Efficient Removal of Heavy Metals from Contaminated Sunflower Straw by an Acid-Assisted Hydrothermal Process
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Sunflower Straw
2.2. Hydrothermal Conversion of Sunflower Straw
2.3. Analytical Methods
2.4. Hydrochar Yield Calculation and Characterization
3. Results
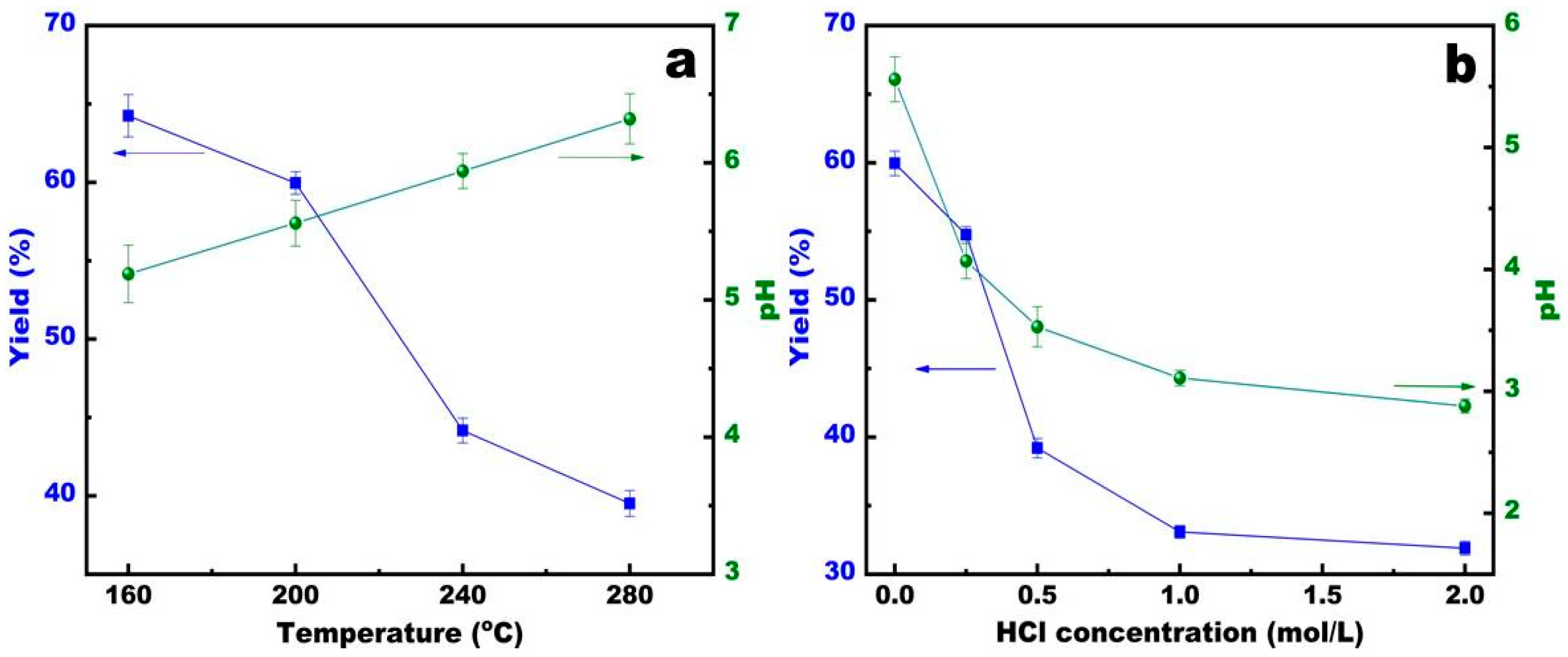
3.1. The Hydrochar Yield and pH
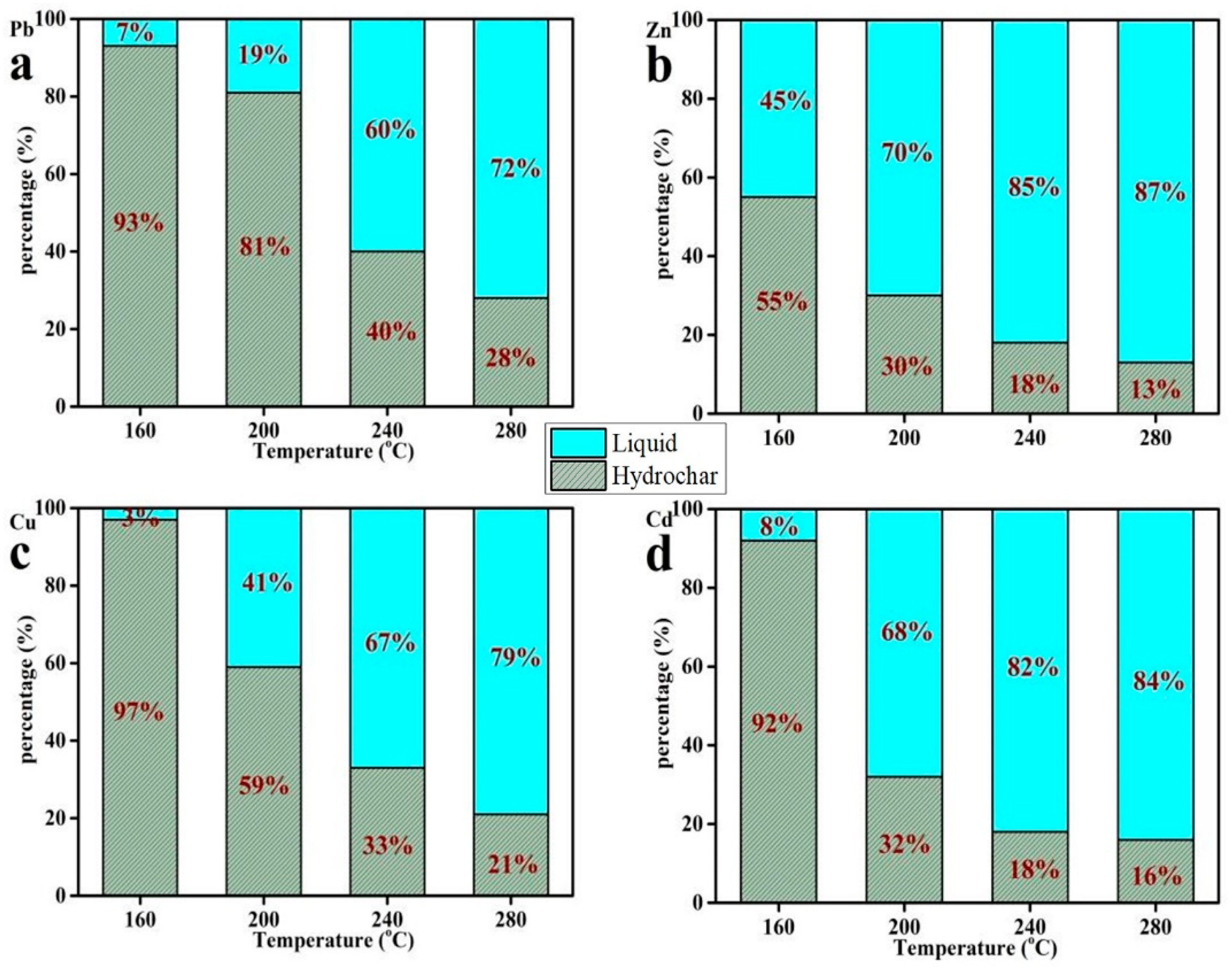
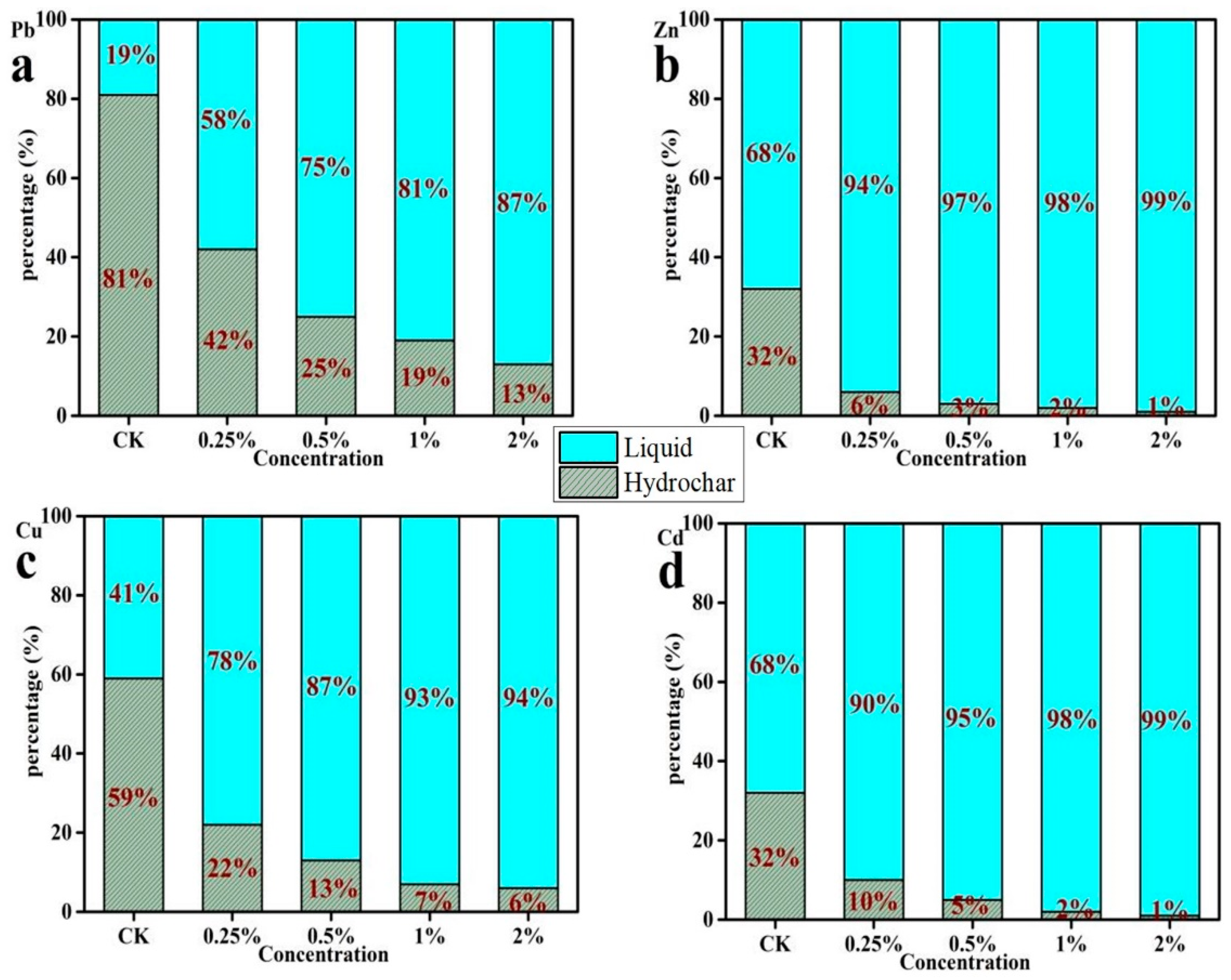
3.2. The Content of Heavy Metals in Hydrochar
3.3. The Content of Heavy Metals in Liquid Phase Products
3.4. The Distribution of Heavy Metals between Hydrochar and Liquid Products during the Hydrothermal Process
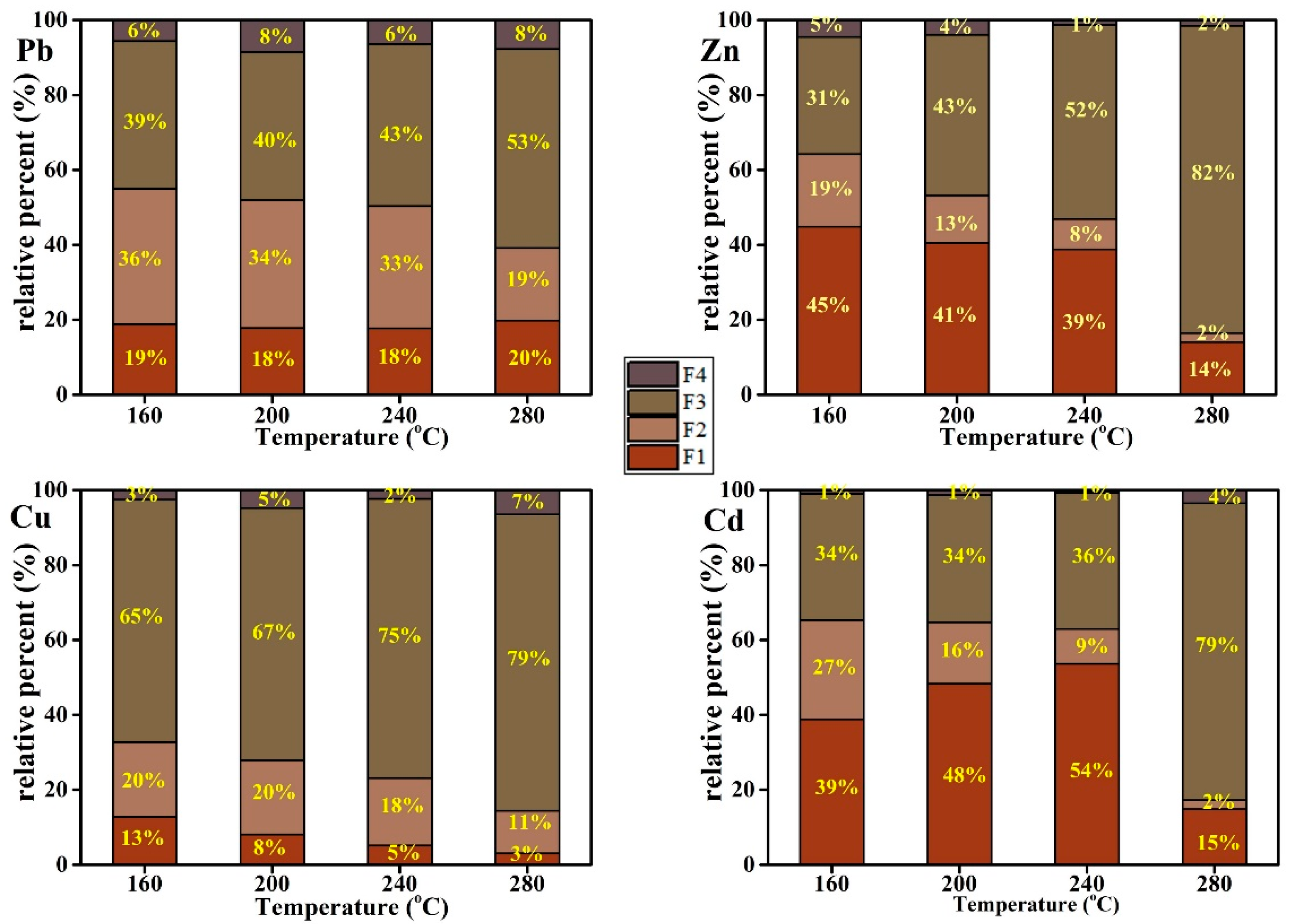
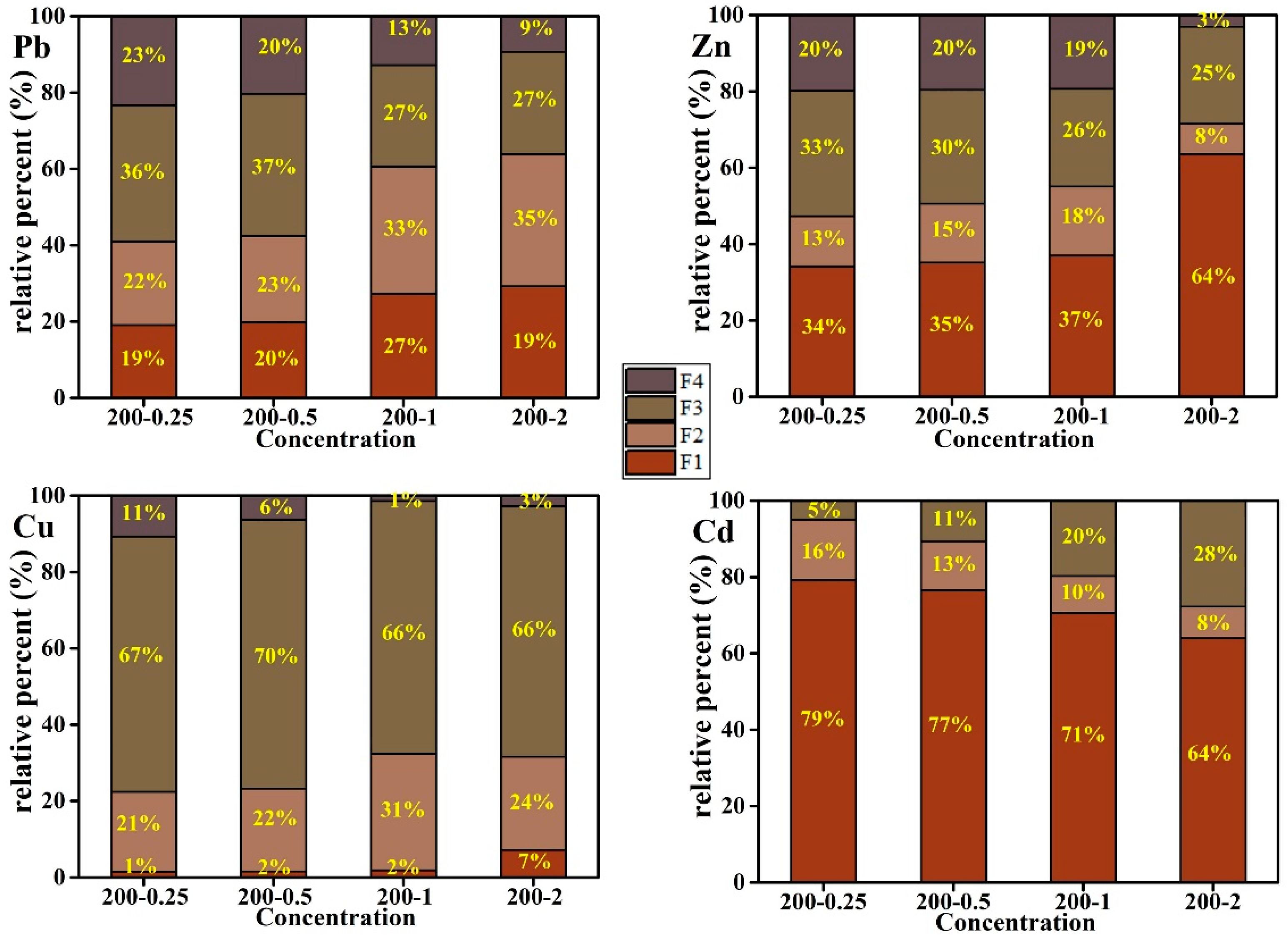
3.5. The Species of Heavy Metals in Hydrochar
3.6. The Surface Structure Characteristics of Hydrochars
4. Discussion
4.1. The Effect of Temperature and HCl Addition on Hydrochar Yield and pH
4.2. The Effect of Temperature and HCl Addition on Heavy Metal Distribution and Species
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, X.; Zhang, J.; Gong, Y.; Liu, Q.; Hou, H. Status of copper accumulation in agricultural soils across China (1985–2016). Chemosphere 2019, 244, 125516. [Google Scholar] [CrossRef] [PubMed]
- Wan, M.; Qu, M.; Hu, W.; Li, W.; Huang, B. Estimation of soil pH using PXRF spectrometry and Vis-NIR spectroscopy for rapid environmental risk assessment of soil heavy metals. Process. Saf. Environ. Prot. 2019, 132, 73–81. [Google Scholar] [CrossRef]
- Wu, W.; Wu, P.; Yang, F.; Sun, D.L.; Zhang, D.X.; Zhou, Y.K. Assessment of heavy metal pollution and human health risks in urban soils around an electronics manufacturing facility. Sci. Total. Environ. 2018, 630, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Zhai, X.; Li, Z.; Huang, B.; Luo, N.; Huang, M.; Zhang, Q.; Zeng, G. Remediation of multiple heavy metal-contaminated soil through the combination of soil washing and in situ immobilization. Sci. Total. Environ. 2018, 635, 92–99. [Google Scholar] [CrossRef]
