Contribution of Solar Radiation and Pollution to Parkinson’s Disease
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Definitions
2.2. Statistical Analysis
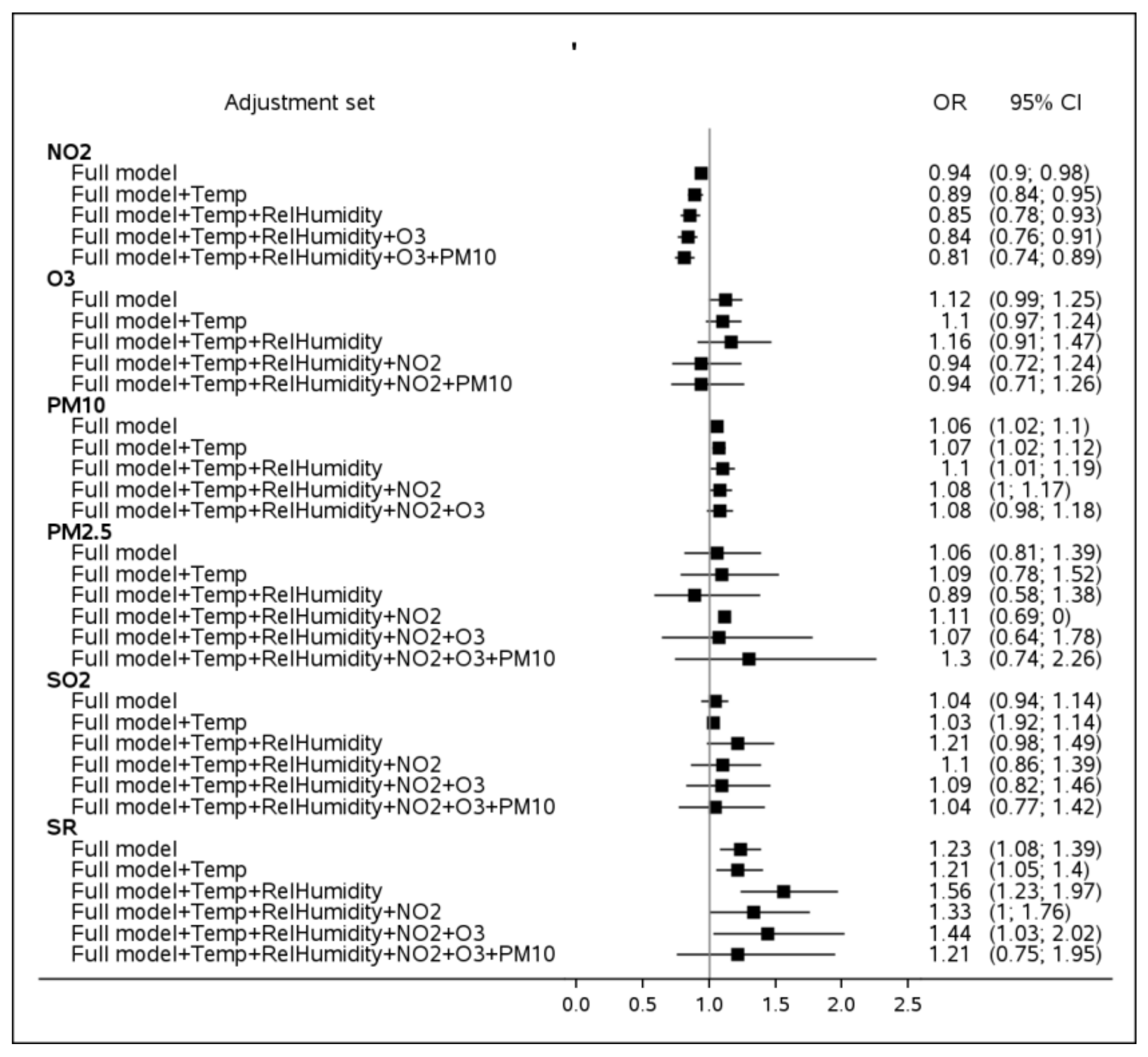
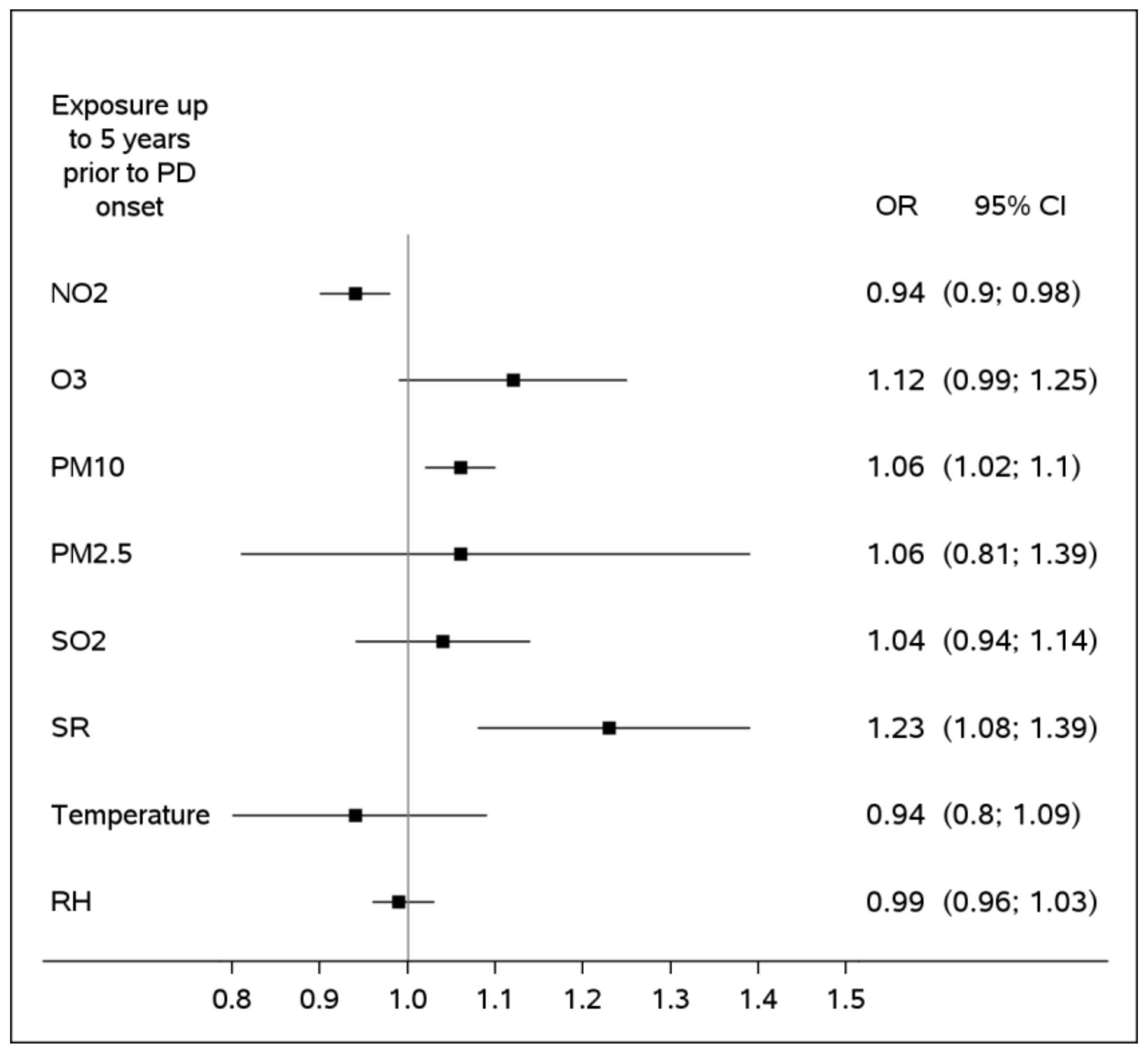
3. Results
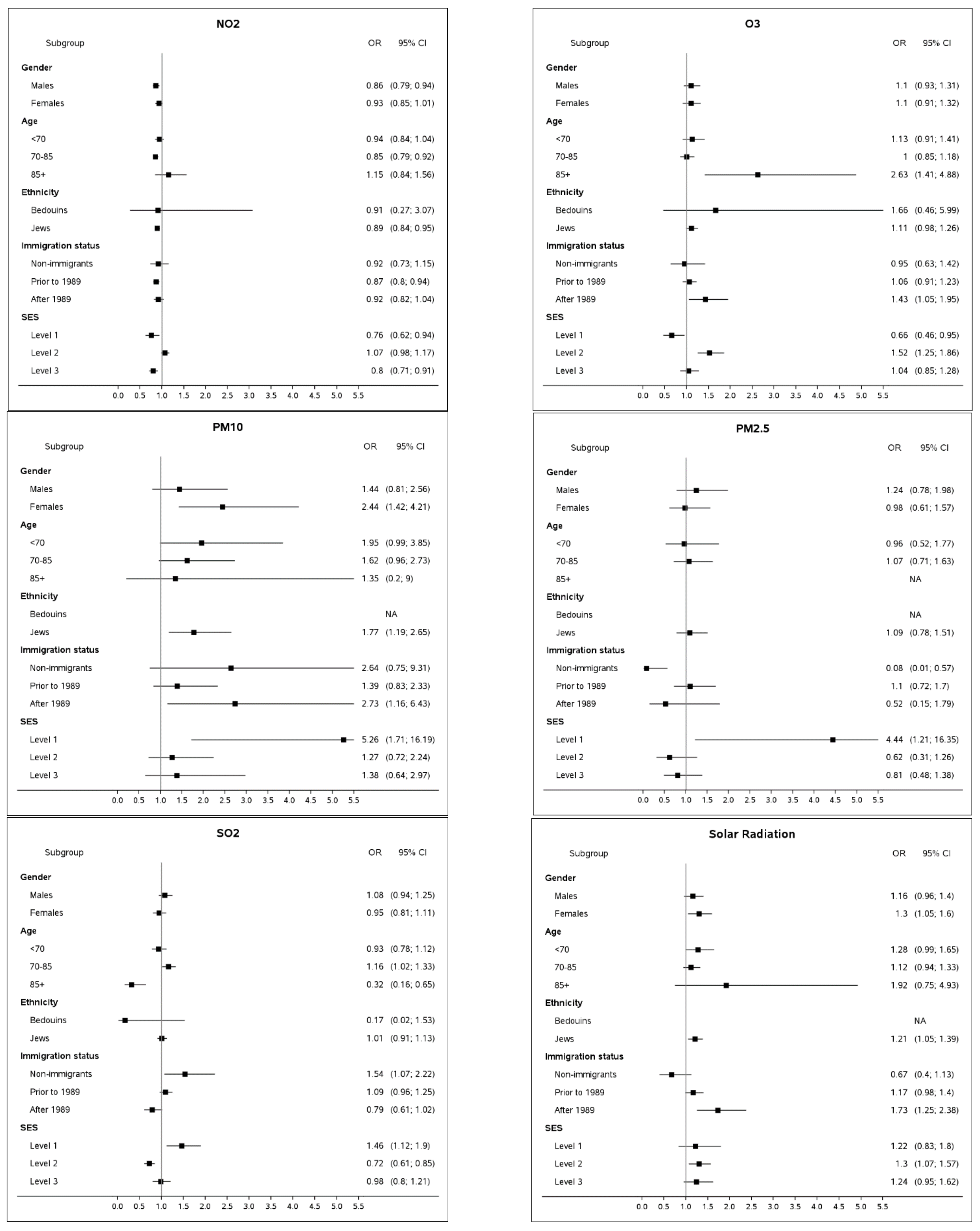
3.1. Subgroup Analyses
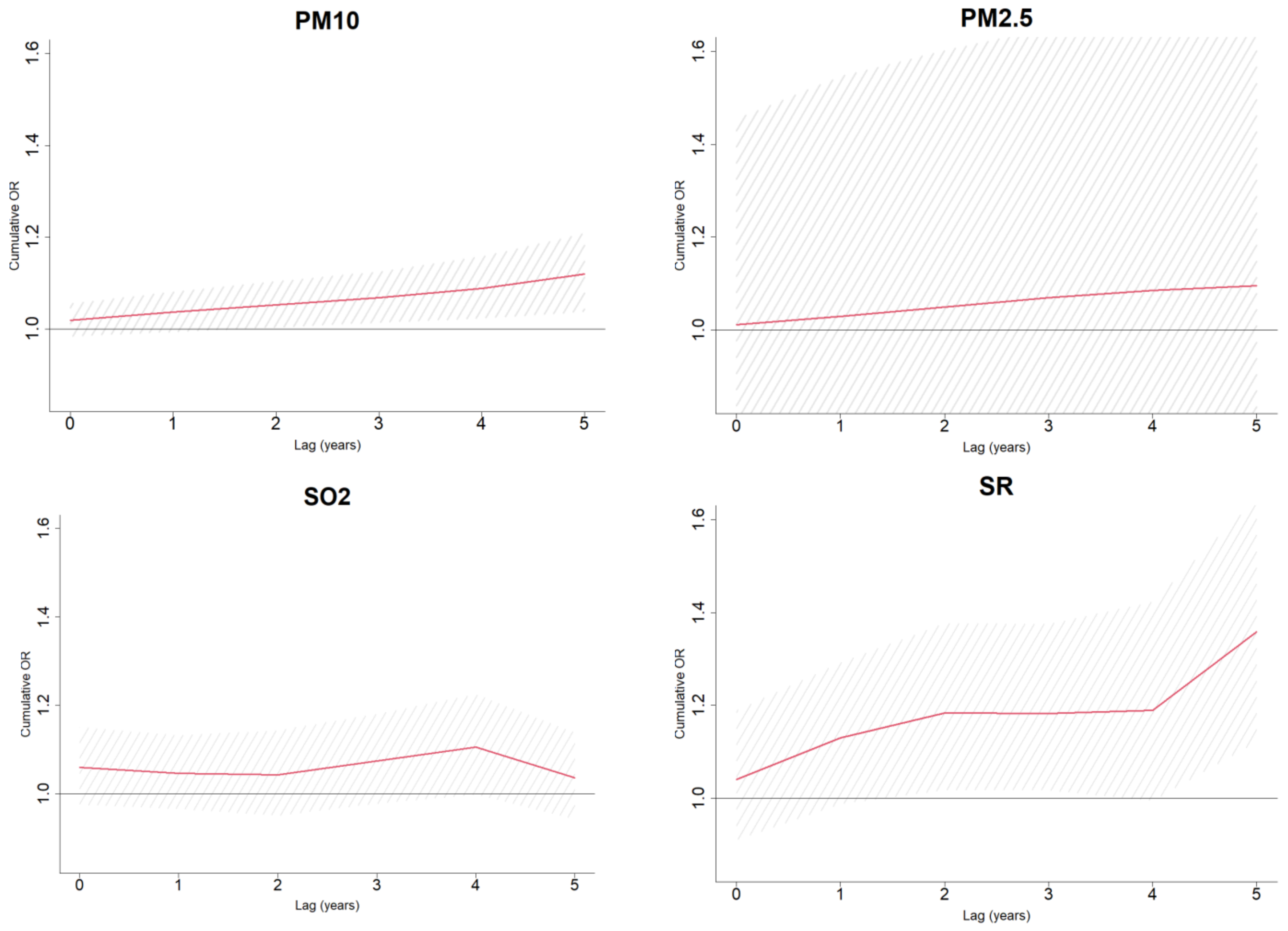
3.2. Sensitivity Analyses
4. Discussion
- Based on the study findings, patients developing PD at relatively young age (<70 years) are likely to be more exposed to PM10, as compared to their controls. Patients diagnosed substantially later (85+) tend to be more exposed to O3. This observation might be related to different etiology of the disease and/or behavioral patterns featuring subjects younger than 70 and older than 85. For instance, Parkinson’s Disease may be more impacted by a genetic composite in patients diagnosed at a younger age, which might be accompanied by triggers different from older patients. Inspection of group younger than 60 at PD onset revealed an even stronger link with PM10, characterized by OR = 3.89 (95%CI: 1.18; 12.76), as compared to OR point estimates of 1.96, 1.62 and 1.35 for groups younger than 70, 70–85 and 85+, respectively. The findings suggest PM10 being a possible risk factor for an early PD onset.
