Respiratory Health Impacts of Outdoor Air Pollution and the Efficacy of Local Risk Communication in Quito, Ecuador
Abstract
1. Introduction
2. Materials and Methods
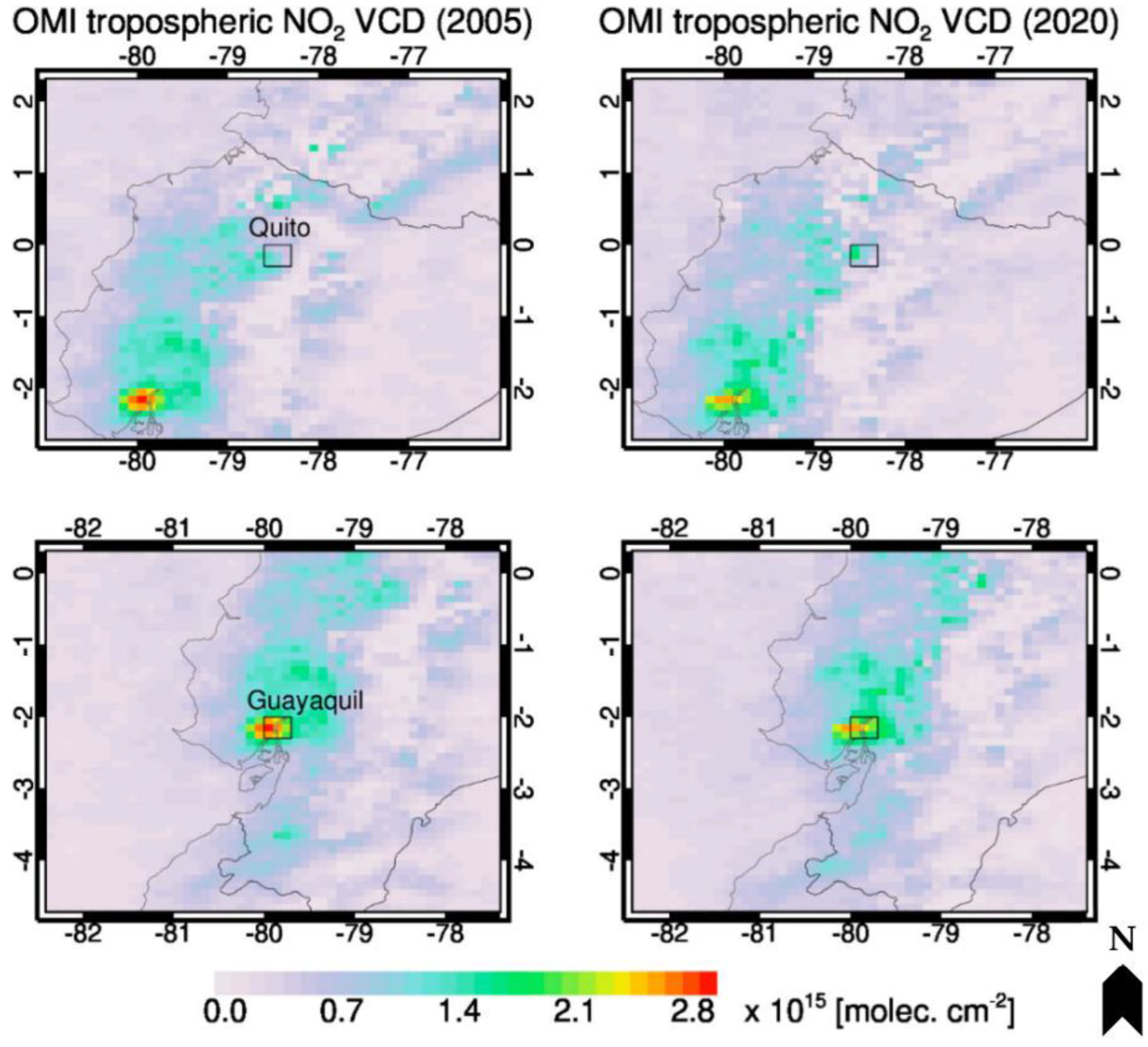
2.1. Exposure Data
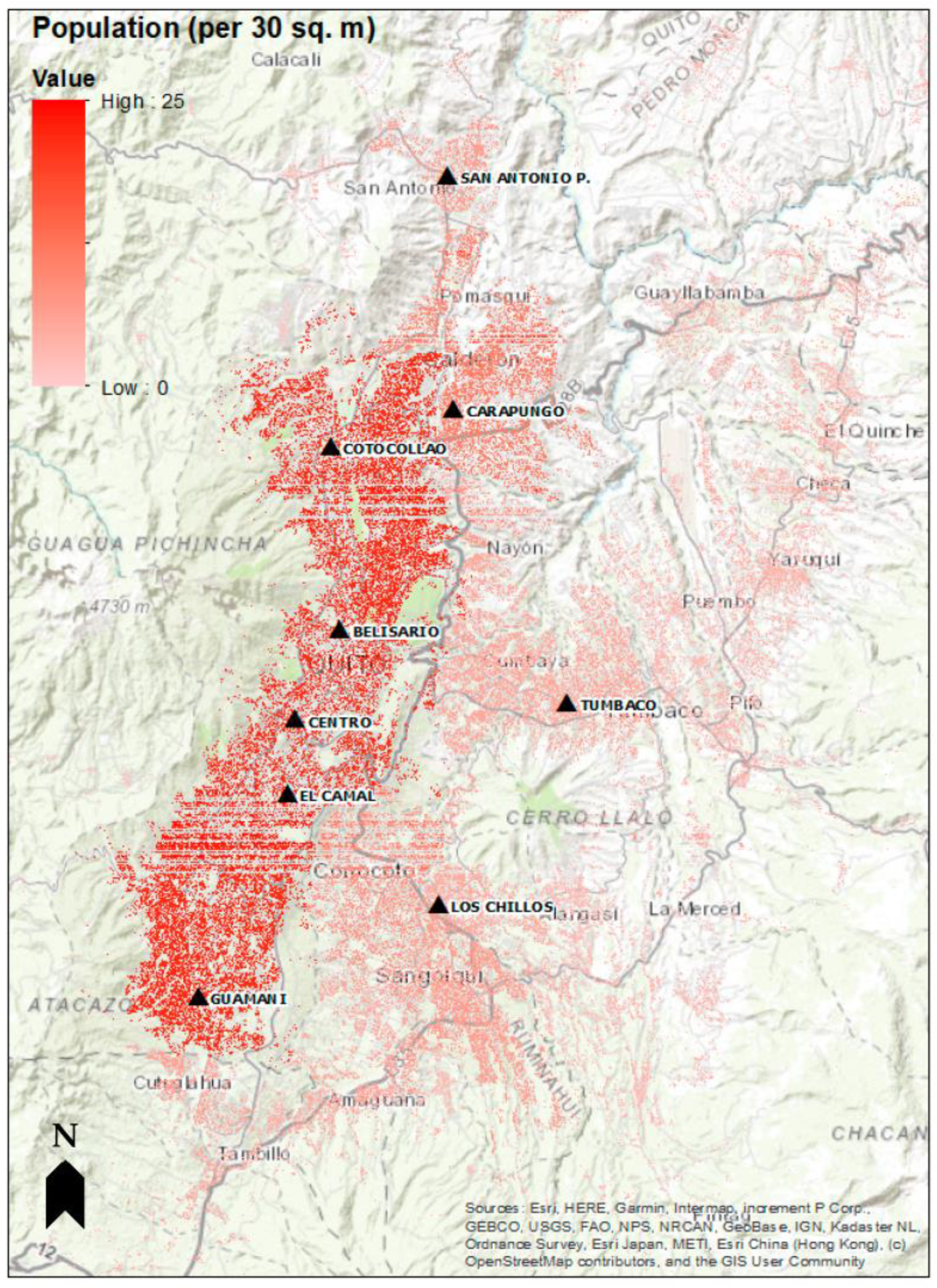
2.2. Health Data
2.3. Model Design
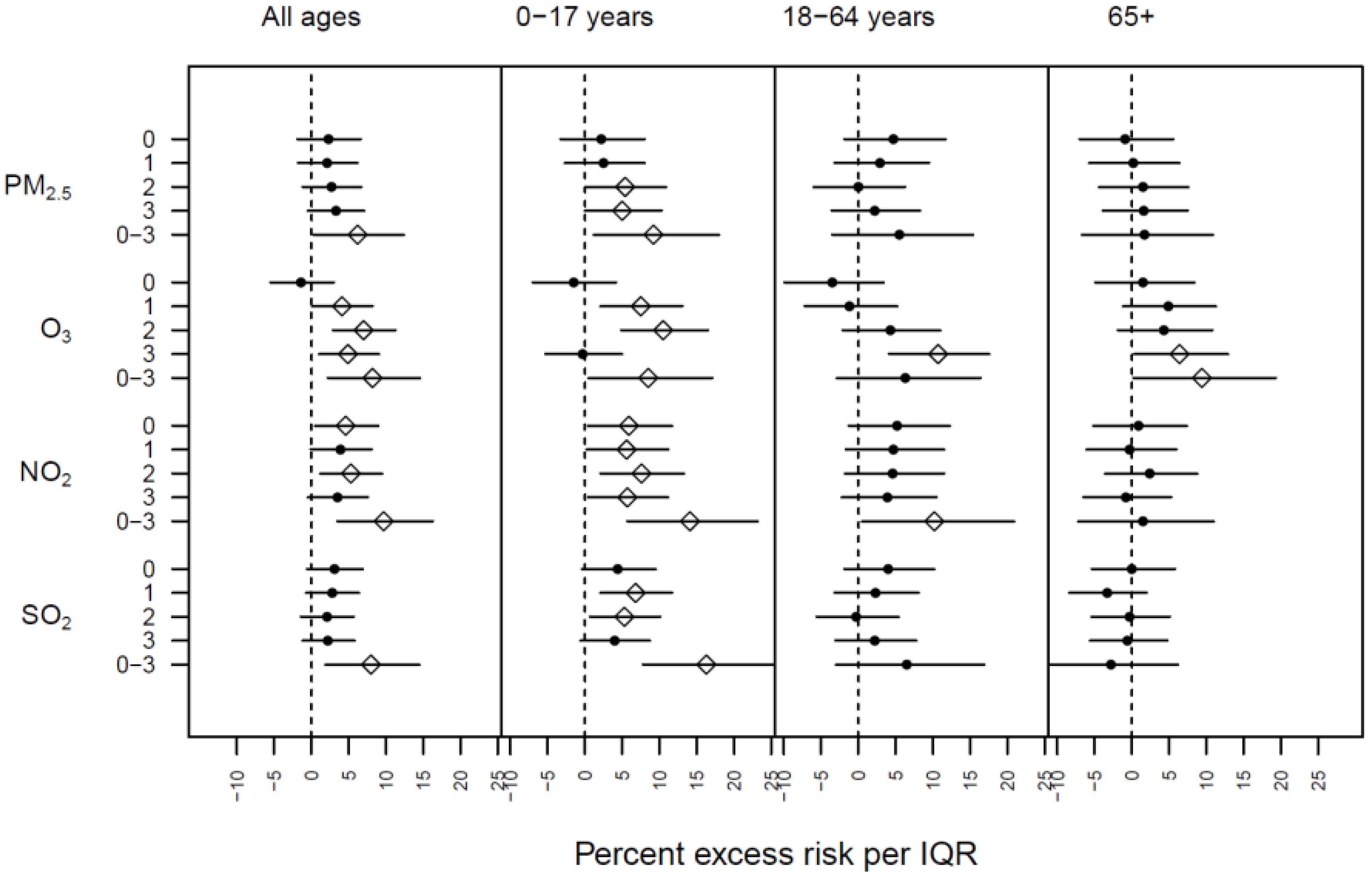
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Minimum | Median | Maximum | IQR | Mean | SD | |
|---|---|---|---|---|---|---|
| PM2.5 (μg/m3) | 6.6 | 17.3 | 32.7 | 6.5 | 17.3 | 4.7 |
| O3 (ppb) | 8.3 | 21.3 | 38.8 | 7.2 | 21.9 | 5.3 |
| NO2 (ppb) | 11.3 | 22 | 37.2 | 6.9 | 22.5 | 4.9 |
| SO2 (ppb) | 0.5 | 1.6 | 3.7 | 1.1 | 1.7 | 0.8 |
| Temp (°C) | 12.3 | 15.5 | 18.6 | 1.3 | 15.5 | 0.9 |
| RH (%) | 26.2 | 69.5 | 92.1 | 17.1 | 67.4 | 11.7 |
