E.; Pawłowski, L. Freezing/Thawing Effects on Anaerobic Digestion of Mixed Sewage Sludge. Bioresour. Technol. 2010, 101, 3466–3473. [Google Scholar] [CrossRef]
- Gao, W. Freezing as a Combined Wastewater Sludge Pretreatment and Conditioning Method. Desalination 2011, 268, 170–173. [Google Scholar] [CrossRef]
- Zawieja, I.; Brzeska, K. Biogas Production in the Methane Fermentation of Excess Sludge Oxidized with Fenton’s Reagent. E3S Web Conf. 2019, 116, 00104. [Google Scholar] [CrossRef]
- Zainuddin, M.F.; Fai, C.K.; Ariff, A.B.; Rios-Solis, L.; Halim, M. Current Pretreatment/Cell Disruption and Extraction Methods Used to Improve Intracellular Lipid Recovery from Oleaginous Yeasts. Microorganisms 2021, 9, 251. [Google Scholar] [CrossRef] [PubMed]
- Bernat, K.; Cydzik-Kwiatkowska, A.; Wojnowska-Baryła, I.; Karczewska, M. Physicochemical Properties and Biogas Productivity of Aerobic Granular Sludge and Activated Sludge. Biochem. Eng. J. 2017, 117, 43–51. [Google Scholar] [CrossRef]
- Cydzik-Kwiatkowska, A.; Bernat, K.; Zielińska, M.; Gusiatin, M.Z.; Wojnowska-Baryła, I.; Kulikowska, D. Valorization of Full-Scale Waste Aerobic Granular Sludge for Biogas Production and the Characteristics of the Digestate. Chemosphere 2022, 303, 135167. [Google Scholar] [CrossRef] [PubMed]
- Nowicka, E.; Machnicka, A.; Grűbel, K. Improving Of Anaerobic Digestion By Dry Ice Disintegration of Surplus Activated Sludge. Proc. ECOpole 2014, 8, 239–247. [Google Scholar] [CrossRef]