- Another observation concerns the new immigrants (arriving in Israel in 1989 and later) who appear to be more vulnerable to exposure to O3 and solar radiation. It is important to mention that the vast majority of the new immigrants in the study population are from the former USSR [41]. This population is used to a significantly colder climate characterized by lower ozone and solar radiation levels; therefore, it can be considered naïve to the semi-arid conditions of the Negev desert region. On the other hand, the dose response effect of SR, i.e., non-existent for Israeli-born participants (OR = 0.67, 95%CI: 0.40; 1.13), moderate for immigrants before 1989 (OR = 1.17, 95%CI: 0.98; 1.40) and relatively high for new immigrants (OR = 1.25, 95%CI: 1.25; 2.38) indicates the adaptation abilities to solar radiation of the population in general.
- Subjects at the lowest SES were more likely to be impacted by SO2, PM10 and PM2.5. Low socioeconomic status has been established as one of the most important risk factors for many health outcomes [42,43]. It should be noted that 12.0% of the lowest SES strata are subjects of Bedouin-Arab origin, a group that comprises just 4.3% of the study group. Approximately a quarter of the Bedouin-Arabs subjects reside in temporary tents and shacks [44] that cannot be hermetically sealed from dust storms (PM10) or anthropogenic pollution (PM2.5 and SO2) when a subject’s home is located in close proximity of a road or industrial zone. Unprotected households combined with hazardous work exposures, poor diet, and other factors characterizing a low SES stratum [43] can potentially increase the susceptibility of the population.
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| ATC Group Name | ATC Code | Generic Name |
|---|---|---|
| Dopa and dopa derivatives | N04BA02 N04BA03 | Levodopa + carbidopa Levodopa + benserazide Levodopa + carbidopa + entacapone |
| Adamantane derivatives | N04BB01 | Amantadine |
| Dopamine agonists | N04BC01 N04BC02 N04BC04 N04BC06 N04BC07 N04BC10 | Bromocriptine Pergolide Ropinirole Cabergoline Apomorphine Lisuride |
| MAO B inhibitors | N04BD01 N04BD02 | Selegiline Rasagiline |
| Other dopaminergic agents | N04BX01 N04BX02 | Tolcapone Entacapone |
| Information on Ambient Pollution within 0–5 Years of PD Onset | Parkinson’s Disease Cases (N = 3343) | Controls (N = 31,324) | p-Value |
|---|---|---|---|
| Mapping quality | |||
| Mapping quality score (highest assigned to full address) | 8.3 ± 2.7 (3343) 9.0 2.0; 10.0 | 8.0 ± 2.8 (31,324) 9.0 2.0; 10.0 | <0.001 |
| Mapping quality score, % (n/N) Full address up to street level Neighborhood Town center | 82.0 (2741/3343) 6.2 (206/3343) 11.9 (396/3343) | 78.7 (24,656/31,324) 7.1 (2222/31,324) 14.2 (4446/31,324) | <0.001 |
| Proportion of years with non-missing measurement | |||
| NO levels, % out of years at follow-up Mean ± SD (n) Median Min; Max | 98.6 ± 11.1 (3343) 100.0 0.0; 100.0 | 98.7 ± 10.5 (31,324) 100.0 0.0; 100.0 | 0.915 |
| NO2 levels, % out of years at follow-up Mean ± SD (n) Median Min; Max | 98.6 ± 11.1 (3343) 100.0 0.0; 100.0 | 98.7 ± 10.5 (31,324) 100.0 0.0; 100.0 | 0.951 |
| O3 levels, % out of years at follow-up Mean ± SD (n) Median Min; Max | 96.3 ± 18.5 (3343) 100.0 0.0; 100.0 | 96.0 ± 19.1 (31,324) 100.0 0.0; 100.0 | 0.917 |
| PM10 levels, % out of years at follow-up Mean ± SD (n) Median Min; Max | 88.9 ± 30.1 (3343) 100.0 0.0; 100.0 | 88.1 ± 30.9 (31,324) 100.0 0.0; 100.0 | 0.072 |
| PM2.5 levels, % out of years at follow-up Mean ± SD (n) Median Min; Max | 49.5 ± 47.2 (3343) 50.0 0.0; 100.0 | 56.6 ± 46.7 (31,324) 83.3 0.0; 100.0 | <0.001 |
| SO2 levels, % out of years at follow-up Mean ± SD (n) Median Min; Max | 97.9 ± 14.2 (3343) 100.0 0.0; 100.0 | 98.0 ± 13.6 (31,324) 100.0 0.0; 100.0 | 0.696 |
| RH levels, % out of years at follow-up Mean ± SD (n) Median Min; Max | 73.4 ± 36.4 (3343) 100.0 0.0; 100.0 | 74.6 ± 37.0 (31,324) 100.0 0.0; 100.0 | 0.103 |
| Temperature levels, % out of years at follow-up Mean ± SD (n) Median Min; Max | 84.3 ± 27.9 (3343) 100.0 0.0; 100.0 | 86.6 ± 26.9 (31,324) 100.0 0.0; 100.0 | <0.001 |