- Song, H.; Kuang, X.; Wei, X.; Luo, S.; Zeng, Q.; Peng, L. The effect of TiO2 nanoparticles size on Cd (II) removal by the paddy crusts from waterbody. J. Environ. Chem. Eng. 2022, 10, 107883. [Google Scholar] [CrossRef]
- Lin, Y.F.; Aarts, M. The molecular mechanism of zinc and cadmium stress response in plants. Cell. Mol. Life Sci. 2012, 69, 3187–3206. [Google Scholar] [CrossRef]
- Ronzan, M.; Piacentini, D.; Fattorini, L.; Rovere, F.D.; Eiche, E.; Riemann, M.; Altamura, M.M.; Falasca, G. Cadmium and arsenic affect root development in Oryza sativa L. negatively interacting with auxin. Environ. Exp. Bot. 2018, 151, 64–75. [Google Scholar] [CrossRef]
- Rizwan, M.; Ali, S.; Rizvi, H.; Rinklebe, J.; Ishaque, W. Phytomanagement of heavy metals in contaminated soils using sunflower: A review. CRC Crit. Rev. Environ. Control 2016, 46, 1498–1528. [Google Scholar] [CrossRef]
- Yang, Y.; Zhou, X.; Tie, B.; Peng, L.; Li, H.; Wang, K.; Zeng, Q. Accepted manuscript comparison of three types of oil crop rotation systems for effective use and remediation of heavy metal contaminated agricultural soil. Chemosphere 2017, 188, 148–156. [Google Scholar] [CrossRef]
- Yuan, J.; Yang, Y.; Zhou, X.-H.; Ge, Y.-C.; Zeng, Q.-R. A new method for simultaneous removal of heavy metals and harmful organics from rape seed meal from metal-contaminated farmland. Sep. Purif. Technol. 2019, 210, 1001–1007. [Google Scholar] [CrossRef]
- Gong, X.; Huang, D.; Liu, Y.; Zeng, G.; Wang, R.; Wei, J.; Huang, C.; Xu, P.; Wan, J.; Zhang, C. Pyrolysis and reutilization of plant residues after phytoremediation of heavy metals contaminated sediments: For heavy metals stabilization and dye adsorption. Bioresour. Technol. 2018, 253, 64–71. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.L.; Wang, R.Z.; Liu, Y.G.; Zeng, G.M.; Huang, C. Application of molecularly imprinted polymers in wastewater treatment: A review. Environ. Ence. Pollut. Res. 2014, 22, 963–977. [Google Scholar] [CrossRef]
