Mn Pretreatment Improves the Physiological Resistance and Root Exudation of Celosia argentea Linn. to Cadmium Stress
Abstract
1. Introduction
2. Materials and Methods
2.1. Seedling Culture
2.2. Hydroponic Experiments
2.2.1. Hydroponic Experiment 1
2.2.2. Hydroponic Experiment 2
2.3. Chemical Analyses
2.4. Statistical Analysis
3. Results and Analysis
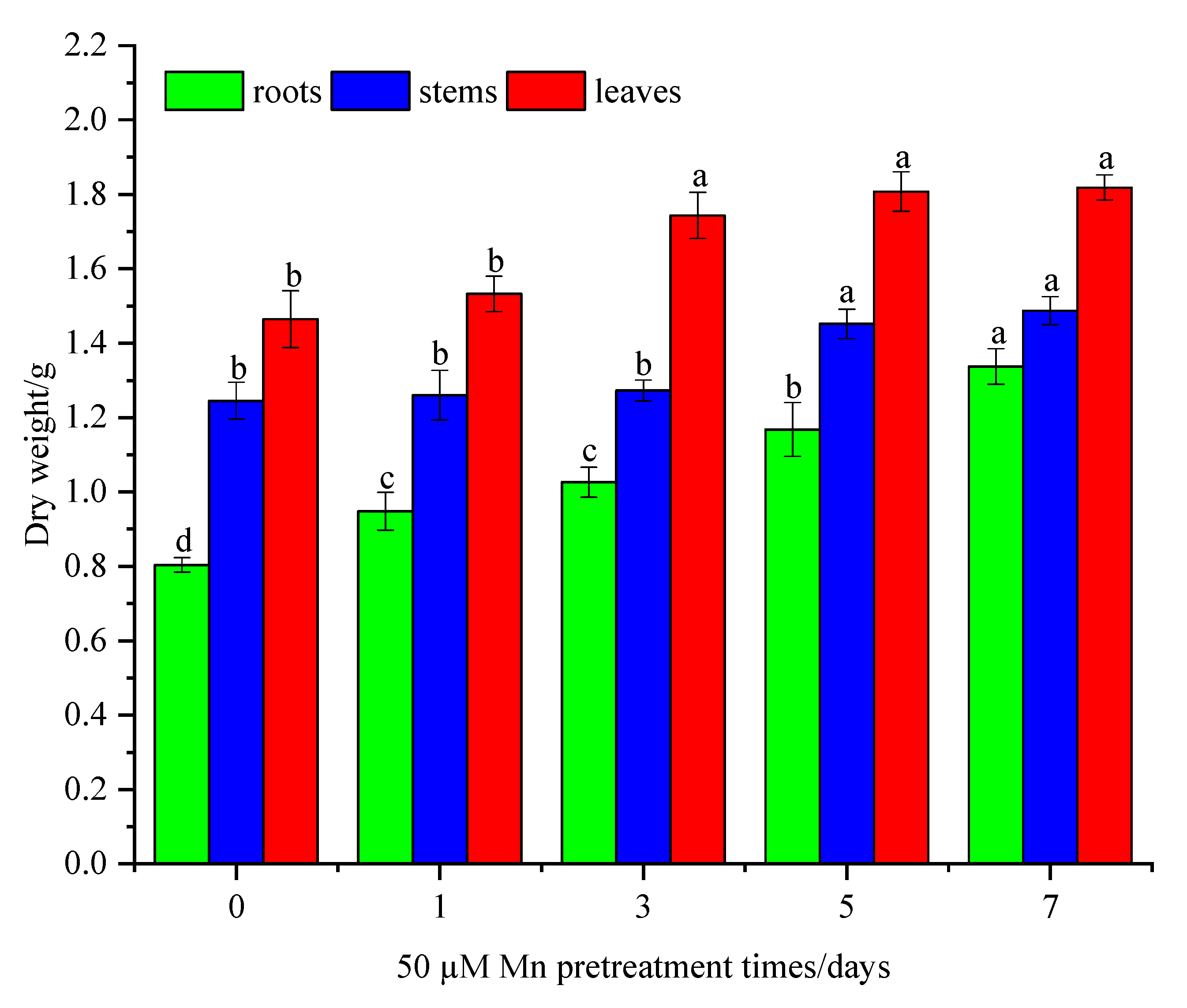
3.1. Mn Pretreatment Increased the Biomass of C. argentea
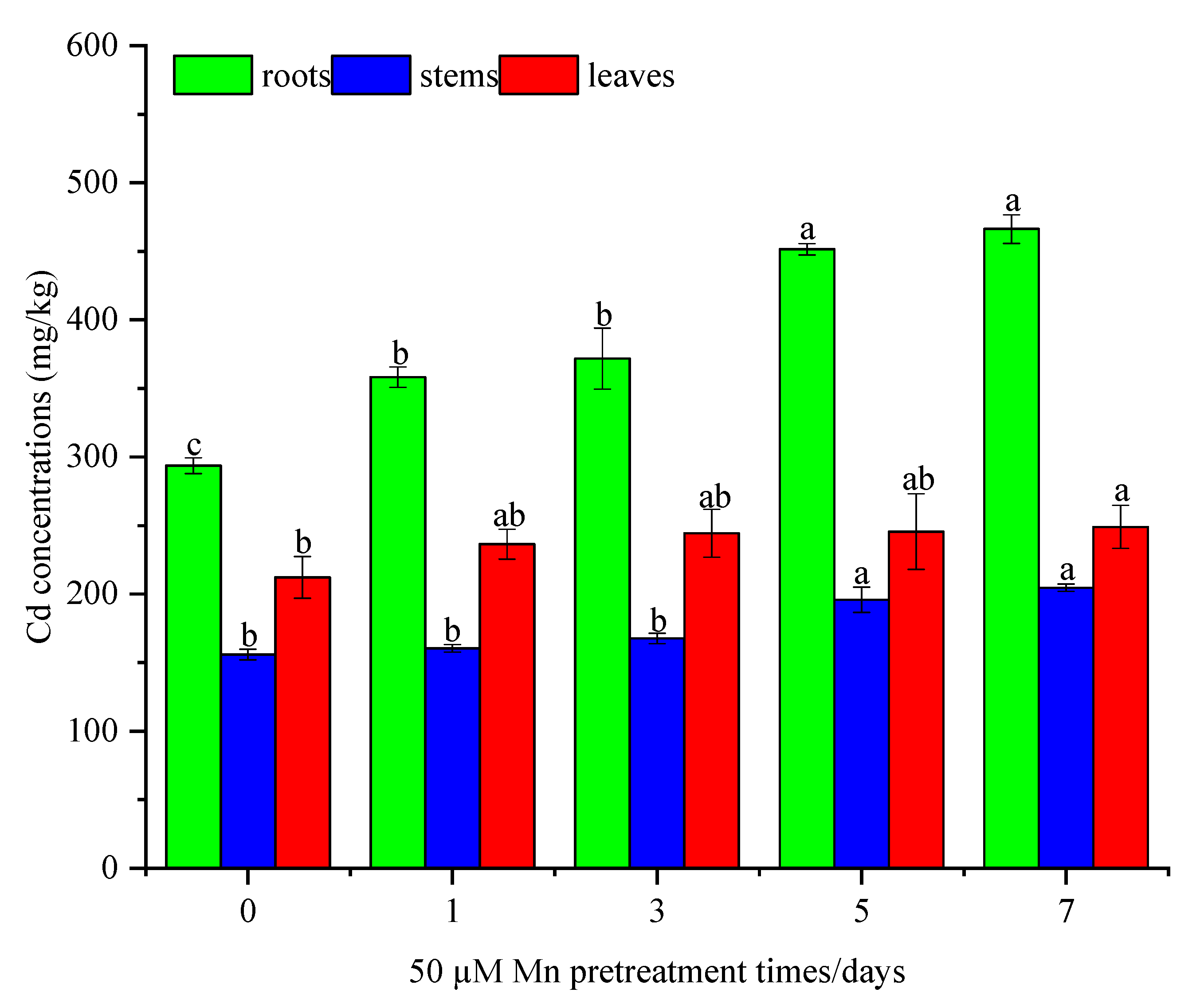
3.2. Mn Pretreatment Stimulated Cd Accumulation of C. argentea
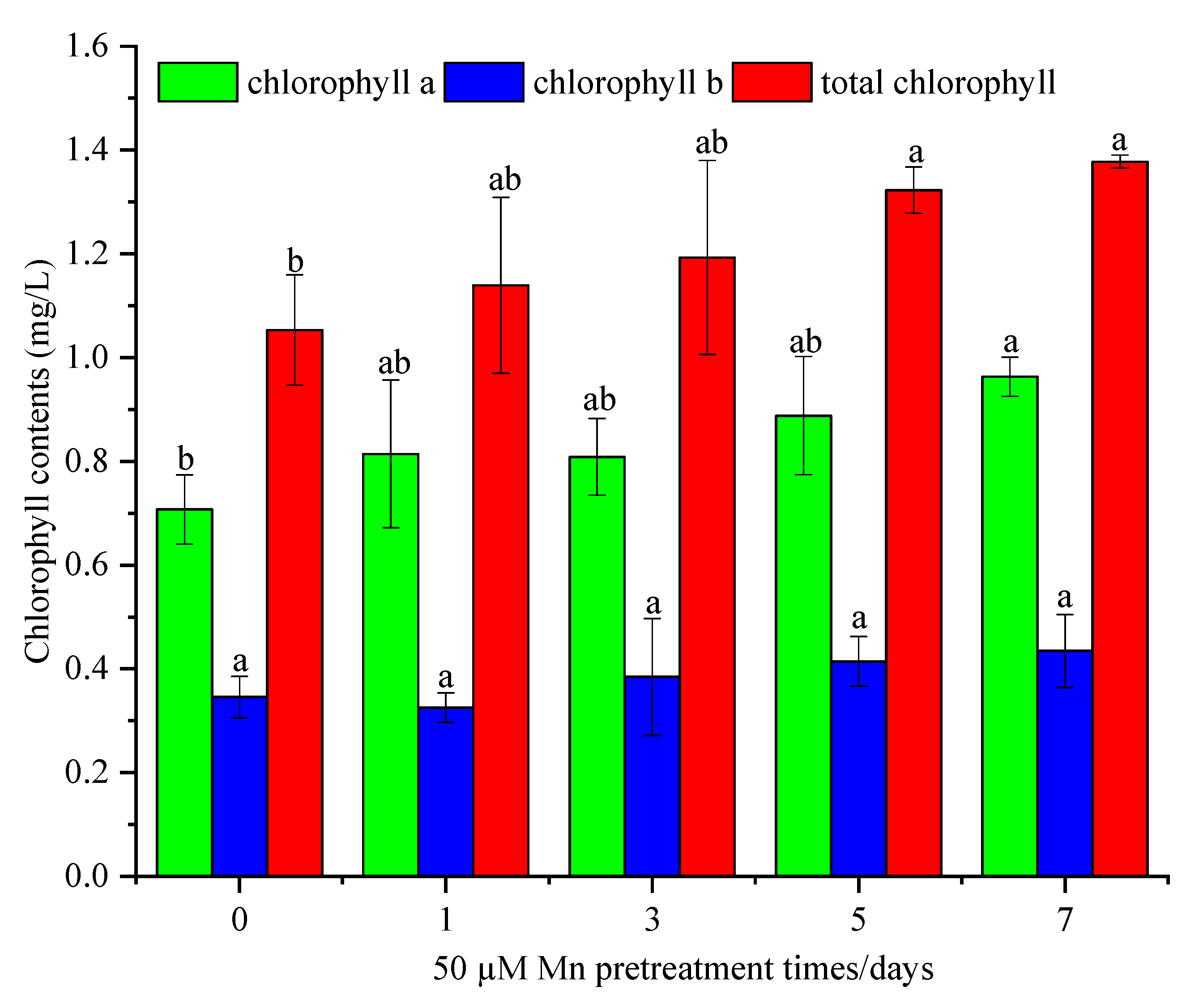
3.3. The Effects of Mn Pretreatment on the Chlorophyll Content of C. argentea under Cd Stress
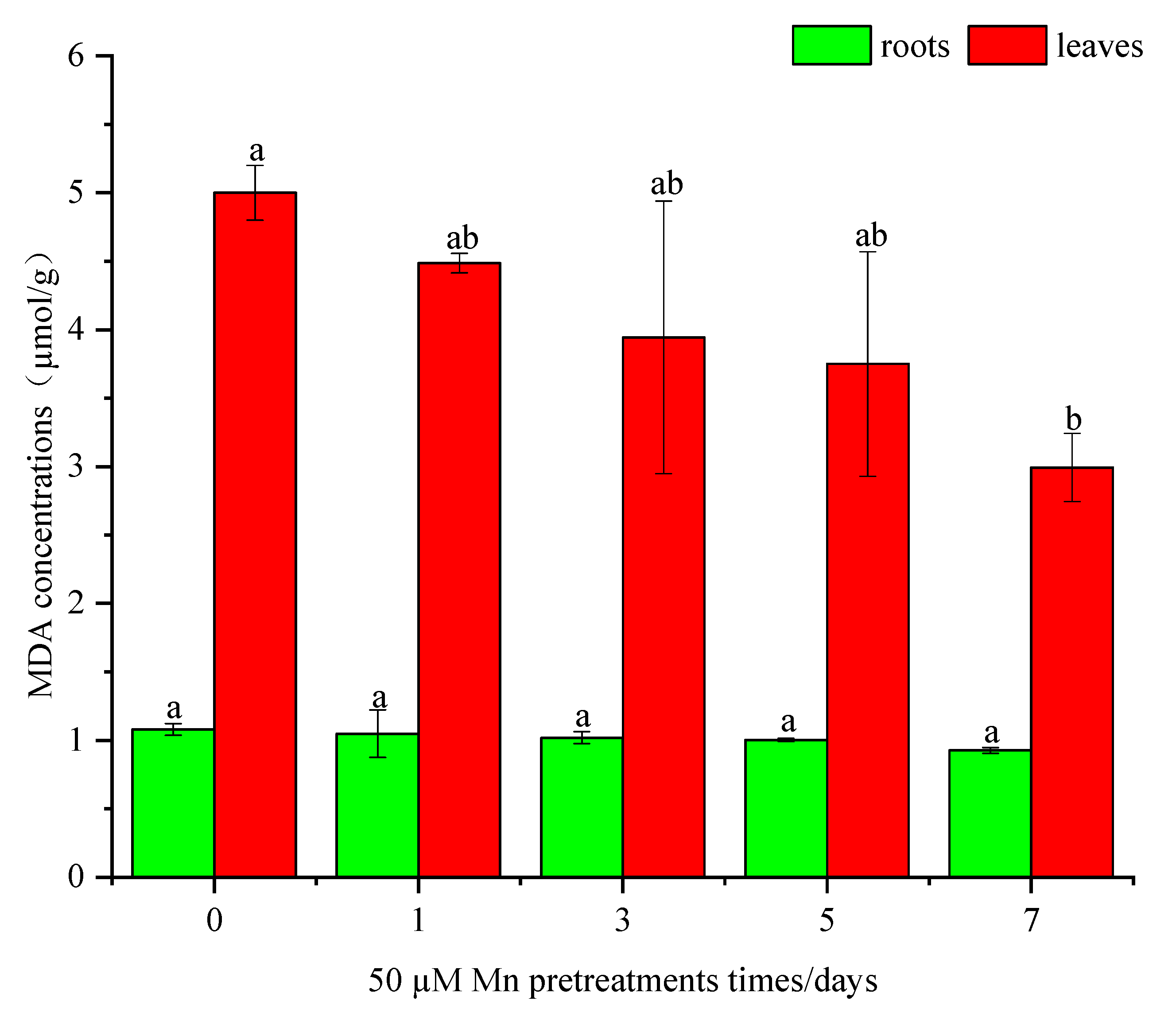
3.4. Mn Pretreatment Caused a Drop in Antioxidant Enzyme Performance and MDA Content in C. argentea under Cd Stress
3.5. Mn Pretreatment Increased Organic Acids in the Root Exudates of C. argentea under Cd Stress
| Organic Acids | Different Treatments | |
|---|---|---|
| 0 μM Mn (Control Group) | 50 μM Mn | |
| Oxalic acid | 0.37 ± 0.12 b | 0.49 ± 0.11 a |
| Tartaric acid | ND | ND |
| Malic acid | 2.58 ± 0.67 b | 3.26 ± 1.02 a |
| Lactic acid | 1.25 ± 0.35 ab | 1.74 ± 0.67 a |
| Acetic acid | ND | 2.97 ± 0.99 |
| Maleic acid | ND | 0.01 ± 0.002 |
| Citric acid | 2.17 ± 0.88 b | 3.37 ± 1.32 a |
| Fumaric acid | ND | 0.01 ± 0.001 |
4. Discussion
4.1. Mn Pretreatment Improves the Physiological Resistance of C. argentea, Thus Increasing Plant Biomass and Cd Bioaccumulation
4.2. Mn Promotes the Exudation of Organic Acids from Roots, Including the Matric and Citric Acids, Thereby Increasing Cd Bioaccumulation in Plants