| SR levels, % out of years at follow-up Mean ± SD (n) Median Min; Max | 79.5 ± 31.6 (3343) 100.0 0.0; 100.0 | 81.4 ± 31.7 (31,324) 100.0 0.0; 100.0 | <0.001 |
| Environmental Factor, Expressed as Exposure to Its Highest Quartile vs. Other Levels, Based on a 5-Year Average | OR 1 | 95%CI | p-Value |
|---|---|---|---|
| NO2 | 0.85 | 0.77; 0.94 | 0.001 |
| O3 | 1.07 | 0.95; 1.20 | 0.286 |
| PM10 | 0.93 | 0.83; 1.03 | 0.165 |
| PM2.5 | 1.08 | 0.91; 1.28 | 0.376 |
| SO2 | 0.84 | 0.77; 0.93 | <0.001 |
| Solar Radiation | 1.18 | 1.03; 1.35 | 0.018 |
| Temperature | 1.04 | 0.90; 1.20 | 0.613 |
| Relative Humidity | 1.17 | 1.04; 1.32 | 0.011 |

References
- Dorsey, E.R.; Sherer, T.; Okun, M.S.; Bloem, B.R. The Emerging Evidence of the Parkinson Pandemic. J. Park. Dis. 2018, 8, S3–S8. [Google Scholar] [CrossRef]
- Pavlou, M.A.S.; Outeiro, T.F. Epigenetics in Parkinson’s Disease. Adv. Exp. Med. Biol. 2017, 978, 363–390. [Google Scholar] [PubMed]
- Fleming, S.M. Mechanisms of Gene-Environment Interactions in Parkinson’s Disease. Curr. Environ. Health Rep. 2017, 4, 192–199. [Google Scholar] [CrossRef] [PubMed]
- Ball, N.; Teo, W.-P.; Chandra, S.; Chapman, J. Parkinson’s Disease and the Environment. Front. Neurol. 2019, 10, 218. [Google Scholar] [CrossRef] [PubMed]
- Dias, V.; Junn, E.; Mouradian, M.M. The role of oxidative stress in Parkinson’s disease. J. Parkinson’s Dis. 2013, 3, 461–491. [Google Scholar] [CrossRef] [PubMed]
- Madetko, N.; Migda, B.; Alster, P.; Turski, P.; Koziorowski, D.; Friedman, A. Platelet-to-lymphocyte ratio and neutrophil-tolymphocyte ratio may reflect differences in PD and MSA-P neuroinflammation patterns. Neurol. Neurochir. Pol. 2022, 56, 148–155. [Google Scholar] [CrossRef] [PubMed]
- Kasdagli, M.I.; Katsouyanni, K.; Dimakopoulou, K.; Samoli, E. Air pollution and Parkinson’s disease: A systematic review and meta-analysis up to 2018. Int. J. Hyg. Environ. Health 2019, 222, 402–409. [Google Scholar] [CrossRef] [PubMed]
- Fu, P.; Guo, X.; Cheung, F.M.H.; Yung, K.K.L. The association between PM2.5 exposure and neurological disorders: A systematic review and meta-analysis. Sci. Total Environ. 2019, 655, 1240–1248. [Google Scholar] [CrossRef]
- Kioumourtzoglou, M.-A.; Wang, Y.; Dominici, F.; Schwartz, J.; Zanobetti, A. Long-Term PM2.5 Exposure and Neurological Hospital Admissions in the Northeastern United States. Environ. Health Perspect. 2016, 124, 23–29. [Google Scholar] [CrossRef]
- Hu, C.Y.; Fang, Y.; Li, F.L.; Dong, B.; Hua, X.G.; Jiang, W.; Zhang, H.; Lyu, Y.; Zhang, X.J. Association between ambient air pollution and Parkinson’s disease: Systematic review and meta-analysis. Environ. Res. 2019, 168, 448–459. [Google Scholar] [CrossRef]
- Yuchi, W.; Sbihi, H.; Davies, H.; Tamburic, L.; Brauer, M. Road proximity, air pollution, noise, green space and neurologic disease incidence: A population-based cohort study. Environ. Health 2020, 19, 8. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.; Burnett, R.T.; Kwong, J.C.; Hystad, P.; van Donkelaar, A.; Brook, J.R.; Copes, R.; Tu, K.; Goldberg, M.S.; Villeneuve, P.J.; et al. Effects of ambient air pollution on incident Parkinson’s disease in Ontario, 2001 to 2013: A population-based cohort study. Int. J. Epidemiol. 2018, 47, 2038–2048. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-Y.; Hung, H.-J.; Chang, K.-H.; Hsu, C.Y.; Muo, C.-H.; Tsai, C.-H.; Wu, T.-N. Long-term exposure to air pollution and the incidence of Parkinson’s disease: A nested case-control study. PLoS ONE 2017, 12, e0182834. [Google Scholar] [CrossRef]
- Liu, R.; Young, M.T.; Chen, J.-C.; Kaufman, J.D.; Chen, H. Ambient Air Pollution Exposures and Risk of Parkinson Disease. Environ. Health Perspect. 2016, 124, 1759–1765. [Google Scholar] [CrossRef] [PubMed]
- Bellou, V.; Belbasis, L.; Tzoulaki, I.; Evangelou, E.; Ioannidis, J.P. Environmental risk factors and Parkinson’s disease: An umbrella review of meta-analyses. Parkinsonism Relat. Disord. 2016, 23, 1–9. [Google Scholar] [CrossRef]