- SAFETY DATA SHEET Carbon Dioxide, solid (Dry Ice) Section 1: Identification of the Substance/Mixture and of the Company/Undertaking. Available online: https://produkte.linde-gas.at/sdb_konform/TE_10022548EN.pdf (accessed on 17 December 2022).
- De Meyer, T.; Hemelsoet, K.; Van Speybroeck, V.; De Clerck, K. Substituent Effects on Absorption Spectra of PH Indicators: An Experimental and Computational Study of Sulfonphthaleine Dyes. Dye Pigment. 2014, 102, 241–250. [Google Scholar] [CrossRef]
- Srisowmeya, G.; Chakravarthy, M.; Nandhini Devi, G. Critical Considerations in Two-Stage Anaerobic Digestion of Food Waste—A Review. Renew. Sustain. Energy Rev. 2020, 119, 109587. [Google Scholar] [CrossRef]
- Ambrose, H.W.; Philip, L.; Suraishkumar, G.K.; Sen, T. Anaerobic Co-Digestion of Mixed Activated Sewage Sludge and Fruit and Vegetable Waste on Two-Stage Digester Stability. Authorea Prepr. 2020. [CrossRef]
- Nkuna, R.; Roopnarain, A.; Adeleke, R. Effects of Organic Loading Rates on Microbial Communities and Biogas Production from Water Hyacinth: A Case of Mono- and Co-Digestion. J. Chem. Technol. Biotechnol. 2019, 94, 1294–1304. [Google Scholar] [CrossRef]
- Matheri, A.N.; Belaid, M.; Seodigeng, T.; Ngila, C.J. Modelling the Kinetic of Biogas Production from Co-Digestion of Pig Waste and Grass Clippings. In Proceedings of the 24th World Congress on Engineering, London, UK, 29 June–1 July 2016. [Google Scholar]