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Oliveria, B.R.M.; Almeida, A.A.F.; Pirovani, C.P.; Barroso, J.P.; de CNeto, C.H.; Santos, N.A.; Ahnert, D.; Baligar, V.C.; Mangabeira, P.A.O. Mitigation of Cd toxicity by Mn in young plants of cacao, evaluated by the proteomic profiles of leaves and roots. Ecotoxicology 2020, 29, 340–358. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.-S.; Zhang, Z. Mechanisms of cadmium phytoremediation and detoxification in plants. Crop. J. 2021, 9, 521–529. [Google Scholar] [CrossRef]
- Wang, W.; Lu, T.; Liu, L.; Yang, X.; Sun, X.; Qiu, G.; Hua, D.; Zhou, D. Zeolite-supported manganese oxides decrease the Cd uptake of wheat plants in Cd-contaminated weakly alkaline arable soils. J. Hazard. Mater. 2021, 419, 126464. [Google Scholar] [CrossRef] [PubMed]
- Yu, G.; Jiang, P.; Fu, X.; Liu, J.; Sunahara, G.I.; Chen, Z.; Xiao, H.; Lin, F.; Wang, X. Phytoextraction of cadmium-contaminated soil by Celosia argentea Linn.: A long-term field study. Environ. Pollut. 2020, 166, 115408. [Google Scholar] [CrossRef]
- Wu, M.; Luo, Q.; Liu, S.; Zhao, Y.; Long, Y.; Pan, Y. Screening ornamental plants to identify potential Cd hyperaccumulators for bioremediation. Ecotoxicol. Environ. Saf. 2018, 162, 35–41. [Google Scholar] [CrossRef]
- Liu, J.; Mo, L.; Zhang, X.; Yao, S.; Wang, Y. Simultaneous hyperaccumulation of cadmium and manganese in Celosia argentea Linn. Int. J. Phytoremediation 2018, 20, 1106–1112. [Google Scholar] [CrossRef]