| Precipitation (mm) | 0.0 | 0.1 | 13.9 | 1.8 | 1.5 | 2.7 |
| Minimum Daily Count | Median Daily Count | Maximum Daily Count | IQR of Daily Count | Mean Daily Count | SD of Daily Count | Total | ||
|---|---|---|---|---|---|---|---|---|
| 2014 | All ages | 1 | 26 | 47 | 11 | 26.3 | 8.1 | 9600 |
| 0–17 years | 0 | 12 | 25 | 8 | 12.3 | 5.4 | 4500 | |
| 18–64 years | 1 | 8 | 17 | 5 | 7.9 | 3.8 | 2900 | |
| 65+ | 0 | 6 | 14 | 4 | 6.0 | 2.6 | 2200 | |
| 2015 | All ages | 1 | 29 | 55 | 15 | 28.4 | 10.0 | 10,366 |
| 0–17 years | 0 | 10 | 28 | 6 | 11.0 | 5.0 | 4033 | |
| 18–64 years | 0 | 10 | 24 | 8 | 10.4 | 5.5 | 3796 | |
| 65+ | 0 | 6 | 16 | 4 | 7.0 | 3.2 | 2537 |

References
- WHO. Billions of people still breathe unhealthy air: New WHO data. Available online: https://www.who.int/news/item/04-04-2022-billions-of-people-still-breathe-unhealthy-air-new-who-data (accessed on 5 July 2022).
