Effect of Combined Kinematic Chain Exercise on Physical Function, Balance Ability, and Gait in Patients with Total Knee Arthroplasty: A Single-Blind Randomized Controlled Trial
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Ethical Statement
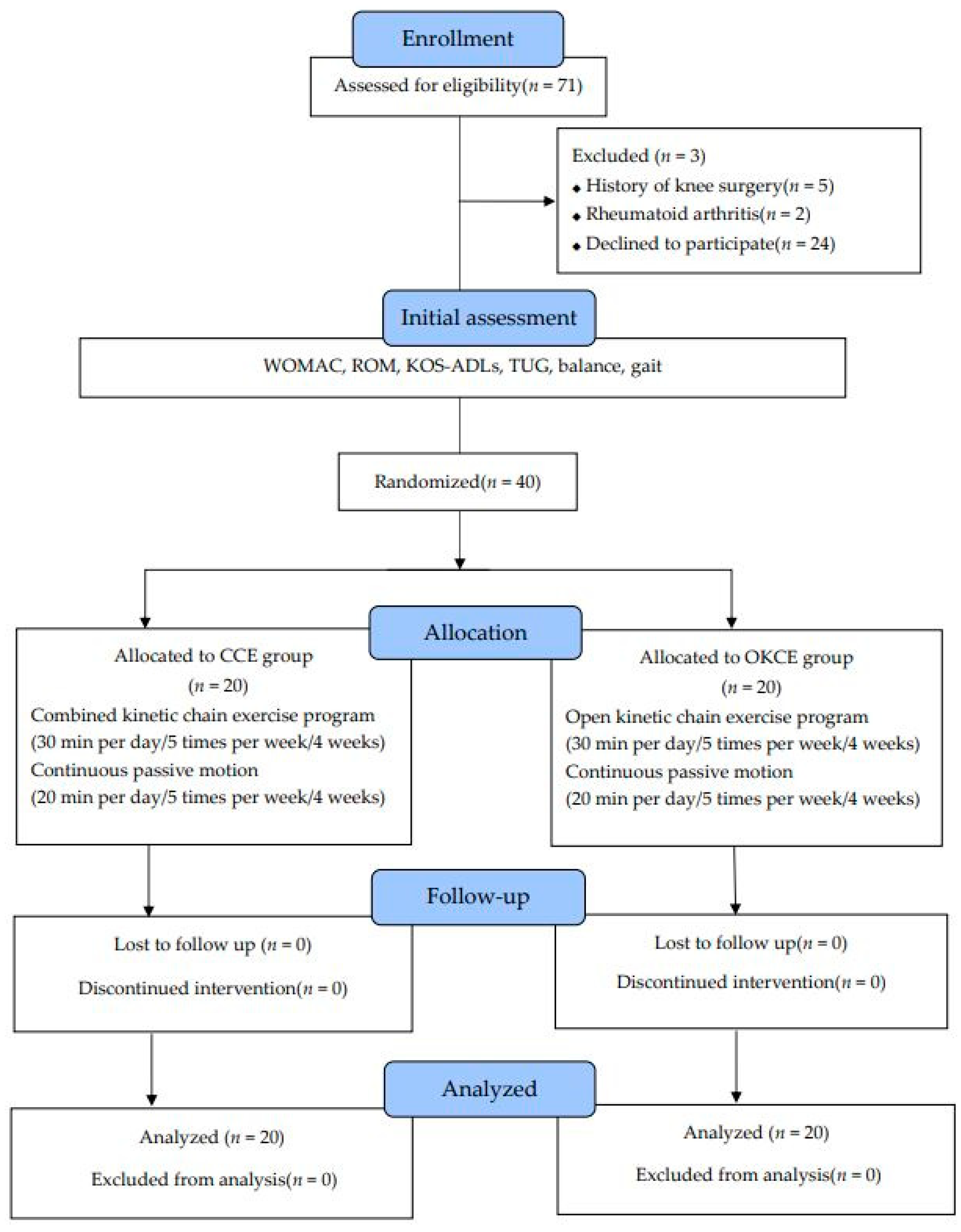
2.3. Study Design
2.4. Study Procedure
2.5. Intervention
2.5.1. Combined Kinetic Chain Exercise Program
2.5.2. Open Kinetic Chain Exercise Program
2.6. Outcome Measurements
2.6.1. Physical Function
2.6.2. Balance
2.6.3. Gait
2.7. Statistical Analysis
3. Results
3.1. Primary Outcomes
3.2. Secondary Outcome
4. Discussion
4.1. Effects of Physical Function
4.2. Effects of Balance
4.3. Effects of Gait
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Crowninshield, R.D.; Rosenberg, A.G.; Sporer, S.M. Changing demographics of patients with total joint replacement. Clin. Orthop. Relat. Res. 2006, 443, 266–272. [Google Scholar] [CrossRef]
- Levinger, P.; Menz, H.B.; Wee, E.; Feller, J.A.; Bartlett, J.R.; Bergman, N.R. Physiological risk factors for falls in people with knee osteoarthritis before and early after knee replacement surgery. Knee Surg. Sport. Traumatol. Arthrosc. 2011, 19, 1082–1089. [Google Scholar] [CrossRef] [PubMed]
- Eymir, M.; Unver, B.; Karatosun, V. Relaxation exercise therapy improves pain, muscle strength, and kinesiophobia following total knee arthroplasty in the short term: A randomized controlled trial. Knee Surg. Sport. Traumatol. Arthrosc. 2022, 30, 2776–2785. [Google Scholar] [CrossRef] [PubMed]