- Godvin Sharmila, V.; Kumar, G.; Sivashanmugham, P.; Piechota, G.; Park, J.H.; Adish Kumar, S.; Rajesh Banu, J. Phase Separated Pretreatment Strategies for Enhanced Waste Activated Sludge Disintegration in Anaerobic Digestion: An Outlook and Recent Trends. Bioresour. Technol. 2022, 363, 127985. [Google Scholar] [CrossRef] [PubMed]
- Ahn, J.Y.; Chang, S.W. Effects of Sludge Concentration and Disintegration/Solubilization Pretreatment Methods on Increasing Anaerobic Biodegradation Efficiency and Biogas Production. Sustainability 2021, 13, 12887. [Google Scholar] [CrossRef]
- Dębowski, M.; Kazimierowicz, J.; Świca, I.; Zieliński, M. Ultrasonic Disintegration to Improve Anaerobic Digestion of Microalgae with Hard Cell Walls—Scenedesmus sp. and Pinnularia sp. Plants 2022, 12, 53. [Google Scholar] [CrossRef] [PubMed]
- Kazimierowicz, J.; Dębowski, M.; Zieliński, M. Effect of Pharmaceutical Sludge Pre-Treatment with Fenton/Fenton-like Reagents on Toxicity and Anaerobic Digestion Efficiency. Int. J. Environ. Res. Public Health 2022, 20, 271. [Google Scholar] [CrossRef] [PubMed]
- Passos, F.; García, J.; Ferrer, I. Impact of Low Temperature Pretreatment on the Anaerobic Digestion of Microalgal Biomass. Bioresour. Technol. 2013, 138, 79–86. [Google Scholar] [CrossRef]
- Barrios, J.A.; Cano, A.; Rivera, F.F.; Cisneros, M.E.; Durán, U. Efficiency of Integrated Electrooxidation and Anaerobic Digestion of Waste Activated Sludge. Biotechnol. Biofuels 2021, 14, 81. [Google Scholar] [CrossRef]