- Vocciante, M.; Caretta, A.; Bua, L.; Bagatin, R.; Franchi, E.; Petruzzelli, G.; Ferro, S. Enhancements in phytoremediation technology: Environmental assessment including different options of biomass disposal and comparison with a consolidated approach. J. Environ. Manag. 2019, 237, 560–568. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Tian, Z.; Cheng, H.; Zhou, H.; Wang, Y. Organic acid leaching was an efficient approach for detoxification of metal-containing plant incineration ash. Environ. Sci. Pollut. Res. 2021, 28, 32721–32732. [Google Scholar] [CrossRef]
- Yang, J.G. Heavy metal removal and crude bio-oil upgrading from Sedum plumbizincicola harvest using hydrothermal upgrading process. Bioresour. Technol. 2010, 101, 7653–7657. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.G.; Tang, C.B.; He, J.; Yang, S.H.; Tang, M.T. Heavy metal removal and crude bio-oil upgrade from Sedum alfredii Hance harvest using hydrothermal upgrading. J. Hazard. Mater. 2010, 179, 1037–1041. [Google Scholar] [CrossRef]
- Feng, Q.; Zhu, X.; Liu, Y.; Quan, S.; Wu, L.; Zhang, S.; Chen, J.; Ren, Z.J. Influences of Temperature and Metal on Subcritical Hydrothermal Liquefaction of Hyperaccumulator: Implications for the Recycling of Hazardous Hyperaccumulators. Environ. Sci. Technol. 2018, 52, 2225–2234. [Google Scholar]
- Lee, J.; Park, K.Y. Conversion of heavy metal-containing biowaste from phytoremediation site to value-added solid fuel through hydrothermal carbonization. Environ. Pollut. 2021, 269, 116127. [Google Scholar] [CrossRef] [PubMed]