- Sabatini, L.; Bosco, F.; Barberis, L.; Camazzola, D.; Bistolfi, A.; Risitano, S.; Massè, A.; Indelli, P.F. Kinetic sensors for ligament balance and kinematic evaluation in anatomic bi-cruciate stabilized total knee arthroplasty. Sensors 2021, 21, 5427. [Google Scholar] [CrossRef] [PubMed]
- Cacciola, G.; Bosco, F.; Giustra, F.; Risitano, S.; Capella, M.; Bistolfi, A.; Massè, A. Learning curve in robotic-assisted total knee arthroplasty: A systematic review of the literature. Appl. Sci. 2022, 12, 11085. [Google Scholar] [CrossRef]
- Castellarin, G.; Merlini, M.; Bettinelli, G.; Riso, R.; Bori, E.; Innocenti, B. Effect of an innovative biofeedback insole on patient rehabilitation after total knee arthroplasty. Appl. Sci. 2022, 12, 2456. [Google Scholar] [CrossRef]
- Hinterwimmer, F.; Lazic, I.; Langer, S.; Suren, C.; Charitou, F.; Hirschmann, M.T.; Matziolis, G.; Seidl, F.; Pohlig, F.; Rueckert, D.; et al. Prediction of complications and surgery duration in primary TKA with high accuracy using machine learning with arthroplasty-specific data. Knee Surg. Sport. Traumatol. Arthrosc. 2022, 1–11. [Google Scholar] [CrossRef]
- Klug, A.; Gramlich, Y.; Rudert, M.; Drees, P.; Hoffmann, R.; Weißenberger, M.; Kutzner, K.P. The projected volume of primary and revision total knee arthroplasty will place an immense burden on future health care systems over the next 30 years. Knee Surg. Sport. Traumatol. Arthrosc. 2021, 29, 3287–3298. [Google Scholar]
- Korea National Statistical Office. Available online: http://www.kostat.go.kr/portal/eng/index.action (accessed on 21 September 2019).