- Palacios, N.; Fitzgerald, K.; Hart, J.E.; Weisskopf, M.; Schwarzschild, M.A.; Ascherio, A.; Laden, F. Air Pollution and Risk of Parkinson’s Disease in a Large Prospective Study of Men. Environ. Health Perspect. 2017, 125, 087011. [Google Scholar] [CrossRef]
- Palacios, N.; Fitzgerald, K.C.; Hart, J.E.; Weisskopf, M.G.; A Schwarzschild, M.; Ascherio, A.; Laden, F. Particulate matter and risk of Parkinson disease in a large prospective study of women. Environ. Health 2014, 13, 80. [Google Scholar] [CrossRef]
- Toro, R.; Downward, G.S.; van der Mark, M.; Brouwer, M.; Huss, A.; Peters, S.; Hoek, G.; Nijssen, P.; Mulleners, W.M.; Sas, A.; et al. Parkinson’s disease and long-term exposure to outdoor air pollution: A matched case-control study in the Netherlands. Environ. Int. 2019, 129, 28–34. [Google Scholar] [CrossRef]
- Lee, P.C.; Raaschou-Nielsen, O.; Lill, C.M.; Bertram, L.; Sinsheimer, J.S.; Hansen, J.; Ritz, B. Gene-environment interactions linking air pollution and inflammation in Parkinson’s disease. Environ. Res. 2016, 151, 713–720. [Google Scholar] [CrossRef]
- Ritz, B.; Lee, P.-C.; Hansen, J.; Lassen, C.F.; Ketzel, M.; Sørensen, M.; Raaschou-Nielsen, O. Traffic-Related Air Pollution and Parkinson’s Disease in Denmark: A Case–Control Study. Environ. Health Perspect. 2016, 124, 351–356. [Google Scholar] [CrossRef]
- Lee, P.C.; Liu, L.L.; Sun, Y.; Chen, Y.A.; Liu, C.C.; Li, C.Y.; Yu, H.L.; Ritz, B. Traffic-related air pollution increased the risk of Parkinson’s disease in Taiwan: A nationwide study. Environ. Int. 2016, 96, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Myung, W.; Kim, D.K.; Kim, S.E.; Kim, C.T.; Kim, H. Short-term air pollution exposure aggravates Parkinson’s disease in a population-based cohort. Sci. Rep. 2017, 7, 44741. [Google Scholar] [CrossRef] [PubMed]
- Fleury, V.; Himsl, R.; Joost, S.; Nicastro, N.; Bereau, M.; Guessous, I.; Burkhard, P.R. Geospatial analysis of individual-based Parkinson’s disease data supports a link with air pollution: A case-control study. Parkinsonism Relat. Disord. 2021, 83, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Gunnarsson, L.-G.; Bodin, L. Occupational Exposures and Neurodegenerative Diseases—A Systematic Literature Review and Meta-Analyses. Int. J. Environ. Res. Public Health 2019, 16, 337. [Google Scholar] [CrossRef] [PubMed]
- van der Mark, M.; Brouwer, M.; Kromhout, H.; Nijssen, P.; Huss, A.; Vermeulen, R. Is pesticide use related to Parkinson disease? Some clues to heterogeneity in study results. Environ. Health Perspect. 2012, 120, 340–347. [Google Scholar] [CrossRef]
- Yan, D.; Zhang, Y.; Liu, L.; Shi, N.; Yan, H. Pesticide exposure and risk of Parkinson’s disease: Dose-response meta-analysis of observational studies. Regul. Toxicol. Pharmacol. 2018, 96, 57–63. [Google Scholar] [CrossRef]
- Zhou, Z.; Zhou, R.; Zhang, Z.; Li, K. The Association Between Vitamin D Status, Vitamin D Supplementation, Sunlight Exposure, and Parkinson’s Disease: A Systematic Review and Meta-Analysis. Med. Sci. Monit. 2019, 25, 666–674. [Google Scholar] [CrossRef]
- Kravietz, A.; Kab, S.; Wald, L.; Dugravot, A.; Singh-Manoux, A.; Moisan, F.; Elbaz, A. Association of UV radiation with Parkinson disease incidence: A nationwide French ecologic study. Environ. Res. 2017, 154, 50–56. [Google Scholar] [CrossRef]
- Rowell, D.; Nghiem, S.; Ramagopalan, S.; Meier, U.-C. Seasonal temperature is associated with Parkinson’s disease prescriptions: An ecological study. Int. J. Biometeorol. 2017, 61, 2205–2211. [Google Scholar] [CrossRef]
- Linares, C.; Martinez-Martin, P.; Rodríguez-Blázquez, C.; Forjaz, M.J.; Carmona, R.; Díaz, J. Effect of heat waves on morbidity and mortality due to Parkinson’s disease in Madrid: A time-series analysis. Environ. Int. 2016, 89–90, 1–6. [Google Scholar] [CrossRef]
- Central Bureau of Statistics. Characterization and Classification of Geographical Units by the Socio-Economic Level of the Population. 2013. Available online: https://www.cbs.gov.il/en/publications/Pages/2017/Characterization-and-Classification-of-Geographical-Units-by-the-Socio-Economic-Level-of-the-Population-2013.aspx (accessed on 8 January 2023).