- Cazorla, M. Air quality over a populated Andean region: Insights from measurements of ozone, NO, and boundary layer depths. Atmos. Pollut. Res. 2016, 7, 66–74. [Google Scholar] [CrossRef]
- Gouveia, N.; Kephart, J.L.; Dronova, I.; McClure, L.; Granados, J.T.; Betancourt, R.M.; O’Ryan, A.C.; Texcalac-Sangrador, J.L.; Martinez-Folgar, K.; Rodriguez, D. Ambient fine particulate matter in Latin American cities: Levels, population exposure, and associated urban factors. Sci. Total Environ. 2021, 772, 145035. [Google Scholar] [CrossRef] [PubMed]
- Cevallos, V.M.; Díaz, V.; Sirois, C.M. Particulate matter air pollution from the city of Quito, Ecuador, activates inflammatory signaling pathways in vitro. Innate Immun. 2017, 23, 392–400. [Google Scholar] [CrossRef] [PubMed]
- Valencia, V.H.; Hertel, O.; Ketzel, M.; Levin, G. Modeling urban background air pollution in Quito, Ecuador. Atmos. Pollut. Res. 2020, 11, 646–666. [Google Scholar] [CrossRef]
- Geddes, J.A.; Martin, R.V.; Boys, B.L.; van Donkelaar, A. Long-term trends worldwide in ambient NO2 concentrations inferred from satellite observations. Environ. Health Perspect. 2016, 124, 281–289. [Google Scholar] [CrossRef] [PubMed]
- WHO. WHO air pollution database. Available online: https://www.who.int/data/gho/data/themes/air-pollution/who-air-quality-database/2014 (accessed on 5 July 2022).
- NASA. Nitrogen Dioxide Trends for World Cities. Available online: https://airquality.gsfc.nasa.gov/no2/world (accessed on 7 July 2023).