- Bian, R.; Li, L.; Shi, W.; Ma, B.; Joseph, S.; Li, L.; Liu, X.; Zheng, J.; Zhang, X.; Cheng, K. Pyrolysis of contaminated wheat straw to stabilize toxic metals in biochar but recycle the extract for agricultural use. Biomass Bioenergy 2018, 118, 32–39. [Google Scholar] [CrossRef]
- Zeng, X.; Xiao, Z.; Zhang, G.; Wang, A.; Li, Z.; Liu, Y.; Wang, H.; Zeng, Q.; Liang, Y.; Zou, D. Speciation and bioavailability of heavy metals in pyrolytic biochar of swine and goat manures. J. Anal. Appl. Pyrolysis 2018, 132, 82–93. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, Y.; Wang, X.; Wu, W.; Cui, X.; Cheng, Z.; Yan, B.; Yang, X.; He, Z.; Chen, G. Hydrothermal conversion of Cd/Zn hyperaccumulator (Sedum alfredii) for heavy metal separation and hydrochar production. J. Hazard. Mater. 2022, 423, 127122. [Google Scholar] [CrossRef]
- Monthioux, M.; Landais, P. Natural and artificial maturations of a coal series: Infrared spectrometry study. Energy Fuels 1988, 2, 794–801. [Google Scholar] [CrossRef]
- Parshetti, G.K.; Hoekman, S.K.; Balasubramanian, R. Chemical, structural and combustion characteristics of carbonaceous products obtained by hydrothermal carbonization of palm empty fruit bunches. Bioresour. Technol. 2013, 135, 683–689. [Google Scholar] [CrossRef] [PubMed]
- Sevilla, M.; Maciá-Agulló, J.; Fuertes, A.B. Hydrothermal carbonization of biomass as a route for the sequestration of CO2: Chemical and structural properties of the carbonized products. Biomass Bioenergy 2011, 35, 3152–3159. [Google Scholar] [CrossRef]
- Chen, J.; Li, S. Characterization of biofuel production from hydrothermal treatment of hyperaccumulator waste (Pteris vittata L.) in sub- and supercritical water. RSC Adv. 2020, 10, 2160–2169. [Google Scholar] [CrossRef]
- Falco, C.; Baccile, N.; Titirici, M.M. Morphological and structural differences between glucose, cellulose and lignocellulosic biomass derived hydrothermal carbons. Green Chem. 2011, 13, 3273–3281. [Google Scholar] [CrossRef]