- Huang, Q.-N.; An, H.; Yang, Y.-J.; Liang, Y.; Shao, G.-S. Effects of Mn-Cd antagonistic interaction on Cd accumulation and major agronomic traits in rice genotypes by different Mn forms. Plant Growth Regul. 2017, 82, 317–331. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, Y.; Chai, T.; Tan, J.; Wang, J.; Feng, S.; Liu, G. Manganese-mitigation of cadmium toxicity to seedling growth of Phytolacca acinosa Roxb. is controlled by the manganese/cadmium molar ratio under hydroponic conditions. Plant Physiol. Biochem. 2013, 73, 144–153. [Google Scholar] [CrossRef]
- Liu, J.; Yu, G.; Jiang, P.; Zhang, X.; Meng, D.; Chen, Z.; Baker, A.J.M.; Qiu, R. Interaction of Mn and Cd during their uptake in Celosia argentea differs between hydroponic and soil systems. Plant Soil 2020, 450, 323–336. [Google Scholar] [CrossRef]
- Fu, J.Y. Effects of Zinc/Manganese on Cadmium Uptake, Transport and Physico-Biochemical Properties of Rice (Oryza sativa L.) under Cadmium Stress. Master’s Thesis, Taiyuan University of Technology, Taiyuan, China, 2019. [Google Scholar]
- Jiang, P.; Zheng, Y.; Liu, J.; Yu, G.; Lin, F. Pathways of cadmium fluxes in the root of the hyperaccumulator Celosia argentea Linn. Environ. Sci. Pollut. Res. 2022, 29, 44413–44421. [Google Scholar] [CrossRef]
- Yu, G.; Liu, J.; Long, Y.; Chen, Z.; Sunahara, G.I.; Jiang, P.; You, S.; Lin, H.; Xiao, H. Phytoextraction of cadmium-contaminated soils: Comparison of plant species and low mo-lecular weight organic acids. Int. J. Phytoremediation 2019, 22, 383–391. [Google Scholar] [CrossRef] [PubMed]