- Bade, M.J.; Kohrt, W.M.; Stevens-Lapsley, J.E. Outcomes before and after total knee arthroplasty compared to healthy adults. J. Orthop. Sport. Phys. Ther. 2010, 40, 559–567. [Google Scholar] [CrossRef]
- Gstoettner, M.; Raschner, C.; Dirnberger, E.; Leimser, H.; Krismer, M. Preoperative proprioceptive training in patients with total knee arthroplasty. Knee 2011, 18, 265–270. [Google Scholar] [CrossRef]
- Piva, S.R.; Gil, A.B.; Almeida, G.J.; DiGioia, A.M., III; Levison, T.J.; Fitzgerald, G.K. A balance exercise program appears to improve function for patients with total knee arthroplasty: A randomized clinical trial. Phys. Ther. 2010, 90, 880–894. [Google Scholar] [CrossRef] [PubMed]
- Pua, Y.H.; Seah, F.J.T.; Seet, F.J.H.; Tan, J.W.M.; Liaw, J.S.C.; Chong, H.C. Sex differences and impact of body mass index on the time course of knee range of motion, knee strength, and gait speed after total knee arthroplasty. Arthritis Care Res. 2015, 67, 1397–1405. [Google Scholar] [CrossRef] [PubMed]
- Bily, W.; Franz, C.; Trimmel, L.; Loefler, S.; Cvecka, J.; Zampieri, S.; Kasche, W.; Sarabon, N.; Zenz, P.; Kern, H. Effects of leg-press training with moderate vibration on muscle strength, pain, and function after total knee arthroplasty: A randomized controlled trial. Arch. Phys. Med. Rehab. 2016, 97, 857–865. [Google Scholar] [CrossRef] [PubMed]
- Domínguez-Navarro, F.; Igual-Camacho, C.; Silvestre-Muñoz, A.; Roig-Casasús, S.; Blasco, J.M. Effects of balance and proprioceptive training on total hip and knee replacement rehabilitation: A systematic review and meta-analysis. Gait Posture 2018, 62, 68–74. [Google Scholar] [CrossRef] [PubMed]
- Peer, M.; Rush, R.; Gallacher, P.; Gleeson, N. Pre-surgery exercise and post-operative physical function of people undergoing knee replacement surgery: A systematic review and meta-analysis of randomized controlled trials. J. Rehabil. Med. 2017, 49, 304–315. [Google Scholar] [CrossRef]
- An, J.; Ryu, H.K.; Lyu, S.J.; Yi, H.J.; Lee, B.H. Effects of preoperative telerehabilitation on muscle strength, range of motion, and functional outcomes in candidates for total knee arthroplasty: A single-blind randomized controlled trial. Int. J. Environ. Res. Public Health 2021, 18, 6071. [Google Scholar] [CrossRef]
- McGinty, G.; Irrgang, J.J.; Pezzullo, D. Biomechanical considerations for rehabilitation of the knee. Clin. Biomech. 2000, 15, 160–166. [Google Scholar] [CrossRef]
- Perriman, A.; Leahy, E.; Semciw, A.I. The effect of open-versus closed-kinetic-chain exercises on anterior tibial laxity, strength, and function following anterior cruciate ligament reconstruction: A systematic review and meta-analysis. J. Orthop. Sport. Phys. Ther. 2018, 48, 552–566. [Google Scholar] [CrossRef]
- Stensdotter, A.-K.; Hodges, P.; Mellor, R.; Sundelin, G.; Häger-Ross, C. Quadriceps activation in closed and in open kinetic chain exercise. Med. Sci. Sport. Exerc. 2003, 35, 2043–2047. [Google Scholar] [CrossRef]
- Balci, P.; Tunay, V.B.; Baltaci, G.; Atay, A.O. The effects of two different closed kinetic chain exercises on muscle strength and proprioception in patients with patellofemoral pain syndrome. Acta Orthop. Traumatol. Turc. 2009, 43, 419–425. [Google Scholar] [CrossRef]
- Negi, D.R.; Rani, P. Comparing OKC (open kinetic chain) with CKC (closed kinetic chain) along with hot pack on quadriceps strength and functional status of women with osteoarthritic knees. IAMR 2012, 1, 20. [Google Scholar]