- Kang, S.; Li, X.; Fan, J.; Chang, J. Characterization of Hydrochars Produced by Hydrothermal Carbonization of Lignin, Cellulose, d-Xylose, and Wood Meal. Ind. Eng. Chem. Res. 2012, 51, 9023–9031. [Google Scholar] [CrossRef]
- Zhang, Y.; Jiang, Q.; Xie, W.; Wang, Y.; Kang, J. Effects of temperature, time and acidity of hydrothermal carbonization on the hydrochar properties and nitrogen recovery from corn stover. Biomass Bioenergy 2019, 122, 175–182. [Google Scholar] [CrossRef]
- Wang, Q.; Hong, L.; Chen, L.; Huang, X. Monodispersed hard carbon spherules with uniform nanopores. Carbon 2001, 39, 2211–2214. [Google Scholar] [CrossRef]
- Fei, Y.H.; Zhao, D.; Liu, Y.; Zhang, W.; Liu, C. Feasibility of sewage sludge derived hydrochars for agricultural application: Nutrients (N, P, K) and potentially toxic elements (Zn, Cu, Pb, Ni, Cd). Chemosphere 2019, 236, 124841. [Google Scholar] [CrossRef]
- Abelleira, J.; Pérez-Elvira, S.; Sánchez-Oneto, J.; Portela, J.R.; Nebot, E. Advanced Thermal Hydrolysis of secondary sewage sludge: A novel process combining thermal hydrolysis and hydrogen peroxide addition. Resour. Conserv. Recycl. 2012, 59, 52–57. [Google Scholar] [CrossRef]
- Lu, X.; Flora, J.R.; Berge, N.D. Influence of process water quality on hydrothermal carbonization of cellulose. Bioresour. Technol. 2014, 154, 229–239. [Google Scholar] [CrossRef] [PubMed]
- Reza, M.T.; Rottler, E.; Herklotz, L.; Wirth, B. Hydrothermal carbonization (HTC) of wheat straw: Influence of feedwater pH prepared by acetic acid and potassium hydroxide. Bioresour. Technol. 2015, 182, 336–344. [Google Scholar] [CrossRef] [PubMed]
- Novak, J.M.; Busscher, W.J.; Laird, D.L.; Ahmedna, M.; Watts, D.W.; Niandou, M. Impact of Biochar Amendment on Fertility of a Southeastern Coastal Plain Soil. Soil Sci. 2009, 174, 105–112. [Google Scholar] [CrossRef]