- Liu, A.; Wang, W.; Zheng, X.; Chen, X.; Fu, W.; Wang, G.; Ji, J.; Jin, C.; Guan, C. Improvement of the Cd and Zn phytoremediation efficiency of rice (Oryza sativa) through the inoculation of a metal-resistant PGPR strain. Chemosphere 2022, 302, 134900. [Google Scholar] [CrossRef] [PubMed]
- Shi, G.; Hu, J.; Ding, F.; Li, S.; Shi, W.; Chen, Y. Exogenous Pseudomonas aeruginosa application improved the phytoremediation efficiency of Lolium multiflorum Lam on Cu–Cd co-contaminated soil. Environ. Technol. Innov. 2022, 27, 102489. [Google Scholar] [CrossRef]
- Jiang, P.P.; Liu, J.; Yu, G.; Lei, L.; Jiang, X. Moderate Mn accumulation enhances growth and alters leaf hormone contents in the hyper-accumulator Celosia argentea Linn. Environ. Exp. Bot. 2021, 191, 104603. [Google Scholar] [CrossRef]
- Ge, J.; Tian, S.; Yu, H.; Zhao, J.; Chen, J.; Pan, L.; Xie, R.; Lu, L. Exogenous application of Mn significantly increased Cd accumulation in the Cd/Zn hyper-accumulator Sedum alfredii. Environ. Pollut. 2021, 278, 116837. [Google Scholar] [CrossRef]
- Gallego, S.M.; Pena, L.B.; Barcia, R.A.; Azpilicueta, C.E.; Iannone, M.F.; Rosales, E.P.; Zawoznik, M.S.; Groppa, M.D.; Benavides, M.P. Unravelling cadmium toxicity and tolerance in plants: Insight into regulatory mechanisms. Environ. Exp. Bot. 2012, 83, 33–46. [Google Scholar] [CrossRef]
- Parmar, P.; Kumari, N.; Sharma, V. Structural and functional alterations in photosynthetic apparatus of plants under cadmium stress. Bot. Stud. 2013, 54, 45. [Google Scholar] [CrossRef]
- Tao, R.; Hu, J.; Cao, C.; Zheng, J.; Zhou, X.; Hu, H.; Ma, Y.; Ye, W.; Ma, Z.; Lu, H. Effect of LMWOAs on maize remediation of cadmium and plumbum pollution in farm-land. Sustainability 2022, 14, 14580. [Google Scholar] [CrossRef]