- Kwon, Y.J.; Park, S.J.; Jefferson, J.; Kim, K. The effect of open and closed kinetic chain exercises on dynamic balance ability of normal healthy adults. J. Phys. Ther. Sci. 2013, 25, 671–674. [Google Scholar] [CrossRef] [PubMed]
- Wirries, N.; Ezechieli, M.; Stimpel, K.; Skutek, M. Impact of continuous passive motion on rehabilitation following total knee arthroplasty. Physiother. Res. Int. 2020, 25, e1869. [Google Scholar] [CrossRef] [PubMed]
- Abbas, C.; Daher, J. Pilot study: Post-operative rehabilitation pathway changes and implementation of functional closed kinetic chain exercise in total hip and total knee replacement patient. J. Bodyw. Mov. Ther. 2017, 21, 823–829. [Google Scholar] [CrossRef] [PubMed]
- Olagbegi, O.M.; Adegoke, B.O.; Odole, A.C. Effectiveness of three modes of kinetic-chain exercises on quadriceps muscle strength and thigh girth among individuals with knee osteoarthritis. Arch. Physiother. 2017, 7, 9. [Google Scholar] [CrossRef]
- Wortley, M.; Zhang, S.; Paquette, M.; Byrd, E.; Baumgartner, L.; Klipple, G. Effects of resistance and Tai Ji training on mobility and symptoms in knee osteoarthritis patients. J. Sport Health Sci. 2013, 2, 209–214. [Google Scholar] [CrossRef]
- Minoonejad, H.; Rajabi, R.; Ebrahimi-Takamjani, E.; Alizadeh, M.; Jamshidi, A.; Azhari, A. Combined open and closed kinetic chain exercises for patellofemoral pain syndrome: A randomized controlled trial. World J. Sport Sci. 2012, 6, 278–285. [Google Scholar]
- Fitz, W.; Shukla, P.; Li, L.; Scott, R.D. Early regain of function and proprioceptive improvement following knee arthroplasty. Arch. Bone Joint Surg. 2018, 6, 523. [Google Scholar]
- Olagbegi, O.M.; Adegoke, B.O.A.; Odole, A.C. Effectiveness of combined chain exercises on pain and function in patients with knee osteoarthritis. Bangladesh J. Med. Sci. 2016, 15, 178–188. [Google Scholar] [CrossRef]
- Thonga, T.; Stasi, S.; Papathanasiou, G. The effect of intensive close-kinetic-chain exercises on functionality and balance confidence after total knee arthroplasty. Cureus 2021, 13, e18965. [Google Scholar] [CrossRef]
- Stevens-Lapsley, J.E.; Petterson, S.C.; Mizner, R.L.; Snyder-Mackler, L. Impact of body mass index on functional performance after total knee arthroplasty. J. Arthroplast. 2010, 25, 1104–1109. [Google Scholar] [CrossRef] [PubMed]
- McConnell, S.; Kolopack, P.; Davis, A.M. The Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC): A review of its utility and measurement properties. Arthritis Care Res. 2001, 45, 453–461. [Google Scholar] [CrossRef]
- Glass, R.; Waddell, J.; Hoogenboom, B. The effects of open versus closed kinetic chain exercises on patients with ACL deficient or reconstructed knees: A systematic review. N. Am. J. Sport. Phys. Ther. 2010, 5, 74. [Google Scholar]
- Risitano, S.; Cacciola, G.; Sabatini, L.; Capella, M.; Bosco, F.; Giustra, F.; Massè, A.; Vaishya, R. Restricted kinematic alignment in primary total knee arthroplasty: A systematic review of radiographic and clinical data. J. Orthop. 2022, 33, 37–43. [Google Scholar] [CrossRef]
- Svensson, M.; Lind, V.; Löfgren Harringe, M. Measurement of knee joint range of motion with a digital goniometer: A reliability study. Physiother. Res. Int. 2019, 24, e1765. [Google Scholar] [CrossRef]