- Cui, X.; Shen, Y.; Yang, Q.; Kawi, S.; He, Z.; Yang, X.; Wang, C.H. Simultaneous syngas and biochar production during heavy metal separation from Cd/Zn hyperaccumulator (Sedum alfredii) by gasification. Chem. Eng. J. 2018, 347, 543–551. [Google Scholar] [CrossRef]
- Zhong, D.; Zhong, Z.; Wu, L.; Xue, H.; Song, Z.; Luo, Y. Thermal Characteristics of Hyperaccumulator and Fate of Heavy Metals during Thermal Treatment of Sedum plumbizincicola. Int. J. Phytoremediation 2015, 17, 766–776. [Google Scholar] [CrossRef]
- Luan, H.; Liu, F.; Long, S.; Liu, Z.; Qi, Y.; Xiao, Z.; Fang, J. The migration, transformation, and risk assessment of heavy metals in residue and bio-oil obtained by the liquefaction of pig manure. Environ. Sci. Pollut. Res. 2021, 28, 15055–15069. [Google Scholar] [CrossRef]
- Wu, Q.; Wang, H.; Zheng, X.; Liu, F.; Xiao, Z. Thermochemical liquefaction of pig manure: Factors influencing on oil. Fuel 2020, 264, 116884. [Google Scholar] [CrossRef]
- Cui, X.; Zhang, J.; Wang, X.; Pan, M.; Lin, Q.; Khan, K.Y.; Yan, B.; Li, T.; He, Z.; Yang, X.; et al. A review on the thermal treatment of heavy metal hyperaccumulator: Fates of heavy metals and generation of products. J. Hazard. Mater. 2021, 405, 123832. [Google Scholar] [CrossRef]
- Lang, Q.; Chen, M.; Guo, Y.; Liu, Z.; Gai, C. Effect of hydrothermal carbonization on heavy metals in swine manure: Speciation, bioavailability and environmental risk. J. Environ. Manag. 2019, 234, 97–103. [Google Scholar] [CrossRef]
- Liu, M.; Duan, Y.; Bikane, K.; Zhao, L. The Migration and Transformation of Heavy Metals in Sewage Sludge during Hydrothermal Carbonization Combined with Combustion. BioMed Res. Int. 2018, 2018, 1913848. [Google Scholar] [CrossRef] [PubMed]
- Song, C.; Yuan, W.; Shan, S.; Ma, Q.; Wang, H. Changes of nutrients and potentially toxic elements during hydrothermal carbonization of pig manure. Chemosphere 2019, 243, 125331. [Google Scholar] [CrossRef] [PubMed]