- Hwang, B.-F.; Jaakkola, J.J. Ozone and Other Air Pollutants and the Risk of Oral Clefts. Environ. Health Perspect. 2008, 116, 1411–1415. [Google Scholar] [CrossRef] [PubMed]
- Chillag-Talmor, O.; Giladi, N.; Linn, S.; Gurevich, T.; El-Ad, B.; Silverman, B.; Friedman, N.; Peretz, C. Estimation of Parkinson’s disease survival in Israeli men and women, using health maintenance organization pharmacy data in a unique approach. J. Neurol. 2013, 260, 62–70. [Google Scholar] [CrossRef] [PubMed]
- Chillag-Talmor, O.; Giladi, N.; Linn, S.; Gurevich, T.; El-Ad, B.; Silverman, B.; Friedman, N.; Peretz, C. Use of a refined drug tracer algorithm to estimate prevalence and incidence of Parkinson’s disease in a large israeli population. J. Parkinson’s Dis. 2011, 1, 35–47. [Google Scholar] [CrossRef] [PubMed]
- Gasparrini, A. Modeling exposure-lag-response associations with distributed lag non-linear models. Stat. Med. 2014, 33, 881–899. [Google Scholar] [CrossRef] [PubMed]
- Gasparrini, A. Distributed Lag Linear and Non-Linear Models in R: The Package dlnm. J. Stat. Softw. 2011, 43, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Wei, F.; Zhang, X.; Wu, M.; Lin, H.; Shui, L.; Jin, M.; Wang, J.; Tang, M.; Chen, K. Air pollution, surrounding green, road proximity and Parkinson’s disease: A prospective cohort study. Environ. Res. 2021, 197, 111170. [Google Scholar] [CrossRef]
- Zhao, N.; Pinault, L.; Toyib, O.; Vanos, J.; Tjepkema, M.; Cakmak, S. Long-term ozone exposure and mortality from neurological diseases in Canada. Environ. Int. 2021, 157, 106817. [Google Scholar] [CrossRef]
- Kirrane, E.F.; Bowman, C.; Davis, J.A.; Hoppin, J.A.; Blair, A.; Chen, H.; Patel, M.M.; Sandler, D.P.; Tanner, C.M.; Vinikoor-Imler, L.; et al. Associations of Ozone and PM2.5 Concentrations with Parkinson’s Disease Among Participants in the Agricultural Health Study. J. Occup. Environ. Med. 2015, 57, 509–517. [Google Scholar] [CrossRef]
- Jo, S.; Kim, Y.-J.; Park, K.W.; Hwang, Y.S.; Lee, S.H.; Kim, B.J.; Chung, S.J. Association of NO2 and Other Air Pollution Exposures with the Risk of Parkinson Disease. JAMA Neurol. 2021, 78, 800–808. [Google Scholar] [CrossRef]
- CBS. Central Bureau of Statistics, Israel. Available online: https://www.cbs.gov.il/en/Pages/default.aspx (accessed on 9 January 2023).
- Hajat, A.; Hsia, C.; O’Neill, M.S. Socioeconomic Disparities and Air Pollution Exposure: A Global Review. Curr. Environ. Health Rep. 2015, 2, 440–450. [Google Scholar] [CrossRef]
- Murray, S. Poverty and health. CMAJ 2006, 174, 923. [Google Scholar] [CrossRef] [PubMed]
- Landau, D.; Novack, L.; Yitshak-Sade, M.; Sarov, B.; Kloog, I.; Hershkovitz, R.; Grotto, I.; Karakis, I. Nitrogen Dioxide pollution and hazardous household environment: What impacts more congenital mal-formations. Chemosphere 2015, 139, 340–348. [Google Scholar] [CrossRef] [PubMed]

| Subjects’ Characteristics | Parkinson’s Disease Cases (N = 3343) | Controls 2 (N = 31,324) | p-Value |
|---|---|---|---|
| Case definition, % (n/N) Diagnosis alone Medication alone Diagnosis and medication Level of certainty in Parkinson’s disease 1, % (n/N) Possible Probable Definite | 24.7 (826/3343) 41.6 (1390/3343) 33.7 (1127/3343) 20.1 (673/3343) 4.9 (164/3343) 75.0 (2506/3343) | ---- ---- | |
| Years of follow-up prior to PD onset year Mean ± SD (n) Median Min; Max | 8.7 ± 5.7 (3343) 8.0 1.0; 21.0 | 8.9 ± 5.7 (31,324) 8.0 1.0; 21.0 | 0.326 |
| Demographical characteristics | |||
| Male, % (n/N) | 55.8 (1865/3343) | 55.6 (19,442/31,324) | ---- |
| Age at diagnosis of the case, years Mean ± SD (n) Median Min; Max | 73.7 ± 10.2 (3343) 75.4 21.3; 108.7 | 73.7 ± 10.0 (31,324) 75.3 20.9; 108.9 | ---- |
| Bedouin, % (n/N) | 4.7 (157/3343) | 4.2 (1318/31,324) | ---- |