- Balasundaram, G.; Vidyarthi, P.K.; Gahlot, P.; Arora, P.; Kumar, V.; Kumar, M.; Kazmi, A.A.; Tyagi, V.K. Energy Feasibility and Life Cycle Assessment of Sludge Pretreatment Methods for Advanced Anaerobic Digestion. Bioresour. Technol. 2022, 357, 127345. [Google Scholar] [CrossRef]
- Kavitha, S.; Rajesh Banu, J.; IvinShaju, C.D.; Kaliappan, S.; Yeom, I.T. Fenton Mediated Ultrasonic Disintegration of Sludge Biomass: Biodegradability Studies, Energetic Assessment, and Its Economic Viability. Bioresour. Technol. 2016, 221, 1–8. [Google Scholar] [CrossRef]

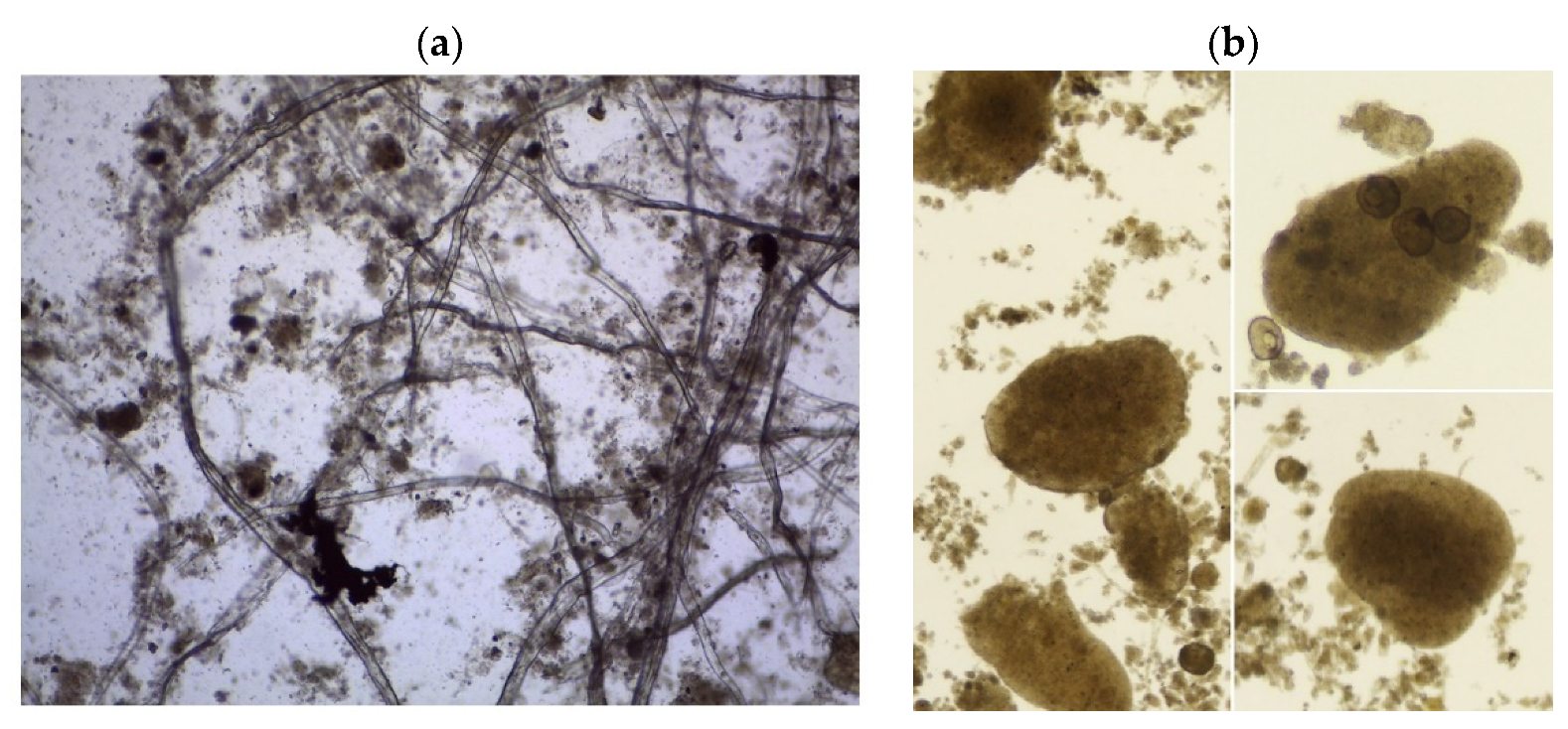
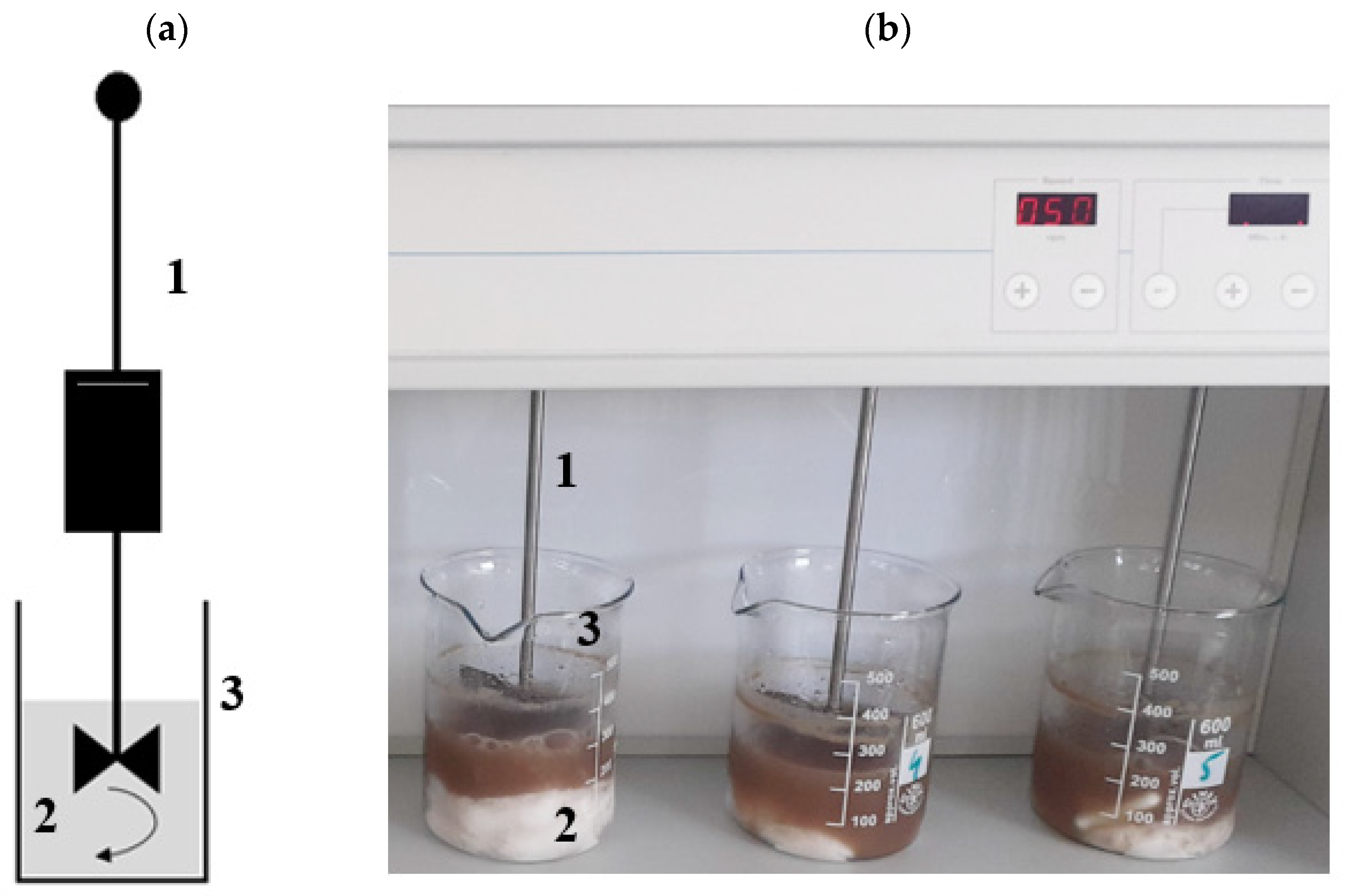
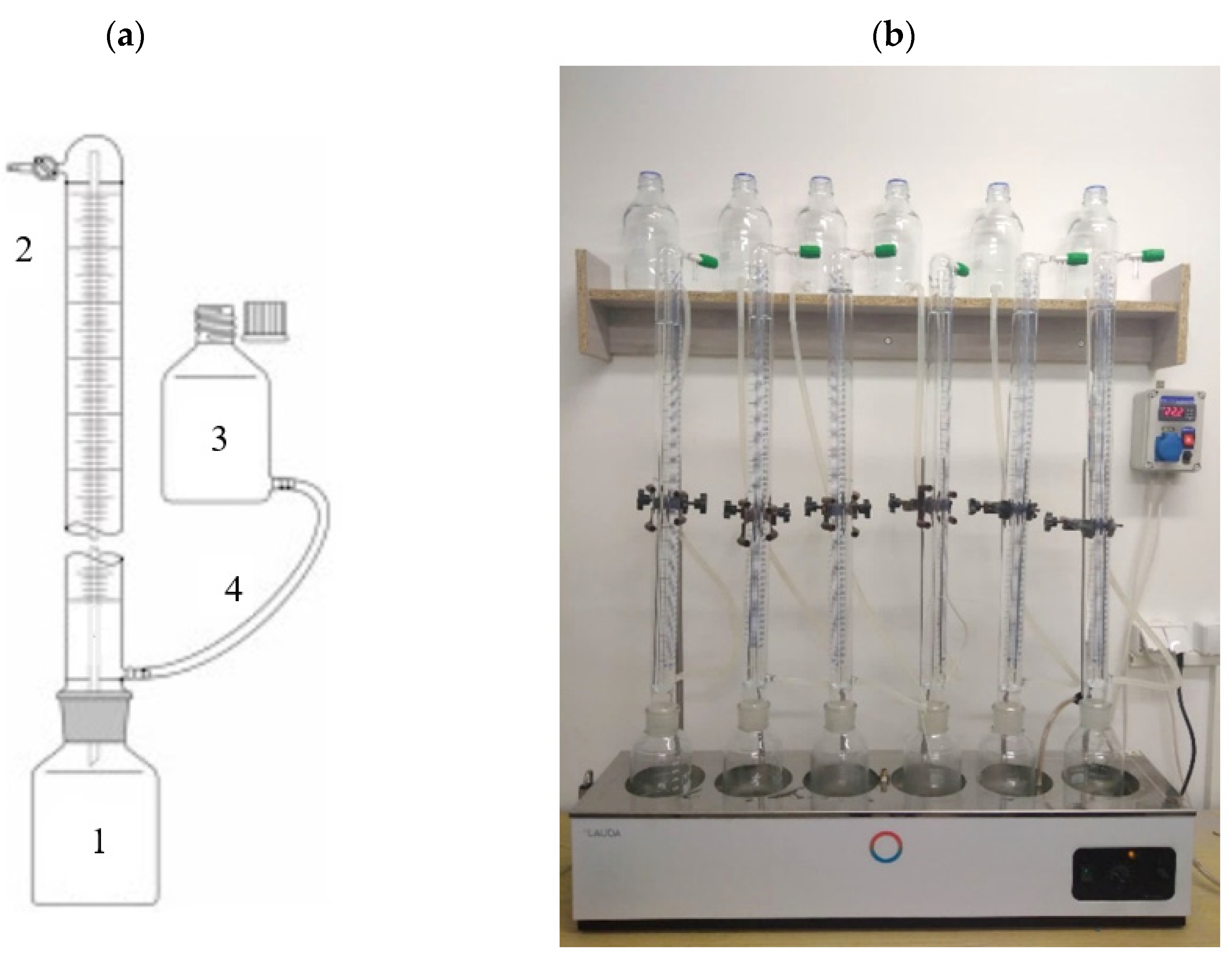
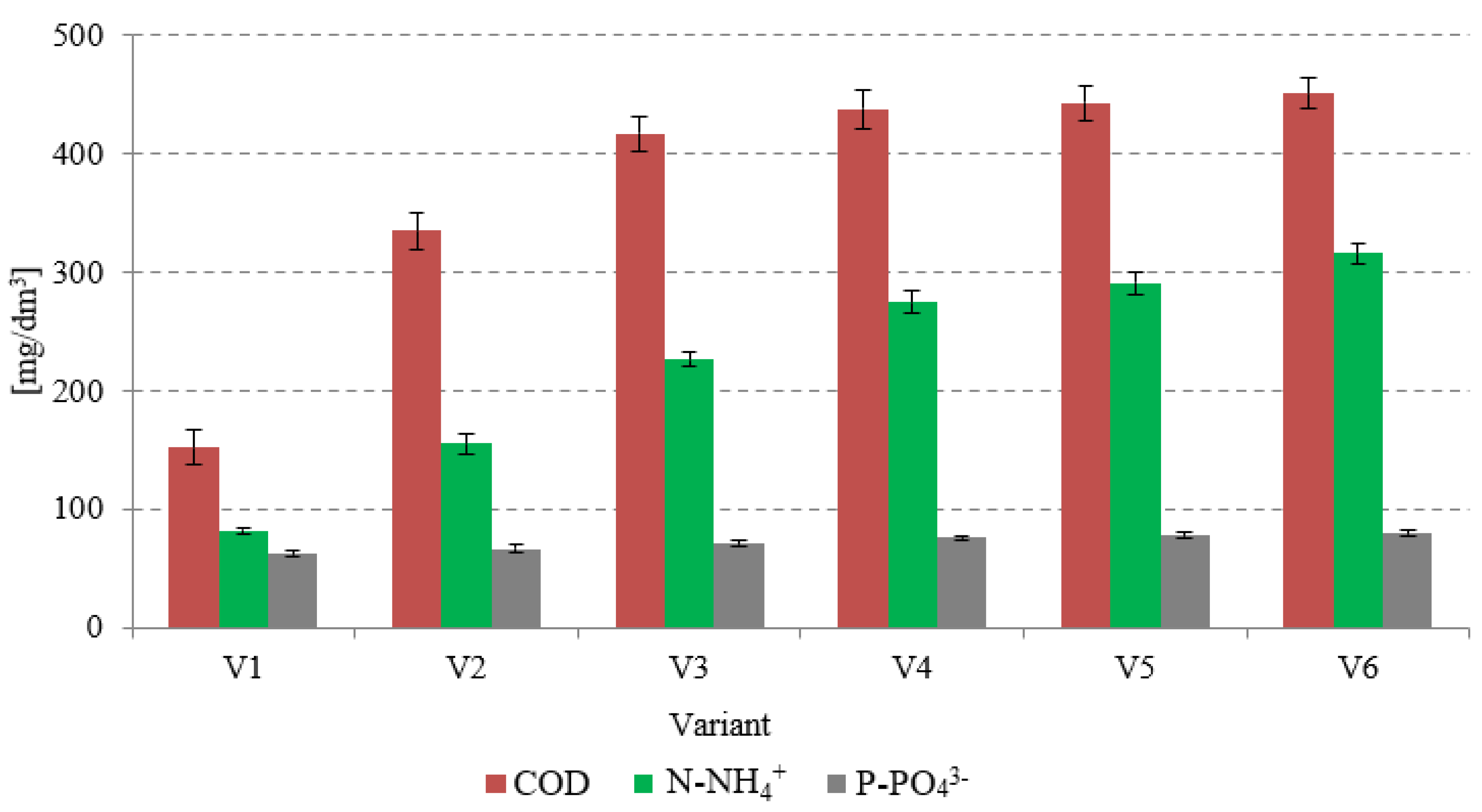

| Indicator | Unit | CAS | AGS | AS |
|---|---|---|---|---|
| pH | - | 7.61 ± 0.1 | 7.80 ± 0.1 | 7.27 ± 0.2 |
| Total solids (TS) | (%) | 4.41 ± 0.1 | 7.42 ± 0.1 | 3.37 ± 0.1 |
| Volatile solids (VS) | (%TS) | 82.62 ± 3.5 | 90.17 ± 5.8 | 66.43 ± 6.1 |
| Mineral solids (MS) | (%TS) | 17.38 ± 1.3 | 9.83 ± 1.1 | 33.57 ± 5.4 |
| Total carbon (TC) | (mg/gTS) | 608 ± 19 | 652 ± 21 | 313 ± 12 |
| Total organic carbon (TOC) | (mg/gTS) | 578 ± 17 | 606 ± 16 | 300 ± 10 |
| Total nitrogen (TN) | (mg/gTS) | 95 ± 6.2 | 100 ± 5.4 | 33.4 ± 3.6 |
| C/N ratio | - | 6.4 ± 0.1 | 6.5 ± 0.1 | 9.37 ± 0.3 |
| Total phosphorus (TP) | (mg/gTS) | 3.2 ± 1.1 | 6.9 ± 1.4 | 1.8 ± 0.1 |
| Variant 1 | Biogas | Methane | |||