- Udayanga, W.C.; Veksha, A.; Giannis, A.; Lisak, G.; Chang, W.C.; Lim, T.T. Fate and distribution of heavy metals during thermal processing of sewage sludge. Fuel 2018, 226, 721–744. [Google Scholar] [CrossRef]
- Zhai, Y.; Liu, X.; Zhu, Y.; Peng, C.; Wang, T.; Zhu, L.; Li, C.; Zeng, G. Hydrothermal carbonization of sewage sludge: The effect of feed-water pH on fate and risk of heavy metals in hydrochars. Bioresour. Technol. 2016, 218, 183–188. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Yao, W.; Li, R.; Ali, A.; Du, J.; Guo, D.; Xiao, R.; Guo, Z.; Zhang, Z.; Awasthi, M.K. Effect of pyrolysis temperature on chemical form, behavior and environmental risk of Zn, Pb and Cd in biochar produced from phytoremediation residue. Bioresour. Technol. 2018, 249, 487–493. [Google Scholar] [CrossRef] [PubMed]
- Devi, P.; Saroha, A.K. Risk analysis of pyrolyzed biochar made from paper mill effluent treatment plant sludge for bioavailability and eco-toxicity of heavy metals. Bioresour. Technol. 2014, 162, 308–315. [Google Scholar] [CrossRef]


| Sample | Concentration of Heavy Metals (mg·kg−1) | |||
|---|---|---|---|---|
| Pb | Zn | Cu | Cd | |
| Untreated | 5.98 ± 0.14 c | 487.48 ± 39.59 a | 14.59 ± 1.24 b | 4.48 ± 0.29 b |
| HTC160 | 8.58 ± 0.38 a | 364.80 ± 11.33 b | 23.63 ± 1.54 a | 6.25 ± 0.15 a |
| HTC200 | 7.30 ± 0.32 b | 220.97 ± 18.24 c | 13.84 ± 0.10 b | 2.37 ± 0.14 c |
| HTC240 | 4.88 ± 0.06 d | 182.32 ± 7.70 cd | 10.90 ± 0.06 c | 1.96 ± 0.06 d |
| HTC280 | 4.08 ± 0.12 e | 146.95 ± 1.46 d | 7.79 ± 0.11 d | 1.80 ± 0.02 d |
| HTC200-0.25 | 4.13 ± 0.06 b | 48.57 ± 1.64 b | 5.27 ± 0.16 b | 0.72 ± 0.03 b |
| HTC200-0.5 | 3.23 ± 0.08 c | 31.27 ± 0.68 bc | 4.57 ± 0.02 c | 0.57 ± 0.02 c |
| HTC200-1 | 2.88 ± 0.14 c | 27.32 ± 1.7 c | 3.03 ± 0.16 d | 0.24 ± 0.02 d |
| HTC200-2 | 2.23 ± 0.15 d | 19.40 ± 0.23 c | 2.63 ± 0.06 e | 0.13 ± 0.01 d |
| Samples | Metal Concentration (mg·L−1) | |||
|---|---|---|---|---|
| Pb | Zn | Cu | Cd | |
