The Effects of Forest Therapy on the Blood Pressure and Salivary Cortisol Levels of Urban Residents: A Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Study Selection
2.3. Data Extraction
2.4. Quality Assessment
2.5. Meta-Analysis
3. Results
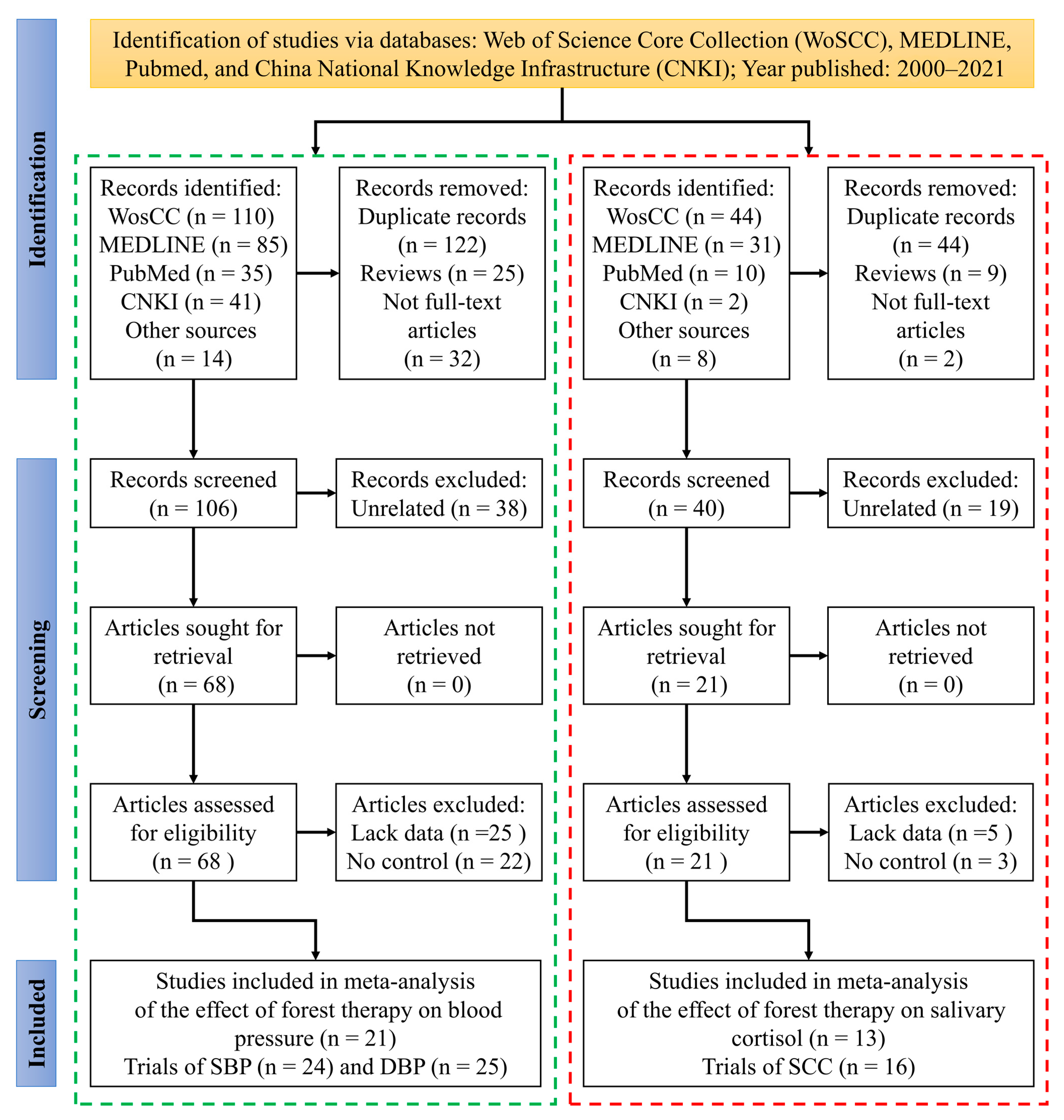
3.1. Study Selection Process
3.2. Study Characteristics and Trial Information
3.3. Risk of Bias
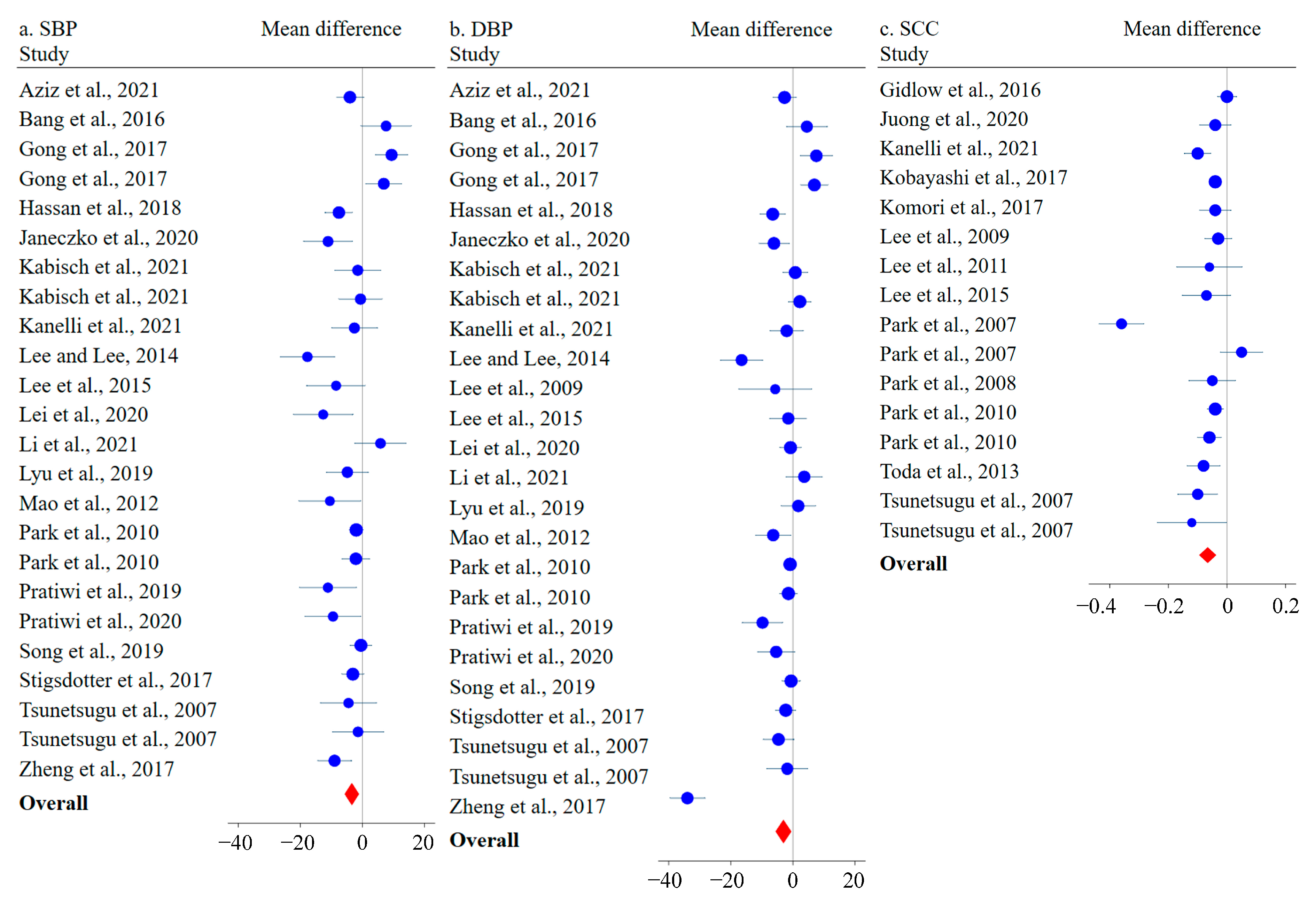
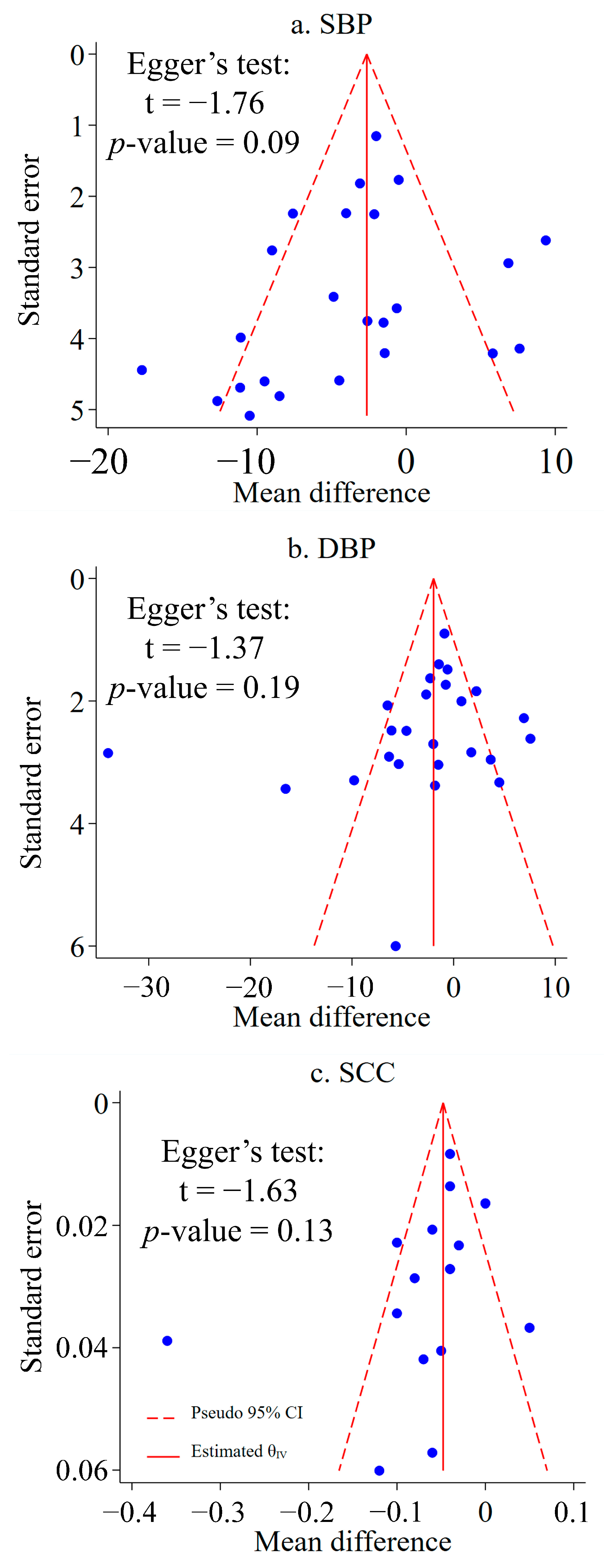
3.4. Meta-Analysis
3.5. Subgroup Analysis
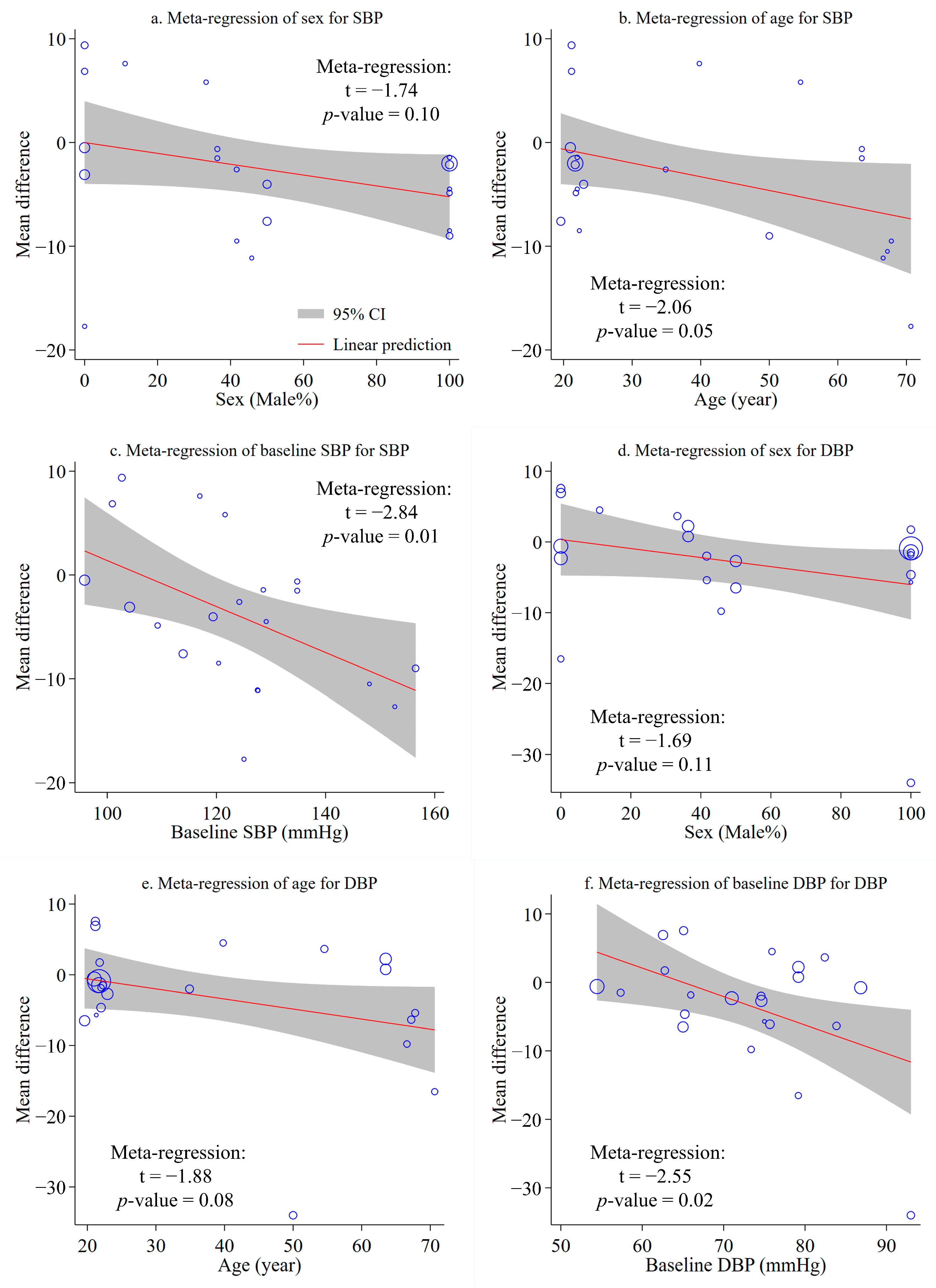
3.6. Meta-Regression Analysis
4. Discussion
4.1. Health Benefits of Forest Therapy to BP and Mental Stress
4.2. Heterogeneity and Its Cause
4.3. Limitations
4.4. Future Research
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pedersen, E. City Dweller Responses to Multiple Stressors Intruding into Their Homes: Noise, Light, Odour, and Vibration. Int. J. Env. Res. Public Health 2015, 12, 3246–3263. [Google Scholar] [CrossRef] [PubMed]
- Krabbendam, L.; van Vugt, M.; Conus, P.; Söderström, O.; Abrahamyan Empson, L.; van Os, J.; Fett, A.-K.J. Understanding Urbanicity: How Interdisciplinary Methods Help to Unravel the Effects of the City on Mental Health. Psychol. Med. 2021, 51, 1099–1110. [Google Scholar] [CrossRef] [PubMed]
- Dzhambov, A.M.; Lercher, P.; Markevych, I.; Browning, M.H.E.M.; Rüdisser, J. Natural and Built Environments and Blood Pressure of Alpine Schoolchildren. Environ. Res. 2022, 204, 111925. [Google Scholar] [CrossRef] [PubMed]
- Mills, K.T.; Stefanescu, A.; He, J. The Global Epidemiology of Hypertension. Nat. Rev. Nephrol. 2020, 16, 223–237. [Google Scholar] [CrossRef]
- World Health Organization. Guideline for the Pharmacological Treatment of Hypertension in Adults; World Health Organization: Geneva, Switzerland, 2021. [Google Scholar]
- Ibrahim, M.M.; Damasceno, A. Hypertension in Developing Countries. Lancet 2012, 380, 611–619. [Google Scholar] [CrossRef]
- Steyn, K.; Bradshaw, D.; Norman, R.; Laubscher, R. Determinants and Treatment of Hypertension in South Africans: The First Demographic and Health Survey. South Afr. Med. J. 2008, 98, 376–380. [Google Scholar]
- Sobngwi, E. Exposure over the Life Course to an Urban Environment and Its Relation with Obesity, Diabetes, and Hypertension in Rural and Urban Cameroon. Int. J. Epidemiol. 2004, 33, 769–776. [Google Scholar] [CrossRef]
- Huang, B.; Xiao, T.; Grekousis, G.; Zhao, H.; He, J.; Dong, G.; Liu, Y. Greenness-Air Pollution-Physical Activity-Hypertension Association among Middle-Aged and Older Adults: Evidence from Urban and Rural China. Environ. Res. 2021, 195, 110836. [Google Scholar] [CrossRef]