- Kapreli, E.; Panelli, G.; Strimpakos, N.; Billis, E.; Zacharopoulos, A.; Athanasopoulos, S. Cross-cultural adaptation of the Greek version of the Knee Outcome Survey–activities of Daily Living Scale (KOS-ADLS). Knee 2011, 18, 424–427. [Google Scholar] [CrossRef]
- Van Alsenoy, K.; Thomson, A.; Burnett, A. Reliability and validity of the Zebris FDM-THQ instrumented treadmill during running trials. Sport. Biomech. 2018, 18, 501–514. [Google Scholar] [CrossRef]
- Coleman, S.; Briffa, N.K.; Carroll, G.; Inderjeeth, C.; Cook, N.; McQuade, J. A randomised controlled trial of a self-management education program for osteoarthritis of the knee delivered by health care professionals. Arthritis Res. Ther. 2012, 14, R21. [Google Scholar] [CrossRef]
- Shumway-Cook, A.; Brauer, S.; Woollacott, M. Predicting the probability for falls in community-dwelling older adults using the Timed Up Go Test. Phys. Ther. 2000, 80, 896–903. [Google Scholar] [CrossRef]
- Nordin, E.; Rosendahl, E.; Lundin-Olsson, L. Timed “Up Go” Test: Reliability in older people dependent in activities of daily living—Focus on cognitive state. Phys. Ther. 2006, 86, 646–655. [Google Scholar] [CrossRef]
- Meier, W.; Mizner, R.; Marcus, R.; Dibble, L.; Peters, C.; Lastayo, P.C. Total knee arthroplasty: Muscle impairments, functional limitations, and recommended rehabilitation approaches. J. Orthop. Sport. Phys. Ther. 2008, 38, 246–256. [Google Scholar] [CrossRef] [PubMed]
- Mizner, R.L.; Petterson, S.C.; Stevens, J.E.; Vandenborne, K.; Snyder-Mackler, L. Early quadriceps strength loss after total knee arthroplasty: The contributions of muscle atrophy and failure of voluntary muscle activation. J. Bone Joint Surg. 2005, 87, 1047. [Google Scholar] [CrossRef] [PubMed]
- Stevens, J.E.; Mizner, R.L.; Snyder-Mackler, L. Quadriceps strength and volitional activation before and after total knee arthroplasty for osteoarthritis. J. Orthop. Res. 2003, 21, 775–779. [Google Scholar] [CrossRef] [PubMed]
- Kehlet, H.; Søballe, K. Fast-track hip and knee replacement—What are the issues? Acta Orthop. 2010, 81, 271. [Google Scholar] [CrossRef]
- MacKay, C.; Clements, N.; Wong, R.; Davis, A.M. A systematic review of estimates of the minimal clinically important difference and patient acceptable symptom state of the Western Ontario and McMaster Universities Osteoarthritis Index in patients who underwent total hip and total knee replacement. Osteoarthr. Cartil. 2019, 27, 1408–1419. [Google Scholar] [CrossRef]
- Collins, N.J.; Misra, D.; Felson, D.T.; Crossley, K.M.; Roos, E.M. Measures of knee function: International Knee Documentation Committee (IKDC) Subjective Knee Evaluation Form, Knee Injury and Osteoarthritis Outcome Score (KOOS), Knee Injury and Osteoarthritis Outcome Score Physical Function Short Form (KOOS-PS), Knee Outcome Survey Activities of Daily Living Scale (KOS-ADL), Lysholm Knee Scoring Scale, Oxford Knee Score (OKS), Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC), Activity Rating Scale (ARS), and Tegner Activity Score (TAS). Arthritis Care Res. 2011, 63, 208–228. [Google Scholar]
- Lim, G.R.; Kwon, E.H.; Kim, D.S.; Kim, J.H.; Park, J.; Choi, E.H. The effects of closed kinetic chain exercise and open kinetic chain exercise on the knee position sense in the normal adults. J. Int. Acad. Phys. Ther. Res. 2010, 1, 126–135. [Google Scholar]