| Immigration status, % (n/N) Immigrated before 1989, % (n/N) Immigrated in 1989 or later, % (n/N) | 64.1 (2143/3343) 26.0 (870/3343) | 61.9 (19,376/31,324) 24.6 (7703/31,324) | <0.001 |
| Socioeconomic Status (0–10) Mean ± SD (n) Median Min; Max Grouped, % (n/N) 0–3 4–5 6+ | 4.9 ± 1.7 (3343) 5.0 0; 10.0 16.4 (548/3343) 53.0 (1772/3343) 30.6 (1023/3343) | 4.7 ± 1.7 (31,324) 4.0 0; 9.0 28.3 (8873/31,324) 40.5 (12,680/31,324) 31.2 (9771/31,324) | <0.001 <0.001 |
| Medical history prior to PD diagnosis of the case, % (n/N) | |||
| Myocardial Infarction Congestive Heart Failure Peripheral Vascular Disease Cerebrovascular Disease, % (n/N) Dementia Chronic Pulmonary Disease Rheumatologic Disease Peptic Ulcer Disease Liver Disease Diabetes Mellitus Hemiplegia or Paraplegia Renal Disease Cancer | 13.2 (440/3343) 7.1 (238/3343) 7.8 (261/3343) 22.5 (753/3343) 3.7 (124/3343) 11.0 (368/3343) 1.9 (62/3343) 5.5 (185/3343) 5.5 (185/3343) 24.5 (819/3343) 5.4 (181/3343) 13.9 (465/3343) 9.7 (324/3343) | 12.8 (3997/31,324) 8.4 (2635/31,324) 7.0 (2201/31,324) 13.6 (4254/31,324) 1.8 (568/31,324) 12.2 (3816/31,324) 1.2 (381/31,324) 5.0 (1566/31,324) 5.4 (1690/31,324) 18.6 (5828/31,324) 3.9 (1208/31,324) 11.6 (3626/31,324) 13.2 (4130/31,324) | 0.335 0.008 0.025 <0.001 <0.001 0.011 <0.001 0.044 0.119 <0.001 <0.001 <0.001 <0.001 |
| Ambient Annual Levels During the Year of PD Onset | Parkinson’s Disease Cases (N = 3343) | Controls (N = 31,324) | Q1 1 | Q2 1 | Q3 1 | IQR 1 |
|---|---|---|---|---|---|---|
| NO2, ppb Mean ± SD (n) Median Min; Max | 9.0 ± 1.8 (3308) 9.5 3.8; 16.0 | 8.9 ± 1.9 (31,059) 9.2 1.1; 17.0 | 7.6 | 9.3 | 10.1 | 2.5 |
| O3, ppm Mean ± SD (n) Median Min; Max | 33.1 ± 4.7 (3233) 32.7 26.3; 82.5 | 32.6 ± 4.5 (30,221) 31.9 26.3; 82.5 | 29.5 | 32.1 | 35.5 | 6 |
| PM10, µg/m3 Mean ± SD (n) Median Min; Max | 39.7 ± 2.2 (3036) 39.8 33.4; 46.3 | 39.8 ± 2.4 (28,296) 39.8 33.4; 46.3 | 38.2 | 39.8 | 40.5 | 2.3 |
| PM2.5 levels, µg/m3 Mean ± SD (n) Median Min; Max | 19.6 ± 3.3 (1879) 19.9 12.1; 26.8 | 19.3 ± 3.4 (19,748) 19.8 12.1; 26.8 | 18.2 | 19.8 | 21.6 | 3.4 |
| SO2, ppm Mean ± SD (n) Median Min; Max | 2.4 ± 1.2 (3279) 2.1 0.3; 11.5 | 2.4 ± 1.3 (30,777) 2.1 0.2; 11.5 | 1.7 | 2.1 | 2.8 | 1.1 |
| RH, % Mean ± SD (n) Median Min; Max | 68.1 ± 5.7 (2833) 71.1 22.0; 78.8 | 68.1 ± 5.7 (26,432) 69.9 22.0; 78.8 | 64.0 | 69.9 | 71.8 | 7.8 |
| Temperature, °C Mean ± SD (n) MedianMin; Max | 19.6 ± 1.4 (3050) 19.5 14.3; 24.9 | 19.5 ± 1.4 (28,636) 19.5 14.3; 24.8 | 19.3 | 19.5 | 20.0 | 0.7 |
| SR, W/m3 Mean ± SD (n) Median Min; Max | 32.9 ± 4.6 (3025) 32.9 25.1; 41.6 | 32.2 ± 4.7 (28,308) 31.1 23.6; 44.8 | 27.6 | 31.1 | 36.7 | 9.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karakis, I.; Yarza, S.; Zlotnik, Y.; Ifergane, G.; Kloog, I.; Grant-Sasson, K.; Novack, L. Contribution of Solar Radiation and Pollution to Parkinson’s Disease. Int. J. Environ. Res. Public Health 2023, 20, 2254. https://doi.org/10.3390/ijerph20032254
Karakis I, Yarza S, Zlotnik Y, Ifergane G, Kloog I, Grant-Sasson K, Novack L. Contribution of Solar Radiation and Pollution to Parkinson’s Disease. International Journal of Environmental Research and Public Health. 2023; 20(3):2254. https://doi.org/10.3390/ijerph20032254
Chicago/Turabian StyleKarakis, Isabella, Shaked Yarza, Yair Zlotnik, Gal Ifergane, Itai Kloog, Kineret Grant-Sasson, and Lena Novack. 2023. "Contribution of Solar Radiation and Pollution to Parkinson’s Disease" International Journal of Environmental Research and Public Health 20, no. 3: 2254. https://doi.org/10.3390/ijerph20032254
APA StyleKarakis, I., Yarza, S., Zlotnik, Y., Ifergane, G., Kloog, I., Grant-Sasson, K., & Novack, L. (2023). Contribution of Solar Radiation and Pollution to Parkinson’s Disease. International Journal of Environmental Research and Public Health, 20(3), 2254. https://doi.org/10.3390/ijerph20032254