- Singh, R.; Suh, I.; Singh, V.; Chaithiraphan, S.; Laothavorn, P.; Sy, R.; Babilonia, N.; Rahman, A.; Sheikh, S.; Tomlinson, B.; et al. Hypertension and Stroke in Asia: Prevalence, Control and Strategies in Developing Countries for Prevention. J. Hum. Hypertens. 2000, 14, 749–763. [Google Scholar] [CrossRef]
- Schulz, M.; Romppel, M.; Grande, G. Built environment and health: A systematic review of studies in Germany. J. Public Health 2018, 40, 8–15. [Google Scholar] [CrossRef]
- Belojević, G.; Jakovljević, B.; Stojanov, V.; Slepčevic, V.Z.; Paunović, K.Z. Nighttime Road-Traffic Noise and Arterial Hypertension in an Urban Population. Hypertens. Res. 2008, 31, 775–781. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bodin, T.; Albin, M.; Ardö, J.; Stroh, E.; Östergren, P.-O.; Björk, J. Road traffic noise and hypertension: Results from a cross-sectional public health survey in southern Sweden. Environ. Health 2009, 8, 38. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.; Perel, P.; Mensah, G.A.; Ezzati, M. Global Epidemiology, Health Burden and Effective Interventions for Elevated Blood Pressure and Hypertension. Nat. Rev. Cardiol. 2021, 18, 785–802. [Google Scholar] [CrossRef]
- Chowdhury, M.A.B.; Epnere, K.; Haque, M.A.; Mkuu, R.S. Urban Rural Differences in Prevalence and Risk Factors of Self-Reported Hypertension among Kenyan Women: A Population-Based Study. J. Hum. Hypertens. 2021, 35, 912–920. [Google Scholar] [CrossRef] [PubMed]
- Jung, W.H.; Woo, J.-M.; Ryu, J.S. Effect of a Forest Therapy Program and the Forest Environment on Female Workers’ Stress. Urban For. Urban Green. 2015, 14, 274–281. [Google Scholar] [CrossRef]
- Shosha, M. Forest Bathing Therapy: The Healing Power of Nature. Int. J. Psychiatry Res. 2021, 4, 1–2. [Google Scholar] [CrossRef]
- Rajoo, K.S.; Karam, D.S.; Abdullah, M.Z. The Physiological and Psychosocial Effects of Forest Therapy: A Systematic Review. Urban For. Urban Green. 2020, 54, 126744. [Google Scholar] [CrossRef]
- Stier-Jarmer, M.; Throner, V.; Kirschneck, M.; Immich, G.; Frisch, D.; Schuh, A. The Psychological and Physical Effects of Forests on Human Health: A Systematic Review of Systematic Reviews and Meta-Analyses. Int. J. Environ. Res. Public Health 2021, 18, 1770. [Google Scholar] [CrossRef]
- Li, Q.; Morimoto, K.; Nakadai, A.; Inagaki, H.; Katsumata, M.; Shimizu, T.; Hirata, Y.; Hirata, K.; Suzuki, H.; Miyazaki, Y.; et al. Forest Bathing Enhances Human Natural Killer Activity and Expression of Anti-Cancer Proteins. Int. J. Immunopathol. Pharmacol. 2007, 20, 3–8. [Google Scholar] [CrossRef]