- Rougier, P.; Farenc, I.; Berger, L. Modifying the gain of the visual feedback affects undisturbed upright stance control. Clin. Biomech. 2004, 19, 858–867. [Google Scholar] [CrossRef]
- Smith, A.J.; Lloyd, D.; Wood, D. Pre-surgery knee joint loading patterns during walking predict the presence and severity of anterior knee pain after total knee arthroplasty. J. Orthop. Res. 2004, 22, 260–266. [Google Scholar] [CrossRef]
- Rajkumar, R.V. Force couple mechanics on femur during closed kinetic chain activities of lower limbs. Int. J. Physiother. Res. 2014, 2, 766–771. [Google Scholar] [CrossRef]
- Perry, J.; Burnfield, J.M. Gait Analysis. Normal and Pathological Function, 2nd ed.; Slack: San Francisco, CA, USA, 2010. [Google Scholar]
- Neptune, R.R.; Sasaki, K.; Kautz, S.A. The effect of walking speed on muscle function and mechanical energetics. Gait Posture 2008, 28, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Au, S.K.; Weber, J.; Herr, H. Biomechanical design of a powered ankle-foot prosthesis. In Proceedings of the 2007 IEEE 10th International Conference on Rehabilitation Robotics, Noordwijk, The Netherlands, 13–15 June 2007. [Google Scholar]
- Kiguchi, K.; Imada, Y. EMG-based control for lower-limb power-assist exoskeletons. In Proceedings of the 2009 IEEE Workshop on Robotic Intelligence in Informationally Structured Space, Nashville, TN, USA, 30 March–2 April 2009. [Google Scholar]
- Han, J.; Waddington, G.; Anson, J.; Adams, R. Level of competitive success achieved by elite athletes and multi-joint proprioceptive ability. J. Sci. Med. Sport 2015, 18, 77–81. [Google Scholar] [CrossRef] [PubMed]
- Ouellet, D.; Moffet, H. Locomotor deficits before and two months after knee arthroplasty. Arthritis Care Res. 2002, 47, 484–493. [Google Scholar] [CrossRef] [PubMed]
- Winter, D.A. Biomechanics and Motor Control of Human Movement; John Wiley Sons: Hoboken, NJ, USA, 2009. [Google Scholar]
- Hesse, S.; Werner, C.; Paul, T.; Bardeleben, A.; Chaler, J. Influence of walking speed on lower limb muscle activity and energy consumption during treadmill walking of hemiparetic patients. Arch. Phys. Med. Rehab. 2001, 82, 1547–1550. [Google Scholar] [CrossRef]
- Mayer, F.; Schlumberger, A.; Van Cingel, R.; Henrotin, Y.; Laube, W.; Schmidtbleicher, D. Training and testing in open versus closed kinetic chain. Isokinet. Exerc. Sci. 2003, 11, 181–187. [Google Scholar] [CrossRef]
- Gremeaux, V.; Duclay, J.; Deley, G.; Philipp, J.; Laroche, D.; Pousson, M. Does eccentric endurance training improve walking capacity in patients with coronary artery disease? A randomized controlled pilot study. Clin. Rehab. 2010, 24, 590–599. [Google Scholar] [CrossRef]
- Umberger, B.R. Stance and swing phase costs in human walking. J. R. Soc. Interface 2010, 7, 1329–1340. [Google Scholar] [CrossRef]
| Week | Contents of Program | Times |
|---|---|---|
| 1st week |
| 10 times, 2 sets |
| 2nd to 3rd week |
| 10 times, 2 sets |
| 4th week |
| 10 times, 2 sets |
| Total exercise time was 30 min with a rest time of 30–40 s between sets. | ||
| Week | Open-Kinetic Chain Exercise Program | Reps |
|---|---|---|
| 1st to 2nd week |
| Each 5 s 10 reps/6 sets |
| 3rd to 4th week |
| Each 5 s 10 reps/6 sets |
| Total exercise time was 30 min with a rest time of 30–40 s between sets. | ||
| Characteristics | CCE Group (n = 20) | OKCE Group (n = 20) | X2/F(p) |
|---|---|---|---|