|---|---|---|---|---|---|
| r | k | %CH4 | r | k | |
| (cm3/d) | (1/d) | (%) | (cm3/d) | (1/d) | |
| 1 | 47.7 | 0.15 | 68.84 ± 2.2 | 22.6 | 0.11 |
| 2 | 56.1 | 0.17 | 70.00 ± 2.1 | 27.5 | 0.12 |
| 3 | 93.7 | 0.22 | 70.14 ± 1.8 | 46.1 | 0.15 |
| 4 | 113.3 | 0.24 | 71.58 ± 1.7 | 58.1 | 0.17 |
| 5 | 92.5 | 0.22 | 63.03 ± 1.3 | 36.7 | 0.14 |
| 6 | 91.2 | 0.21 | 45.80 ± 2.1 | 19.1 | 0.10 |
| V1 | V2 | V3 | V4 | V5 | V6 | |
|---|---|---|---|---|---|---|
| pH of AGS after pre-treatment with SCO2 | 7.80 ± 0.1 | 7.51 ± 0.1 | 7.21 ± 0.1 | 6.93 ± 0.1 | 6.42 ± 0.1 | 6.31 ± 0.1 |
| pH of AGS + inoculum | 7.48 ± 0.1 | 7.36 ± 0.1 | 7.24 ± 0.1 | 7.12 ± 0.1 | 6.93 ± 0.1 | 6.89 ± 0.1 |
| pH after AD | 7.01 ± 0.1 | 6.86 ± 0.1 | 6.80 ± 0.1 | 6.75 ± 0.1 | 6.44 ± 0.1 | 6.30 ± 0.1 |
| FOS/TAC | 0.42 ± 0.04 | 0.42 ± 0.03 | 0.41 ± 0.04 | 0.41 ± 0.05 | 0.40 ± 0.03 | 0.40 ± 0.02 |
| Taxonomic Group | V1 | V2 | V3 | V4 | V5 | V6 |
|---|---|---|---|---|---|---|
| Bacteria (EUB338) | 69 ± 10 | 70 ± 11 | 69 ± 12 | 69 ± 10 | 69 ± 11 | 68 ± 10 |
| Archaea (ARC915) | 24 ± 6 | 24 ± 4 | 25 ± 7 | 25 ± 11 | 19 ± 9 | 18 ± 8 |
| Methanosarcinaceae (MSMX860) | 10 ± 4 | 11 ± 4 | 12 ± 4 | 12 ± 5 | 11 ± 4 | 10 ± 6 |
| Methanosaeta (MX825) | 5 ± 2 | 8 ± 3 | 8 ± 3 | 9 ± 4 | 7 ± 3 | 5 ± 2 |
| Variant | SCO2/AGS | ρAGS | MAGS | VAGS | ρSCO2 | VSCO2 | MSCO2 | PSCO2 | WSCO2 | Es | Ymethane | Ymethane | CVmethane | Eout | Enout | Enet | Enet |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| - | kg/dm3 | kg | dm3 | kg/dm3 | dm3 | kg | M | kg/h | Wh | dm3/kgVS | dm3/kgFM | Wh/dm3 | Wh | Wh | Wh | kWh/tonTS | |