- Antonelli, M.; Donelli, D.; Carlone, L.; Maggini, V.; Firenzuoli, F.; Bedeschi, E. Effects of Forest Bathing (Shinrin-Yoku) on Individual Well-Being: An Umbrella Review. Int. J. Environ. Health Res. 2021, 32, 1842–1867. [Google Scholar] [CrossRef]
- Smyth, J.M.; Ockenfels, M.C.; Gorin, A.A.; Catley, D.; Porter, L.S.; Kirschbaum, C.; Hellhammer, D.H.; Stone, A.A. Individual Differences in the Diurnal Cycle of Cortisol. Psychoneuroendocrinology 1997, 22, 89–105. [Google Scholar] [CrossRef] [PubMed]
- Antonelli, M.; Barbieri, G.; Donelli, D. Effects of Forest Bathing (Shinrin-Yoku) on Levels of Cortisol as a Stress Biomarker: A Systematic Review and Meta-Analysis. Int. J. Biometeorol. 2019, 63, 1117–1134. [Google Scholar] [CrossRef]
- Van Aken, M.O.; Romijn, J.A.; Miltenburg, J.A.; Lentjes, E.G.W.M. Automated Measurement of Salivary Cortisol. Clin. Chem. 2003, 49, 1408–1409. [Google Scholar] [CrossRef] [PubMed]
- Tyrvaeinen, L.; Ojala, A.; Korpela, K.; Lanki, T.; Tsunetsugu, Y.; Kagawa, T. The influence of urban green environments on stress relief measures: A field experiment. J. Environ. Psychol. 2014, 38, 1–9. [Google Scholar] [CrossRef]
- Triguero-Mas, M.; Gidlow, C.J.; Martı’nez, D.; de Bont, J.; Carrasco-Turigas, G.; Martínez-Íñiguez, T.; Hurst, G.; Masterson, D.; Donaire-Gonzalez, D.; Seto, E.; et al. The effect of randomised exposure to different types of natural outdoor environments compared to exposure to an urban environment on people with indications of psychological distress in Catalonia. PLoS ONE 2017, 12, e0172200. [Google Scholar] [CrossRef]
- Deeks, J.J.; Higgins, J.P.; Altman, D.G. Analysing Data and Undertaking Meta-analyses. In Cochrane Handbook for Systematic Reviews of Interventions; Wiley: Hoboken, NJ, USA, 2019; pp. 241–284. [Google Scholar]
- Glass, G.V. Primary, Secondary, and Meta-Analysis of Research. Educ. Res. 1976, 5, 3–8. [Google Scholar] [CrossRef]
- McKenzie, J.E.; Beller, E.M.; Forbes, A.B. Introduction to Systematic Reviews and Meta-Analysis. Respirology 2016, 21, 626–637. [Google Scholar] [CrossRef]
- Kotera, Y.; Richardson, M.; Sheffield, D. Effects of Shinrin-Yoku (Forest Bathing) and Nature Therapy on Mental Health: A Systematic Review and Meta-Analysis. Int. J. Ment. Health Addict. 2022, 20, 337–361. [Google Scholar] [CrossRef]