| Surgery side (left/right) | 10/10 | 9/11 | 0.309 (0.759) |
| Age (years) | 71.60 ± 3.53 | 70.04 ± 2.47 | 1.244 (0.221) |
| Height (cm) | 152.47 ± 6.05 | 151.34 ± 5.82 | 0.599 (0.605) |
| Weight (kg) | 61.08 ± 8.67 | 59.57 ± 9.54 | 0.522 (0.553) |
| BMI (kg/m2) | 26.15 ± 2.89 | 25.92 ± 3.30 | 0.237 (0.814) |
| Variable | CCE Group (n = 20) | OKCE Group (n = 20) | t (p) | Time | Group | Time × Group | |
|---|---|---|---|---|---|---|---|
| F (p) | F (p) | F (p) | |||||
| WOMAC (Pain) | Pretest | 12.60 ± 1.66 | 11.85 ± 2.05 | 1.266 (0.213) | 304.42 (0.000) | 6.060 (0.018) | 26.725 (0.000) |
| Posttest | 3.85 ± 1.66 | 7.10 ± 2.55 | |||||
| Mean difference | 8.75 ± 2.51 | 4.75 ± 2.38 | 5.170 (0.000) | ||||
| 95% CI | (7.575 to 9.925) | (3.653 to 5.865) | |||||
| t (p) | 15.587 (0.000) | 8.920 (0.001) | |||||
| WOMAC (Stiffness) | Pretest | 4.95 ± 1.05 | 4.40 ± 0.88 | 1.793 (0.081) | |||
| Posttest | 1.50 ± 0.68 | 2.65 ± 0.81 | |||||
| Mean difference | 3.45 ± 1.35 | 1.75 ± 1.11 | 4.325 (0.000) | 175.04 (0.000) | 2.447 (0.126) | 18.709 (0.000) | |
| 95% CI | (2.815 to 4.085) | (1.227 to 2.273) | |||||
| t (p) | 11.376 (0.000) | 7.000 (0.000) | |||||
| WOMAC (Physical function) | Pretest | 48.60 ± 6.93 | 47.70 ± 9.02 | 0.354 (0.725) | |||
| Posttest | 24.20 ± 5.46 | 31.25 ± 7.03 | |||||
| Mean difference | 24.40 ± 8.48 | 16.45 ± 7.02 | 3.227 (0.003) | 275.01 (0.000) | 2.555 (0.118) | 10.416 (0.003) | |
| 95% CI | (20.49 to 28.38) | (13.16 to 19.74) | |||||
| t (p) | 12.856 (0.000) | 10.476 (0.000) | |||||
| ROM | Pretest | 108.02 ± 7.07 | 109.92 ± 9.90 | –0.696 (0.491) | |||
| Posttest | 137.92 ± 3.15 | 132.78 ± 6.70 | |||||
| Mean difference | 29.90 ± 8.32 | 22.86 ± 7.47 | 2.815 (0.008) | 444.98 (0.000) | 0.751 (0.392) | 7.923 (0.008) | |
| 95% CI | (26.00 to 33.795) | (19.36 to 26.36) | |||||
| t (p) | –16.065 (0.000) | –13.682 (0.000) | |||||
| KOS-ADLs | Pretest | 24.80 ± 10.10 | 30.20 ± 9.42 | –1.748 (0.089) | |||
| Posttest | 46.10 ± 8.99 | 43.80 ± 8.50 | |||||
| Mean difference | 21.30 ± 8.46 | 13.60 ± 8.81 | 2.817 (0.008) | 163.03 (0.000) | 0.357 (0.554) | 7.936 (0.008) | |
| 95% CI | (16.065 to 25.263) | (9.47 to 17.73) | |||||
| t (p) | –11.250 (0.000) | –6.899 (0.000) | |||||
| Variable | CCE Group (n = 20) | OKCE Group (n = 20) | t (p) | Time | Group | Time × Group | |
|---|---|---|---|---|---|---|---|
| F (p) | F (p) | F (p) | |||||
| CEA (mm2) | Pretest | 456.42 ± 188.43 | 517.72 ± 231.21 | –0.919 (0.364) | |||
| Posttest | 285.30 ± 212.74 | 403.15 ± 248.05 | |||||
| Mean difference | 171.11 ± 76.02 | 114.57 ± 89.09 | 2.159 (0.037) | 304.42 (0.000) | 6.060 (0.018) | 26.725 (0.000) | |
| 95% CI | (135.54 to 206.70) | (72.88 to 156.27) | |||||
| t (p) | 10.066 (0.000) | 5.751 (0.000) | |||||
| CPL (mm) | Pretest | 130.20 ± 32.68 | 127.28 ± 38.69 | 0.258 (0.798) | |||
| Posttest | 99.29 ± 29.95 | 109.22 ± 37.00 | |||||
| Mean difference | 30.90 ± 15.30 | 18.05 ± 13.23 | 2.842 (0.007) | 119.00 (0.000) | 1.699 (0.200) | 4.661 (0.037) | |
| 95% CI | (23.75 to 38.07) | (11.86 to 24.25) | |||||
| t (p) | 9.031 (0.000) | 6.102 (0.000) | |||||
| CAV (mm/sec) | Pretest | 14.49 ± 3.12 | 14.56 ± 4.75 | –0.053 (0.958) | |||
| Posttest | 10.24 ± 3.26 | 11.75 ± 4.08 | |||||
| Mean difference | 4.24 ± 1.32 | 2.80 ± 1.49 | 3.228 (0.003) | 117.13 (0.000) | 0.106 (0.746) | 8.076 (0.007) | |
| 95% CI | (3.63 to 4.87) | (2.11 to 3.51) | |||||