- Lu, L.-L.; Tian, S.-K.; Yang, X.-E.; Peng, H.-Y.; Li, T.-Q. Improved cadmium uptake and accumulation in the hyperaccumulator Sedum alfredii: The impact of citric acid and tartaric acid. J. Zhejiang Univ. B 2013, 14, 106–114. [Google Scholar] [CrossRef]
- Jiang, P.; Liu, J.; Chen, M.; Yu, G.; You, S.; Li, J. Exogenous selenium improves the physiological resistance of cucumber to cadmium stress. Toxicol. Environ. Chem. 2020, 102, 455–472. [Google Scholar] [CrossRef]
- Song, M.; Fan, S.; Pang, C.; Wei, H.; Yu, S. Genetic analysis of the antioxidant enzymes, methane dicarboxylic aldehyde (MDA) and chlorophyll content in leaves of the short season cotton (Gossypium hirsutum L.). Euphytica 2014, 198, 153–162. [Google Scholar] [CrossRef]
- O’Sullivan, J.B.; Plozza, T.; Stefanelli, D.; Jing, J.; Tang, C. Elevated CO2 and phosphorus deficiency interactively enhance root exuda-tion in Lupinus albus L. Plant Soil 2021, 465, 229–243. [Google Scholar] [CrossRef]
- Zornoza, P.; Sánchez-Pardo, B.O.; Carpena, R. Interaction and accumulation of manganese and cadmium in the manga-nese accumulator Lupinus albus. J. Plant Physiol. 2010, 167, 1027–1032. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Li, R.; Lu, Y.; Bai, Z. Sustainable management of cadmium-contaminated soils as affected by exogenous application of nutrients: A review. J. Environ. Manag. 2021, 295, 113081. [Google Scholar] [CrossRef]
- Ramos, I.; Esteban, E.; Lucena, J.J.; Gárate, A. Cadmium uptake and subcellular distribution in plants of Lactuca sp. Cd–Mn interaction. Plant Sci. 2002, 162, 761–767. [Google Scholar] [CrossRef]