- Ideno, Y.; Hayashi, K.; Abe, Y.; Ueda, K.; Iso, H.; Noda, M.; Lee, J.S.; Suzuki, S. Blood Pressure-Lowering Effect of Shinrin-Yoku (Forest Bathing): A Systematic Review and Meta-Analysis. BMC Complement. Altern. Med. 2017, 17, 409. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; PRISMA Group. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. BMJ 2009, 339, b2535. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Fletcher, B.; Gheorghe, A.; Moore, D.; Wilson, S.; Damery, S. Improving the Recruitment Activity of Clinicians in Randomised Controlled Trials: A Systematic Review. BMJ Open 2012, 2, e000496. [Google Scholar] [CrossRef] [PubMed]
- Yao, W.; Zhang, X.; Gong, Q. The Effect of Exposure to the Natural Environment on Stress Reduction: A Meta-Analysis. Urban For. Urban Green. 2021, 57, 126932. [Google Scholar] [CrossRef]
- Higgins, J.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.; Welch, V. Cochrane Handbook for Systematic Reviews of Interventions Version 6.3.; Cochrane: London, UK, 2022. [Google Scholar]
- Sterne, J.A.C.; Hernán, M.A.; Reeves, B.C.; Savović, J.; Berkman, N.D.; Viswanathan, M.; Henry, D.; Altman, D.G.; Ansari, M.T.; Boutron, I.; et al. ROBINS-I: A Tool for Assessing Risk of Bias in Non-Randomised Studies of Interventions. BMJ 2016, 355, i4919. [Google Scholar] [CrossRef]
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.-Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A Revised Tool for Assessing Risk of Bias in Randomised Trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef] [PubMed]
- McGuinness, L.A.; Higgins, J.P.T. Risk-of-bias VISualization (Robvis): An R Package and Shiny Web App for Visualizing Risk-of-bias Assessments. Res. Synth. Methods 2021, 12, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.; Li, T.; Deeks, J. Choosing Effect Measures and Computing Estimates of Effect. In Cochrane Handbook for Systematic Reviews of Interventions; Higgins, J., Thomas, J., Chandler, J., Cumpston, M., Li, T., Page, M., Welch, V., Eds.; Cochrane: London, UK, 2022. [Google Scholar]
- Higgins, J.P.T. Measuring Inconsistency in Meta-Analyses. BMJ 2003, 327, 557–560. [Google Scholar] [CrossRef]
- Borenstein, M. In a meta-analysis, the I-squared statistic does not tell us how much the effect size varies. J. Clin. Epidemiol. 2022. [Google Scholar] [CrossRef]
- Egger, M.; Smith, G.D.; Schneider, M.; Minder, C. Bias in Meta-Analysis Detected by a Simple, Graphical Test. BMJ 1997, 315, 629–634. [Google Scholar] [CrossRef]
- Deng, H. PythonMeta, Python Module of Meta-Analysis. Available online: http://www.Pymeta.com (accessed on 17 August 2022).