| 1 | 0 | 1.03 | 1.03 | 1 | 1.56 | 0 | 0 | 4500 | 1090 | 0 | 213 ± 12 | 14.88 ± 1.5 | 9.17 | 136.48 ± 1.5 | 0 | 0 | 0 |
| 2 | 0.1 | 0.1 | 0.156 | 0.64404 | 235 ± 15 | 16.42 ± 2.2 | 150.58 ± 2.2 | 14.10 ± 1.4 | 13.45 ± 1.4 | 176.02 ± 18 | |||||||
| 3 | 0.2 | 0.2 | 0.312 | 1.28807 | 304 ± 11 | 21.24 ± 1.8 | 194.79 ± 1.8 | 58.31 ± 1.6 | 57.02 ± 1.6 | 746.08 ± 21 | |||||||
| 4 | 0.3 | 0.3 | 0.468 | 1.93211 | 341 ± 13 | 23.83 ± 1.6 | 218.49 ± 1.6 | 82.02 ± 1.5 | 80.08 ± 1.5 | 1047.85 ± 20 | |||||||
| 5 | 0.4 | 0.4 | 0.624 | 2.57615 | 271 ± 10 | 18.94 ± 2.0 | 173.64 ± 2.0 | 37.16 ± 1.7 | 34.59 ± 1.7 | 452.56 ± 22 | |||||||
| 6 | 0.5 | 0.5 | 0.78 | 3.22018 | 196 ± 12 | 13.70 ± 1.8 | 125.59 ± 1.8 | −10.89 ± 1.6 | −14.11 ± 1.6 | −184.66 ± 21 |
| Variant | Enet | Energy Price | Energy Value | Price of EU Carbon Permits | SCO2 | Value of SCO2 | Profit | Profit |
|---|---|---|---|---|---|---|---|---|
| Wh | EUR/kWh | EUR | EUR/kg | kg | EUR | EUR | EUR/tonTS | |
| 1 | 0 | 0.2266 | 0.00000 | 0.0517 | 0 | 0 | 0 | 0 |
| 2 | 13.45 ± 1.4 | 0.00302 | 0.156 | 0.00807 | 0.01108 | 144.99 | ||
| 3 | 57.02 ± 1.6 | 0.01291 | 0.312 | 0.01613 | 0.02905 | 380.04 | ||
| 4 | 80.08 ± 1.5 | 0.01815 | 0.468 | 0.02420 | 0.04234 | 554.05 | ||
| 5 | 34.59 ± 1.7 | 0.00788 | 0.624 | 0.03226 | 0.04014 | 525.24 | ||