- de la Luz Mora, M.; Rosas, A.; Ribera, A.; Rengel, Z. Differential tolerance to Mn toxicity in perennial ryegrass genotypes: Involve-ment of antioxidative enzymes and root exudation of carboxylates. Plant Soil 2009, 320, 79–89. [Google Scholar] [CrossRef]
- Luo, Q.; Sun, L.N.; Hu, X.M. Metabolomics Study on Root Exudates of Cadmium Hyperaccumulator Sedum Alfredii. Chin. J. Anal. Chem. 2015, 43, 7–12. [Google Scholar] [CrossRef]
- Bali, A.S.; Sidhu, G.P.S.; Kumar, V. Root exudates ameliorate cadmium tolerance in plants: A review. Environ. Chem. Lett. 2020, 18, 1243–1275. [Google Scholar] [CrossRef]
- Arsenov, D.; Zupunski, M.; Borisev, M.; Nikolic, N.; Orlovic, S.; Pilipovic, A. Exogenously applied citric acid enhances antioxidant defense and phytoextraction of cadmium by willows (Salix spp.). Water Air Soil Pollut. 2017, 228, 221. [Google Scholar] [CrossRef]
- Nascimento, C.W.A.; Hesterberg, D.; Tappero, R. Effects of exogenous citric acid on the concentration and spatial distribu-tion of Ni, Zn, Co, Cr, Mn and Fe in leaves of Noccaea caerulescens grown on a serpentine soil. J. Hazard. Mater. 2020, 398, 122992. [Google Scholar] [CrossRef]
- Elkhatib, E.; Thabet, A.G.; Mahdy, A.M. Phytoremediation of cadmium contaminated soils: Role of organic complexing agents in cadmium phytoextraction. Land Contam. Reclam. 2001, 9, 359–366. [Google Scholar]
- Zu, Y.; Li, Y.; Min, H.; Zhan, F.; Qin, L.; Wang, J. Subcellular distribution and chemical form of Pb in hyperaccumulator Arenaria orbiculata and response of root exudates to Pb addition. Front. Environ. Sci. Eng. 2014, 9, 250–258. [Google Scholar] [CrossRef]