- Abdul Aziz, N.A.; Shian, L.Y.; Mokhtar, M.D.M.; Raman, T.L.; Saikim, F.H.; Chen, W.; Nordin, N.M. Effectiveness of Urban Green Space on Undergraduates’ Stress Relief in Tropical City: A Field Experiment in Kuala Lumpur. Urban For. Urban Green. 2021, 63, 127236. [Google Scholar] [CrossRef]
- Bang, K.S.; Lee, I.S.; Kim, S.J.; Song, M.K.; Park, S.E. The Effects of Urban Forest-Walking Program on Health Promotion Behavior, Physical Health, Depression, and Quality of Life: A Randomized Controlled Trial of Office-Workers. J. Korean Acad. Nurs. 2016, 46, 140. [Google Scholar] [CrossRef] [PubMed]
- Gidlow, C.J.; Jones, M.V.; Hurst, G.; Masterson, D.; Clark-Carter, D.; Tarvainen, M.P.; Smith, G.; Nieuwenhuijsen, M. Where to Put Your Best Foot Forward: Psycho-Physiological Responses to Walking in Natural and Urban Environments. J. Environ. Psychol. 2016, 45, 22–29. [Google Scholar] [CrossRef]
- Gong, M.; Wu, J.; Nan, H. An Empirical Study on the Effects of Viewing Forest on Human Physical and Mental Health. J. Beijing For. Univ. (Soc. Sci.) 2017, 16, 44–51. [Google Scholar]
- Hassan, A.; Tao, J.; Li, G.; Jiang, M.; Aii, L.; Zhihui, J.; Zongfang, L.; Qibing, C. Effects of Walking in Bamboo Forest and City Environments on Brainwave Activity in Young Adults. Evid.-Based Complement. Altern. Med. 2018, 2018, 9653857. [Google Scholar] [CrossRef] [PubMed]
- Janeczko, E.; Bielinis, E.; Wójcik, R.; Woźnicka, M.; Kędziora, W.; Łukowski, A.; Elsadek, M.; Szyc, K.; Janeczko, K. When Urban Environment Is Restorative: The Effect of Walking in Suburbs and Forests on Psychological and Physiological Relaxation of Young Polish Adults. Forests 2020, 11, 591. [Google Scholar] [CrossRef]
- Joung, D.; Lee, B.; Lee, J.; Lee, C.; Koo, S.; Park, C.; Kim, S.; Kagawa, T.; Park, B.J. Measures to Promote Rural Healthcare Tourism with a Scientific Evidence-Based Approach. Int. J. Environ. Res. Public Health 2020, 17, 3266. [Google Scholar] [CrossRef] [PubMed]
- Kabisch, N.; Püffel, C.; Masztalerz, O.; Hemmerling, J.; Kraemer, R. Physiological and Psychological Effects of Visits to Different Urban Green and Street Environments in Older People: A Field Experiment in a Dense Inner-City Area. Landsc. Urban Plan 2021, 207, 103998. [Google Scholar] [CrossRef]
- Kanelli, A.A.; Dimitrakopoulos, P.G.; Fyllas, N.M.; Chrousos, G.P.; Kalantzi, O.-I. Engaging the Senses: The Association of Urban Green Space with General Health and Well-Being in Urban Residents. Sustainability 2021, 13, 7322. [Google Scholar] [CrossRef]
- Kobayashi, H.; Song, C.; Ikei, H.; Park, B.-J.; Lee, J.; Kagawa, T.; Miyazaki, Y. Population-Based Study on the Effect of a Forest Environment on Salivary Cortisol Concentration. Int. J. Environ. Res. Public Health 2017, 14, 931. [Google Scholar] [CrossRef]
- Komori, T.; Mitsui, M.; Togashi, K.; Matsui, J.; Kato, T.; Uei, D.; Shibayama, A.; Yamato, K.; Okumura, H.; Kinoshita, F. Relaxation Effect of a 2-Hour Walk in Kumano-Kodo Forest. J. Neurol. Neurosci. 2017, 8, 174. [Google Scholar] [CrossRef]
- Lee, J.-Y.; Lee, D.-C. Cardiac and Pulmonary Benefits of Forest Walking versus City Walking in Elderly Women: A Randomised, Controlled, Open-Label Trial. Eur. J. Integr. Med. 2014, 6, 5–11. [Google Scholar] [CrossRef]
- Lee, J.; Park, B.-J.; Tsunetsugu, Y.; Kagawa, T.; Miyazaki, Y. Restorative Effects of Viewing Real Forest Landscapes, Based on a Comparison with Urban Landscapes. Scand. J. For. Res. 2009, 24, 227–234. [Google Scholar] [CrossRef]
- Lee, J.; Park, B.-J.; Tsunetsugu, Y.; Ohira, T.; Kagawa, T.; Miyazaki, Y. Effect of Forest Bathing on Physiological and Psychological Responses in Young Japanese Male Subjects. Public Health 2011, 125, 93–100. [Google Scholar] [CrossRef]
- Lee, J.; Park, B.-J.; Ohira, T.; Kagawa, T.; Miyazaki, Y. Acute Effects of Exposure to a Traditional Rural Environment on Urban Dwellers: A Crossover Field Study in Terraced Farmland. Int. J. Environ. Res. Public Health 2015, 12, 1874–1893. [Google Scholar] [CrossRef] [PubMed]
- Lei, H.; Zhi, Y.; Zhang, B.; Liu, X.; Wei, X.; Zhang, A.; Pan, R. Effect of Forest Therapy on Blood Pressure and Related Factors in Elderly Patients with Hypertension. J. West China For. Sci. 2020, 49, 46–52. [Google Scholar]
- Li, H.; Liu, H.; Yang, Z.; Bi, S.; Cao, Y.; Zhang, G. The Effects of Green and Urban Walking in Different Time Frames on Physio-Psychological Responses of Middle-Aged and Older People in Chengdu, China. Int. J. Environ. Res. Public Health 2020, 18, 90. [Google Scholar] [CrossRef] [PubMed]