| t (p) | 3.228 (0.003) | 8.407 (0.000) | |||||
| Variable | CCE Group (n = 20) | OKCE Group (n = 20) | t (p) | Time | Group | Time × Group | |
|---|---|---|---|---|---|---|---|
| F (p) | F (p) | F (p) | |||||
| TUG (s) | Pretest | 13.92 ± 2.41 | 13.05 ± 1.76 | 1.299 (0.202) | |||
| Posttest | 10.47 ± 1.45 | 11.19 ± 2.14 | |||||
| Mean difference | 3.45 ± 1.41 | 1.86 ± 1.36 | 3.626 (0.001) | 146.42 (0.000) | 0.015 (0.903) | 13.150 (0.001) | |
| 95% CI | (2.79 to 4.12) | (1.22 to 2.50) | |||||
| t (p) | 10.916 (0.000) | 6.108 (0.000) | |||||
| Velocity (km/h) | Pretest | 2.32 ± 0.44 | 2.49 ± 0.63 | –0.981 (0.333) | |||
| Posttest | 2.68 ± 0.41 | 2.65 ± 0.61 | |||||
| Mean difference | 0.35 ± 0.26 | 0.15 ± 0.24 | 2.526 (0.016) | 249.99 (0.000) | 0.430 (0.516) | 10.421 (0.003) | |
| 95% CI | (0.23 to 0.48) | (0.03 to 0.26) | |||||
| t (p) | –5.998 (0.000) | –2.764 (0.013) | |||||
| Cadence (step/min) | Pretest | 98.49 ± 7.47 | 102.18 ± 8.55 | –1.450 (0.155) | |||
| Posttest | 107.68 ± 6.99 | 104.81 ± 8.27 | |||||
| Mean difference | 9.18 ± 4.15 | 2.63 ± 3.58 | 5.337 (0.000) | 92.683 (0.000) | 0.029 (0.886) | 28.485 (0.000) | |
| 95% CI | (7.24 to 11.13) | (0.96 to 4.30) | |||||
| t (p) | –9.879 (0.000) | –3.285 (0.004) | |||||
| Step length (cm) | Pretest | 37.38 ± 5.71 | 39.54 ± 4.85 | –1.286 (0.206) | |||
| Posttest | 39.89 ± 5.92 | 40.41 ± 4.57 | |||||
| Mean difference | 2.49 ± 1.48 | 0.86 ± 1.24 | 3.766 (0.001) | 49.146 (0.000) | 1.060 (0.310) | 9.533 (0.004) | |
| 95% CI | (1.81 to 3.19) | (0.28 to 1.45) | |||||
| t (p) | –7.509 (0.000) | –3.114 (0.006) | |||||
| Stride length (cm) | Pretest | 77.99 ± 13.53 | 84.08 ± 11.78 | –1.516 (0.138) | |||
| Posttest | 84.19 ± 14.90 | 86.48 ± 11.53 | |||||
| Mean difference | 6.19 ± 3.39 | 2.40 ± 4.31 | 3.088 (0.004) | 60.206 (0.000) | 0.657 (0.423) | 14.183 (0.001) | |
| 95% CI | (4.61 to 8.16) | (0.39 to 4.43) | |||||
| t (p) | –8.155 (0.000) | –2.498 (0.022) | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
An, J.; Son, Y.-W.; Lee, B.-H. Effect of Combined Kinematic Chain Exercise on Physical Function, Balance Ability, and Gait in Patients with Total Knee Arthroplasty: A Single-Blind Randomized Controlled Trial. Int. J. Environ. Res. Public Health 2023, 20, 3524. https://doi.org/10.3390/ijerph20043524
An J, Son Y-W, Lee B-H. Effect of Combined Kinematic Chain Exercise on Physical Function, Balance Ability, and Gait in Patients with Total Knee Arthroplasty: A Single-Blind Randomized Controlled Trial. International Journal of Environmental Research and Public Health. 2023; 20(4):3524. https://doi.org/10.3390/ijerph20043524
Chicago/Turabian StyleAn, Jungae, Young-Wan Son, and Byoung-Hee Lee. 2023. "Effect of Combined Kinematic Chain Exercise on Physical Function, Balance Ability, and Gait in Patients with Total Knee Arthroplasty: A Single-Blind Randomized Controlled Trial" International Journal of Environmental Research and Public Health 20, no. 4: 3524. https://doi.org/10.3390/ijerph20043524
APA StyleAn, J., Son, Y.-W., & Lee, B.-H. (2023). Effect of Combined Kinematic Chain Exercise on Physical Function, Balance Ability, and Gait in Patients with Total Knee Arthroplasty: A Single-Blind Randomized Controlled Trial. International Journal of Environmental Research and Public Health, 20(4), 3524. https://doi.org/10.3390/ijerph20043524