- Peláez, L.M.G.; Santos, J.M.; de Almeida Albuquerque, T.T.; Reis, N.C., Jr.; Andreão, W.L.; de Fátima Andrade, M. Air quality status and trends over large cities in South America. Environ. Sci. Policy 2020, 114, 422–435. [Google Scholar] [CrossRef]
- Wen, X.J.; Balluz, L.; Mokdad, A. Association between media alerts of air quality index and change of outdoor activity among adult asthma in six states, BRFSS, 2005. J. Community Health 2009, 34, 40–46. [Google Scholar] [CrossRef]
- Borbet, T.C.; Gladson, L.A.; Cromar, K.R. Assessing air quality index awareness and use in Mexico City. BMC Public Health 2018, 18, 538. [Google Scholar] [CrossRef]
- Delmas, M.A.; Kohli, A. Can apps make air pollution visible? Learning about health impacts through engagement with air quality information. J. Bus. Ethics 2020, 161, 279–302. [Google Scholar] [CrossRef]
- Cromar, K.R.; Gladson, L.A.; Ewart, G. Trends in Excess Morbidity and Mortality Associated with Air Pollution above American Thoracic Society-Recommended Standards, 2008–2017. Ann. Am. Thorac. Soc. 2019, 16, 836–845. [Google Scholar] [CrossRef]
- Perlmutt, L.; Stieb, D.; Cromar, K. Accuracy of quantification of risk using a single-pollutant Air Quality Index. J. Expo. Sci. Environ. Epidemiol. 2017, 27, 24–32. [Google Scholar] [CrossRef] [PubMed]
- Brauer, M.; Brook, J.R.; Christidis, T.; Chu, Y.; Crouse, D.L.; Erickson, A. Mortality–Air pollution associations in low-exposure environments (MAPLE): Phase 2. Health Eff. Inst. Tech. Rep. Res. Rep. 2022, 2022, 212. [Google Scholar]
- Chen, R.; Wang, X.; Meng, X.; Hua, J.; Zhou, Z.; Chen, B.; Kan, H. Communicating air pollution-related health risks to the public: An application of the Air Quality Health Index in Shanghai, China. Environ. Int. 2013, 51, 168–173. [Google Scholar] [CrossRef] [PubMed]
- Stieb, D.M.; Burnett, R.T.; Smith-Doiron, M.; Brion, O.; Shin, H.H.; Economou, V. A new multipollutant, no-threshold air quality health index based on short-term associations observed in daily time-series analyses. J. Air Waste Manag. Assoc. 2008, 58, 435–450. [Google Scholar] [CrossRef]
- Wong, T.W.; Tam, W.W.S.; Yu, I.T.S.; Lau, A.K.H.; Pang, S.W.; Wong, A.H. Developing a risk-based air quality health index. Atmos. Environ. 2013, 76, 52–58. [Google Scholar] [CrossRef]
- Cairncross, E.K.; John, J.; Zunckel, M. A novel air pollution index based on the relative risk of daily mortality associated with short-term exposure to common air pollutants. Atmos. Environ. 2007, 41, 8442–8454. [Google Scholar] [CrossRef]
- Li, X.; Xiao, J.; Lin, H.; Liu, T.; Qian, Z.; Zeng, W.; Guo, L.; Ma, W. The construction and validity analysis of AQHI based on mortality risk: A case study in Guangzhou, China. Environ. Pollut. 2017, 220, 487–494. [Google Scholar] [CrossRef]
- To, T.; Shen, S.; Atenafu, E.G.; Guan, J.; McLimont, S.; Stocks, B.; Licskai, C. The air quality health index and asthma morbidity: A population-based study. Environ. Health Perspect. 2013, 121, 46–52. [Google Scholar] [CrossRef]
- Chen, H.; Li, Q.; Kaufman, J.S.; Wang, J.; Copes, R.; Su, Y.; Benmarhnia, T. Effect of air quality alerts on human health: A regression discontinuity analysis in Toronto, Canada. Lancet Planet. Health. 2018, 2, e19–e26. [Google Scholar] [CrossRef]