- Lyu, B.; Zeng, C.; Xie, S.; Li, D.; Lin, W.; Li, N.; Jiang, M.; Liu, S.; Chen, Q. Benefits of a Three-Day Bamboo Forest Therapy Session on the Psychophysiology and Immune System Responses of Male College Students. Int. J. Environ. Res. Public Health 2019, 16, 4991. [Google Scholar] [CrossRef]
- Mao, G.X.; Cao, Y.B.; Lan, X.G.; He, Z.H.; Chen, Z.M.; Wang, Y.Z.; Hu, X.L.; Lv, Y.D.; Wang, G.F.; Yan, J. Therapeutic Effect of Forest Bathing on Human Hypertension in the Elderly. J. Cardiol. 2012, 60, 495–502. [Google Scholar] [CrossRef]
- Park, B.J.; Tsunetsugu, Y.; Kasetani, T.; Hirano, H.; Kagawa, T.; Sato, M.; Miyazaki, Y. Physiological Effects of Shinrin-Yoku (Taking in the Atmosphere of the Forest)—Using Salivary Cortisol and Cerebral Activity as Indicators—. J. Physiol. Anthropol. 2007, 26, 123–128. [Google Scholar] [CrossRef]
- Park, B.J.; Tsunetsugu, Y.; Ishii, H.; Furuhashi, S.; Hirano, H.; Kagawa, T.; Miyazaki, Y. Physiological Effects of Shinrin-Yoku (Taking in the Atmosphere of the Forest) in a Mixed Forest in Shinano Town, Japan. Scand. J. For. Res. 2008, 23, 278–283. [Google Scholar] [CrossRef]
- Park, B.J.; Tsunetsugu, Y.; Kasetani, T.; Kagawa, T.; Miyazaki, Y. The Physiological Effects of Shinrin-Yoku (Taking in the Forest Atmosphere or Forest Bathing): Evidence from Field Experiments in 24 Forests across Japan. Environ. Health Prev. Med. 2010, 15, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Pratiwi, P.I.; Xiang, Q.; Furuya, K. Physiological and Psychological Effects of Viewing Urban Parks in Different Seasons in Adults. Int. J. Environ. Res. Public Health 2019, 16, 4279. [Google Scholar] [CrossRef] [PubMed]
- Pratiwi, P.I.; Xiang, Q.; Furuya, K. Physiological and Psychological Effects of Walking in Urban Parks and Its Imagery in Different Seasons in Middle-Aged and Older Adults: Evidence from Matsudo City, Japan. Sustainability 2020, 12, 4003. [Google Scholar] [CrossRef]
- Song, C.; Ikei, H.; Kagawa, T.; Miyazaki, Y. Physiological and Psychological Effects of Viewing Forests on Young Women. Forests 2019, 10, 635. [Google Scholar] [CrossRef]
- Stigsdotter, U.K.; Corazon, S.S.; Sidenius, U.; Kristiansen, J.; Grahn, P. It Is Not All Bad for the Grey City—A Crossover Study on Physiological and Psychological Restoration in a Forest and an Urban Environment. Health Place 2017, 46, 145–154. [Google Scholar] [CrossRef] [PubMed]
- Toda, M.; Den, R.; Hasegawa-Ohira, M.; Morimoto, K. Effects of Woodland Walking on Salivary Stress Markers Cortisol and Chromogranin A. Complement Ther. Med. 2013, 21, 29–34. [Google Scholar] [CrossRef]
- Tsunetsugu, Y.; Park, B.-J.; Ishii, H.; Hirano, H.; Kagawa, T.; Miyazaki, Y. Physiological Effects of Shinrin-Yoku (Taking in the Atmosphere of the Forest) in an Old-Growth Broadleaf Forest in Yamagata Prefecture, Japan. J. Physiol. Anthropol. 2007, 26, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.; Mo, D.; Lan, F.; Chen, C.; Long, C. Effects of Forest Bathing on Blood Pressure, Blood Lipids and Heart Function in Patients with Hypertension. Chin. J. Conval. Med. 2017, 26, 449–451. [Google Scholar]
- Kondo, M.C.; Jacoby, S.F.; South, E.C. Does Spending Time Outdoors Reduce Stress? A Review of Real-Time Stress Response to Outdoor Environments. Health Place 2018, 51, 136–150. [Google Scholar] [CrossRef]
- Li, H.; Zhang, X.; Bi, S.; Cao, Y.; Zhang, G. Psychological Benefits of Green Exercise in Wild or Urban Greenspaces: A Meta-Analysis of Controlled Trials. Urban For. Urban Green. 2022, 68, 127458. [Google Scholar] [CrossRef]
- Wen, Y.; Yan, Q.; Pan, Y.; Gu, X.; Liu, Y. Medical Empirical Research on Forest Bathing (Shinrin-Yoku): A Systematic Review. Environ. Health Prev. Med. 2019, 24, 70. [Google Scholar] [CrossRef] [PubMed]
- Rajoo, K.S.; Karam, D.S.; Wook, N.-F.; Abdullah, M.-Z. Forest Therapy: An Environmental Approach to Managing Stress in Middle-Aged Working Women. Urban For. Urban Green. 2020, 55, 126853. [Google Scholar] [CrossRef]
- Li, Q.; Kobayashi, M.; Kumeda, S.; Ochiai, T.; Miura, T.; Kagawa, T.; Imai, M.; Wang, Z.; Otsuka, T.; Kawada, T. Effects of Forest Bathing on Cardiovascular and Metabolic Parameters in Middle-Aged Males. Evid.-Based Complement. Altern. Med. 2016, 2016, 2587381. [Google Scholar] [CrossRef]
- Bielinis, E.; Jaroszewska, A.; Łukowski, A.; Takayama, N. The Effects of a Forest Therapy Programme on Mental Hospital Patients with Affective and Psychotic Disorders. Int. J. Environ. Res. Public Health 2019, 17, 118. [Google Scholar] [CrossRef] [PubMed]