| HTC160 | 0.04 ± 0.01 e | 19.55 ± 0.58 f | 0.04 ± 0.001 g | 0.04 ± 0.004 g |
| HTC200 | 0.10 ± 0.01 e | 30.27 ± 0.14 e | 0.58 ± 0.03 f | 0.30 ± 0.01 f |
| HTC240 | 0.32 ± 0.01 d | 36.48 ± 0.91 d | 0.96 ± 0.01 e | 0.35 ± 0.01 e |
| HTC280 | 0.41 ± 0.01 b | 39.91 ± 0.52 c | 1.14 ± 0.01 dc | 0.37 ± 0.003 d |
| HTC200-0.25 | 0.31 ± 0.01 d | 41.65 ± 0.24 c | 1.00 ± 0.02 dc | 0.36 ± 0.004 d |
| HTC200-0.5 | 0.39 ± 0.01 c | 45.48 ± 0.91 b | 1.22 ± 0.05 b | 0.39 ± 0.003 c |
| HTC200-1 | 0.42 ± 0.01 b | 47.36 ± 0.18 a | 1.30 ± 0.02 ab | 0.42 ± 0.01 b |
| HTC200-2 | 0.47 ± 0.01 a | 48.09 ± 0.17 a | 1.35 ± 0.04 a | 0.44 ± 0.01 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, H.; Zhou, J.; He, S.; Ma, Q.; Peng, L.; Yin, M.; Lin, H.; Zeng, Q. Efficient Removal of Heavy Metals from Contaminated Sunflower Straw by an Acid-Assisted Hydrothermal Process. Int. J. Environ. Res. Public Health 2023, 20, 1311. https://doi.org/10.3390/ijerph20021311
Song H, Zhou J, He S, Ma Q, Peng L, Yin M, Lin H, Zeng Q. Efficient Removal of Heavy Metals from Contaminated Sunflower Straw by an Acid-Assisted Hydrothermal Process. International Journal of Environmental Research and Public Health. 2023; 20(2):1311. https://doi.org/10.3390/ijerph20021311
Chicago/Turabian StyleSong, Huijuan, Jun Zhou, Shilong He, Qiao Ma, Liang Peng, Miaogen Yin, Hui Lin, and Qingru Zeng. 2023. "Efficient Removal of Heavy Metals from Contaminated Sunflower Straw by an Acid-Assisted Hydrothermal Process" International Journal of Environmental Research and Public Health 20, no. 2: 1311. https://doi.org/10.3390/ijerph20021311
APA StyleSong, H., Zhou, J., He, S., Ma, Q., Peng, L., Yin, M., Lin, H., & Zeng, Q. (2023). Efficient Removal of Heavy Metals from Contaminated Sunflower Straw by an Acid-Assisted Hydrothermal Process. International Journal of Environmental Research and Public Health, 20(2), 1311. https://doi.org/10.3390/ijerph20021311