- Cromar, K.; Gladson, L.; Palomera, M.J.; Perlmutt, L. Development of a health-based index to identify the association between air pollution and health effects in Mexico City. Atmosphere 2021, 12, 372. [Google Scholar] [CrossRef]
- Laumbach, R.J.; Cromar, K.R.; Adamkiewicz, G.; Carlsten, C.; Charpin, D.; Chan, W.R.; de Nazelle, A.; Forastiere, F.; Goldstein, J.; Gumy, S.; et al. Personal Interventions for Reducing Exposure and Risk for Outdoor Air Pollution: An Official American Thoracic Society Workshop Report. Ann. Am. Thorac. Soc. 2021, 18, 1435–1443. [Google Scholar] [CrossRef] [PubMed]
- Ward, A.L.S.; Beatty, T.K. Who responds to air quality alerts? Environ. Resour. Econ. 2016, 65, 487–511. [Google Scholar] [CrossRef]
- Neidell, M. Air quality warnings and outdoor activities: Evidence from Southern California using a regression discontinuity design. J. Epidemiol. Community Health 2010, 64, 921–926. [Google Scholar] [CrossRef] [PubMed]
- McDermott, M.; Srivastava, R.; Croskell, S. Awareness of and compliance with air pollution advisories: A comparison of parents of asthmatics with other parents. J. Asthma 2006, 43, 235–239. [Google Scholar] [CrossRef]
- Van Buuren, S.; Groothuis-Oudshoorn, K. MICE: Multivariate Imputation by Chained Equations in R. J. Stat. Softw. 2011, 45, 1–67. [Google Scholar] [CrossRef]
- Lepeule, J.; Litonjua, A.A.; Gasparrini, A.; Koutrakis, P.; Sparrow, D.; Vokonas, P.S.; Schwartz, J. Lung function association with outdoor temperature and relative humidity and its interaction with air pollution in the elderly. Environ. Res. 2018, 165, 110–117. [Google Scholar] [CrossRef]
- Zhu, Z.; Qiao, Y.; Liu, Q.; Lin, C.; Dang, E.; Fu, W.; Wang, G.; Dong, J. The impact of meteorological conditions on Air Quality Index under different urbanization gradients: A case from Taipei. Environ. Dev. Sustain. 2021, 23, 3994–4010. [Google Scholar] [CrossRef]
- Li, Y.; Chen, Y.; Karimian, H.; Tao, T. Spatiotemporal analysis of air quality and its relationship with meteorological factors in the Yangtze River Delta. J. Elem. 2020, 25, 1059–1075. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2013. [Google Scholar]
- Moorman, J.E. National Surveillance for Asthma—United States, 1980–2004; U.S. Department of Health and Human Services: Atlanta, GA, USA, 2007. [Google Scholar]
- Trasande, L.; Thurston, G.D. The role of air pollution in asthma and other pediatric morbidities. J. Allergy Clin. Immunol. 2005, 115, 689–699. [Google Scholar] [CrossRef]
- Kim, J.J. Ambient air pollution: Health hazards to children. Pediatrics 2004, 114, 1699–1707. [Google Scholar]
- Gilliland, F.D. Outdoor air pollution, genetic susceptibility, and asthma management: Opportunities for intervention to reduce the burden of asthma. Pediatrics 2009, 123 (Suppl. S3), S168–S173. [Google Scholar] [CrossRef]
- Selgrade, M.K.; Plopper, C.G.; Gilmour, M.I.; Conolly, R.B.; Foos, B.S. Assessing the health effects and risks associated with children’s inhalation exposures—Asthma and allergy. J. Toxicol. Environ. Health Part A 2007, 71, 196–207. [Google Scholar] [CrossRef] [PubMed]
- Bateson, T.F.; Schwartz, J. Children’s response to air pollutants. J. Toxicol. Environ. Health Part A 2007, 71, 238–243. [Google Scholar] [CrossRef] [PubMed]
- Wesson, K.; Fann, N.; Morris, M.; Fox, T.; Hubbell, B. A multi–pollutant, risk–based approach to air quality management: Case study for Detroit. Atmos. Pollut. Res. 2010, 1, 296–304. [Google Scholar] [CrossRef]