- Kaptchuk, T.J.; Kelley, J.M.; Conboy, L.A.; Davis, R.B.; Kerr, C.E.; Jacobson, E.E.; Kirsch, I.; Schyner, R.N.; Nam, B.H.; Nguyen, L.T.; et al. Components of Placebo Effect: Randomised Controlled Trial in Patients with Irritable Bowel Syndrome. BMJ 2008, 336, 999–1003. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Otsuka, T.; Kobayashi, M.; Wakayama, Y.; Inagaki, H.; Katsumata, M.; Hirata, Y.; Li, Y.; Hirata, K.; Shimizu, T.; et al. Acute Effects of Walking in Forest Environments on Cardiovascular and Metabolic Parameters. Eur. J. Appl. Physiol. 2011, 111, 2845–2853. [Google Scholar] [CrossRef] [PubMed]
- Hunter, M.R.; Gillespie, B.W.; Chen, S.Y.-P. Urban Nature Experiences Reduce Stress in the Context of Daily Life Based on Salivary Biomarkers. Front. Psychol. 2019, 10, 722. [Google Scholar] [CrossRef]
- Ibrahim, H.N.; Hebert, S.A.; Murad, D.N.; Adrogue, H.E.; Nguyen, D.T.; Graviss, E.A.; Nguyen, H.; Matas, A. Outcomes of Hypertensive Kidney Donors Using Current and Past Hypertension Definitions. Kidney Int. Rep. 2021, 6, 1242–1253. [Google Scholar] [CrossRef]
- Zhu, S.; Hu, F.; He, S.; Qiu, Q.; Su, Y.; He, Q.; Li, J. Comprehensive Evaluation of Healthcare Benefits of Different Forest Types: A Case Study in Shimen National Forest Park, China. Forests 2021, 12, 207. [Google Scholar] [CrossRef]
- Kim, J.G.; Shin, W.S. Forest Therapy Alone or with a Guide: Is There a Difference between Self-Guided Forest Therapy and Guided Forest Therapy Programs? Int. J. Environ. Res. Public Health 2021, 18, 6957. [Google Scholar] [CrossRef]
| Criteria | Inclusion |
|---|---|
| P (Participants) | Adults living in urban areas, regardless of their health status |
| I (Intervention) | All types of forest therapy activities (real forest-based seated viewing, walking, or multi-session program) |
| C (Comparison) | Visiting urban environments (urban environment-based seated viewing, walking, or multi-session program) |
| O (Outcomes) | Measurement of the participants’ SBP, and/or DBP, and/or SCC after intervention |
| Reference | Design (RCT/Non-RCT) | Study Location | Participants’ Characteristics | Intervention Procedure (Sessions and Duration) | Outcomes | |||
|---|---|---|---|---|---|---|---|---|
| Sex; Male% | Mean Age (SD) or Range; Year | SBP | DBP | SCC | ||||
| Abdul Aziz et al., 2021 [45] | Crossover RCT | Malaysia | 50 | 22.93 (1.0); 20–25 | Forest walking (20 min) | ○ | ○ | |
| Bang et al., 2016 [46] | Parallel RCT | Korea | 7.4 | 39.8 | Forest walking (5 weeks) | ○ | ○ | |
| Gidlow et al., 2016 [47] | Crossover RCT | United Kingdom | 65 | 47.9 (11.6) | Forest walking (30 min) | ○ | ||
| Gong et al., 2017 [48] | Parallel RCT | China | 0 | 21.17 (1.46); 19–24 | 1. Seated viewing (30 min) | ○ | ○ | |
| 2. Forest walking (60 min) | ||||||||
| Hassan et al., 2018 [49] | Crossover RCT | China | 50 | 19.6 (1.42); 19–24 | Forest walking (15 min) | ○ | ○ | |
| Janeczko et al., 2020 [50] | Parallel RCT | Poland | / | 19–24 | Forest walking (30 min) | ○ | ○ | |
| Joung et al., 2020 [51] | Non-RCT | Korea | 62.5 | 20.9 (1.3) | Forest walking (15 min) | ○ | ||
| Kabisch et al., 2021 [52] | Parallel RCT | Germany | 36.4 | 63.5 (4.2); 55–70 | 1. Seated viewing (15 min) | ○ | ○ | |
| 2. Seated viewing (15 min) + walking (30 min) | ||||||||
| Kanelli et al., 2021 [53] | Non-RCT | Greece | 41.7 | 34.9 (11.0) | Forest walking (60 min) | ○ | ○ | ○ |
| Kobayashi et al., 2017 [54] | Crossover RCT | Japan | 0 | 70.7; 60–80 | Seated viewing (15 min) | ○ | ||
| Komori et al., 2017 [55] | Crossover RCT | Japan | 100 | 31.5 (5.6) | Forest walking (2 h) | ○ | ||
| Lee and Lee, 2014 [56] | Parallel RCT | Japan | 0 | 70.65; 60–80 | Forest walking (60 min) | ○ | ○ | |
| Lee et al., 2009 [57] | Crossover RCT | Japan | 100 | 21.3 (1.1); 20–23 | Seated viewing (15 min) | ○ | ○ | |
| Lee et al., 2011 [58] | Cross-over RCT | Japan | 100 | 21.2 (0.9) | Seated viewing (15 min) | ○ | ||
| Lee et al., 2015 [59] | Crossover RCT | Japan | 100 | 22.3 (1.3) | Seated viewing (15 min) | ○ | ○ | ○ |
| Lei et al., 2020 [60] | Parallel RCT | China | / | 60–70 | Forest bathing program (5 days) | ○ | ○ | |
| Li et al., 2020 [61] | Parallel RCT | China | 33.3 | 54.56; 40–71 | Forest walking (15 min) | ○ | ○ | |
| Lyu et al., 2019 [62] | Parallel RCT | China | 100 | 21.7; 19–24 | Forest therapy program (3 days) | ○ | ○ | |
| Mao et al., 2012 [63] | Parallel RCT | China | / | 66.6; 60–75 | Forest bathing program (7 days) | ○ | ○ | |
| Park et al., 2007 [64] | Crossover RCT | Japan | 100 | 22.8 (1.4) | 1. Seated viewing (20 min) | ○ | ||