| 6 | −14.11 ± 1.6 | −0.00322 | 0.78 | 0.04033 | 0.03710 | 485.48 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kazimierowicz, J.; Dębowski, M.; Zieliński, M. Technological, Ecological, and Energy-Economic Aspects of Using Solidified Carbon Dioxide for Aerobic Granular Sludge Pre-Treatment Prior to Anaerobic Digestion. Int. J. Environ. Res. Public Health 2023, 20, 4234. https://doi.org/10.3390/ijerph20054234
Kazimierowicz J, Dębowski M, Zieliński M. Technological, Ecological, and Energy-Economic Aspects of Using Solidified Carbon Dioxide for Aerobic Granular Sludge Pre-Treatment Prior to Anaerobic Digestion. International Journal of Environmental Research and Public Health. 2023; 20(5):4234. https://doi.org/10.3390/ijerph20054234
Chicago/Turabian StyleKazimierowicz, Joanna, Marcin Dębowski, and Marcin Zieliński. 2023. "Technological, Ecological, and Energy-Economic Aspects of Using Solidified Carbon Dioxide for Aerobic Granular Sludge Pre-Treatment Prior to Anaerobic Digestion" International Journal of Environmental Research and Public Health 20, no. 5: 4234. https://doi.org/10.3390/ijerph20054234
APA StyleKazimierowicz, J., Dębowski, M., & Zieliński, M. (2023). Technological, Ecological, and Energy-Economic Aspects of Using Solidified Carbon Dioxide for Aerobic Granular Sludge Pre-Treatment Prior to Anaerobic Digestion. International Journal of Environmental Research and Public Health, 20(5), 4234. https://doi.org/10.3390/ijerph20054234