- Li, Z.-R.; Wang, J.-X.; An, L.-Z.; Tan, J.-B.; Zhan, F.-D.; Wu, J.; Zu, Y.-Q. Effect of root exudates of intercropping Vicia faba and Arabis alpina on accumulation and sub-cellular distribution of lead and cadmium. Int. J. Phytoremediation 2019, 21, 4–13. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
You, S.; Deng, Z.; Chen, M.; Zheng, Y.; Liu, J.; Jiang, P. Mn Pretreatment Improves the Physiological Resistance and Root Exudation of Celosia argentea Linn. to Cadmium Stress. Int. J. Environ. Res. Public Health 2023, 20, 1065. https://doi.org/10.3390/ijerph20021065
You S, Deng Z, Chen M, Zheng Y, Liu J, Jiang P. Mn Pretreatment Improves the Physiological Resistance and Root Exudation of Celosia argentea Linn. to Cadmium Stress. International Journal of Environmental Research and Public Health. 2023; 20(2):1065. https://doi.org/10.3390/ijerph20021065
Chicago/Turabian StyleYou, Shaohong, Zhenliang Deng, Mouyixing Chen, Yingyi Zheng, Jiu Liu, and Pingping Jiang. 2023. "Mn Pretreatment Improves the Physiological Resistance and Root Exudation of Celosia argentea Linn. to Cadmium Stress" International Journal of Environmental Research and Public Health 20, no. 2: 1065. https://doi.org/10.3390/ijerph20021065
APA StyleYou, S., Deng, Z., Chen, M., Zheng, Y., Liu, J., & Jiang, P. (2023). Mn Pretreatment Improves the Physiological Resistance and Root Exudation of Celosia argentea Linn. to Cadmium Stress. International Journal of Environmental Research and Public Health, 20(2), 1065. https://doi.org/10.3390/ijerph20021065