| 2. Forest walking (20 min) | ||||||||
| Park et al., 2008 [65] | Crossover RCT | Japan | 100 | 21.3 (1.1) | Seated viewing (15 min) | ○ | ||
| Park et al., 2010 [66] | Crossover RCT | Japan | 100 | 21.7 (1.5) | 1. Seated viewing (average 14 min) | ○ | ○ | ○ |
| 2. Forest walking (average 16 min) | ||||||||
| Pratiwi et al., 2019 [67] | Crossover RCT | Japan | 45.8 | 66.6 | Seated viewing (11–15 min) | ○ | ○ | |
| Pratiwi et al., 2020 [68] | Crossover RCT | Japan | 41.7 | 67.8 | Forest walking (11–15 min) | ○ | ○ | |
| Song et al., 2019 [69] | Crossover RCT | China | 0 | 21.0 (1.3) | Seated viewing (15 min) | ○ | ○ | |
| Stigsdotter et al., 2017 [70] | Non-RCT | Denmark | 0 | 20–36 | Seated viewing (50 min) + walking (15 min) | ○ | ○ | |
| Toda et al., 2013 [71] | Non-RCT | Japan | 100 | 67.6 (2.8); 64–74 | Seated viewing (45 min) | ○ | ||
| Tsunetsugu et al., 2007 [72] | Crossover RCT | Japan | 100 | 22.0 (1.0); 21–23 | 1. Seated viewing (15 min) | ○ | ○ | ○ |
| 2. Forest walking (15 min) | ||||||||
| Zheng et al., 2017 [73] | Parallel RCT | China | 100 | 50 | Forest bathing program (20 days) | ○ | ○ | |
| Outcomes | Subgroup Analysis | Number of Studies | Number of Participants | Effect MD (95% CI) | 95% PI | Heterogeneity (τ2) | Heterogeneity (I2; %) | p-Value |
|---|---|---|---|---|---|---|---|---|
| SBP | Overall | 24 | 2246 | −3.44 (−5.74, −1.14) | (−13.30, 6.42) | 21.2229 | 72.87 | <0.01 |
| Design-based subgroup | 0.79 | |||||||
| RCT | 22 | 2096 | −3.55 (−6.12, −0.99) | 75.21 | <0.01 | |||
| Non-RCT | 2 | 150 | −3.00 (−6.21, −0.20) | 0.00 | 0.90 | |||
| Session-based subgroup | 0.36 | |||||||
| Seated viewing | 7 | 896 | −1.89 (−5.23, 1.46) | 60.96 | 0.02 | |||
| Walking or multi-session program | 17 | 1350 | −4.04 (−7.14, −0.94) | 75.80 | <0.01 | |||
| Duration-based subgroup | 0.53 | |||||||
| <20 min | 11 | 1292 | −2.72 (−4.84, −0.60) | 37.87 | 0.10 | |||
| ≥20 min | 13 | 954 | −4.25 (−8.47, −0.02) | 82.49 | <0.01 | |||
| DBP | Overall | 25 | 2270 | −3.07 (−5.59, −0.54) | (−15.54, 9.41) | 34.7231 | 88.59 | 0.02 |
| Design-based subgroup | 0.63 | |||||||
| RCT | 23 | 2120 | −3.17 (−5.94, −0.39) | 89.53 | <0.01 | |||
| Non-RCT | 2 | 150 | −2.22 (−4.96, 0.52) | 0.00 | 0.92 | |||
| Session-based subgroup | 0.25 | |||||||
| Seated viewing | 8 | 920 | −1.13 (−3.80, 1.54) | 69.08 | <0.01 | |||
| Walking or multi-session program | 17 | 1350 | −3.81 (−7.51, −0.11) | 91.15 | <0.01 | |||
| Duration-based subgroup | 0.35 | |||||||
| <20 min | 12 | 1316 | −1.60 (−3.33, 0.12) | 52.77 | 0.02 | |||
| ≥20 min | 13 | 954 | −4.18 (−9.33, 0.98) | 93.42 | <0.01 | |||
| SCC | Overall | 16 | 1786 | −0.07 (−0.10, −0.04) | (−0.18, 0.05) | 0.0026 | 83.85 | <0.01 |
| Design-based subgroup | 0.67 | |||||||
| RCT | 13 | 1654 | −0.06 (−0.10, −0.03) | 86.02 | <0.01 | |||
| Non-RCT | 3 | 132 | −0.08 (−0.11, −0.04) | 30.66 | 0.24 | |||
| Session-based subgroup | 0.16 | |||||||
| Seated viewing | 8 | 1346 | −0.09 (−0.14, −0.04) | 89.80 | <0.01 | |||
| Walking or multi-session program | 8 | 440 | −0.05 (−0.08, −0.01) | 70.17 | <0.01 | |||
| Duration-based subgroup | 0.36 | |||||||
| <20 min | 10 | 1538 | −0.04 (−0.06, −0.03) | 0.00 | 0.74 | |||
| ≥20 min | 6 | 248 | −0.09 (−0.17, 0.00) | 94.18 | <0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qiu, Q.; Yang, L.; He, M.; Gao, W.; Mar, H.; Li, J.; Wang, G. The Effects of Forest Therapy on the Blood Pressure and Salivary Cortisol Levels of Urban Residents: A Meta-Analysis. Int. J. Environ. Res. Public Health 2023, 20, 458. https://doi.org/10.3390/ijerph20010458
Qiu Q, Yang L, He M, Gao W, Mar H, Li J, Wang G. The Effects of Forest Therapy on the Blood Pressure and Salivary Cortisol Levels of Urban Residents: A Meta-Analysis. International Journal of Environmental Research and Public Health. 2023; 20(1):458. https://doi.org/10.3390/ijerph20010458
Chicago/Turabian StyleQiu, Quan, Ling Yang, Mei He, Wen Gao, Harrison Mar, Jiyue Li, and Guangyu Wang. 2023. "The Effects of Forest Therapy on the Blood Pressure and Salivary Cortisol Levels of Urban Residents: A Meta-Analysis" International Journal of Environmental Research and Public Health 20, no. 1: 458. https://doi.org/10.3390/ijerph20010458
APA StyleQiu, Q., Yang, L., He, M., Gao, W., Mar, H., Li, J., & Wang, G. (2023). The Effects of Forest Therapy on the Blood Pressure and Salivary Cortisol Levels of Urban Residents: A Meta-Analysis. International Journal of Environmental Research and Public Health, 20(1), 458. https://doi.org/10.3390/ijerph20010458