- Tolbert, P.E.; Klein, M.; Peel, J.L.; Sarnat, S.E.; Sarnat, J.A. Multipollutant modeling issues in a study of ambient air quality and emergency department visits in Atlanta. J. Expo. Sci. Environ. Epidemiol. 2007, 17, S29–S35. [Google Scholar] [CrossRef] [PubMed]
- Vedal, S.; Kaufman, J.D. What does multi-pollutant air pollution research mean? Am. J. Respir. Crit. Care Med. 2011, 183, 4–6. [Google Scholar] [CrossRef] [PubMed]
- Thurston, G.D.; Ahn, J.; Cromar, K.R.; Shao, Y.; Reynolds, H.R.; Jerrett, M.; Lim, C.C.; Shanley, R.; Park, Y.; Hayes, R.B. Ambient particulate matter air pollution exposure and mortality in the NIH-AARP diet and health cohort. Environ. Health Perspect. 2016, 124, 484–490. [Google Scholar] [CrossRef]
- Perlmutt, L.D.; Cromar, K.R. Comparing associations of respiratory risk for the EPA Air Quality Index and health-based air quality indices. Atmos. Environ. 2019, 202, 1–7. [Google Scholar] [CrossRef]
- Gladson, L.A.; Cromar, K.R.; Ghazipura, M.; Knowland, K.E.; Keller, C.A.; Duncan, B. Communicating respiratory health risk among children using a global air quality index. Environ. Int. 2022, 159, 107023. [Google Scholar] [CrossRef]

| Contaminant (μg/m3) | Mathematical Expressions for Each Concentration (C) Range | |||
|---|---|---|---|---|
| O3, 8 h maximum | 0 < C ≤ 100 | 100 < C ≤ 200 | 200 < C ≤ 600 | 600 < C |
| index values = C | index values = C | index values = 0.5C + 100 | index values = 0.5C + 100 | |
| NO2, 1 h maximum | 0 < C ≤ 200 | 200 < C ≤ 1000 | 1000 < C ≤ 3000 | 3000 < C |
| index values = 0.50C | index values = 0.125C + 75.00 | index values = 0.1C + 100 | index values = 0.1C + 100 | |
| SO2, 24 h average | 0 < C ≤ 62.5 | 62.5 < C ≤ 125 | 125 < C ≤ 200 | 200 < C |
| index values = 0.8C | index values = 1.333C − 66.667 | index values = 0.125C + 175 | index values = 0.125C + 175 | |
| PM2.5, 24 h average | 0 < C ≤ 50 | 50 < C ≤ 250 | 250 < C | |
| index values = 2C | index values = C + 50 | index values = C + 50 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, J.; Gladson, L.; Díaz Suárez, V.; Cromar, K. Respiratory Health Impacts of Outdoor Air Pollution and the Efficacy of Local Risk Communication in Quito, Ecuador. Int. J. Environ. Res. Public Health 2023, 20, 6326. https://doi.org/10.3390/ijerph20146326
Zhou J, Gladson L, Díaz Suárez V, Cromar K. Respiratory Health Impacts of Outdoor Air Pollution and the Efficacy of Local Risk Communication in Quito, Ecuador. International Journal of Environmental Research and Public Health. 2023; 20(14):6326. https://doi.org/10.3390/ijerph20146326
Chicago/Turabian StyleZhou, Jiang, Laura Gladson, Valeria Díaz Suárez, and Kevin Cromar. 2023. "Respiratory Health Impacts of Outdoor Air Pollution and the Efficacy of Local Risk Communication in Quito, Ecuador" International Journal of Environmental Research and Public Health 20, no. 14: 6326. https://doi.org/10.3390/ijerph20146326
APA StyleZhou, J., Gladson, L., Díaz Suárez, V., & Cromar, K. (2023). Respiratory Health Impacts of Outdoor Air Pollution and the Efficacy of Local Risk Communication in Quito, Ecuador. International Journal of Environmental Research and Public Health, 20(14), 6326. https://doi.org/10.3390/ijerph20146326







