Looking Towards 2030: Strengthening the Environmental Health in Childhood–Adolescent Cancer Survivor Programs
Abstract
1. Introduction
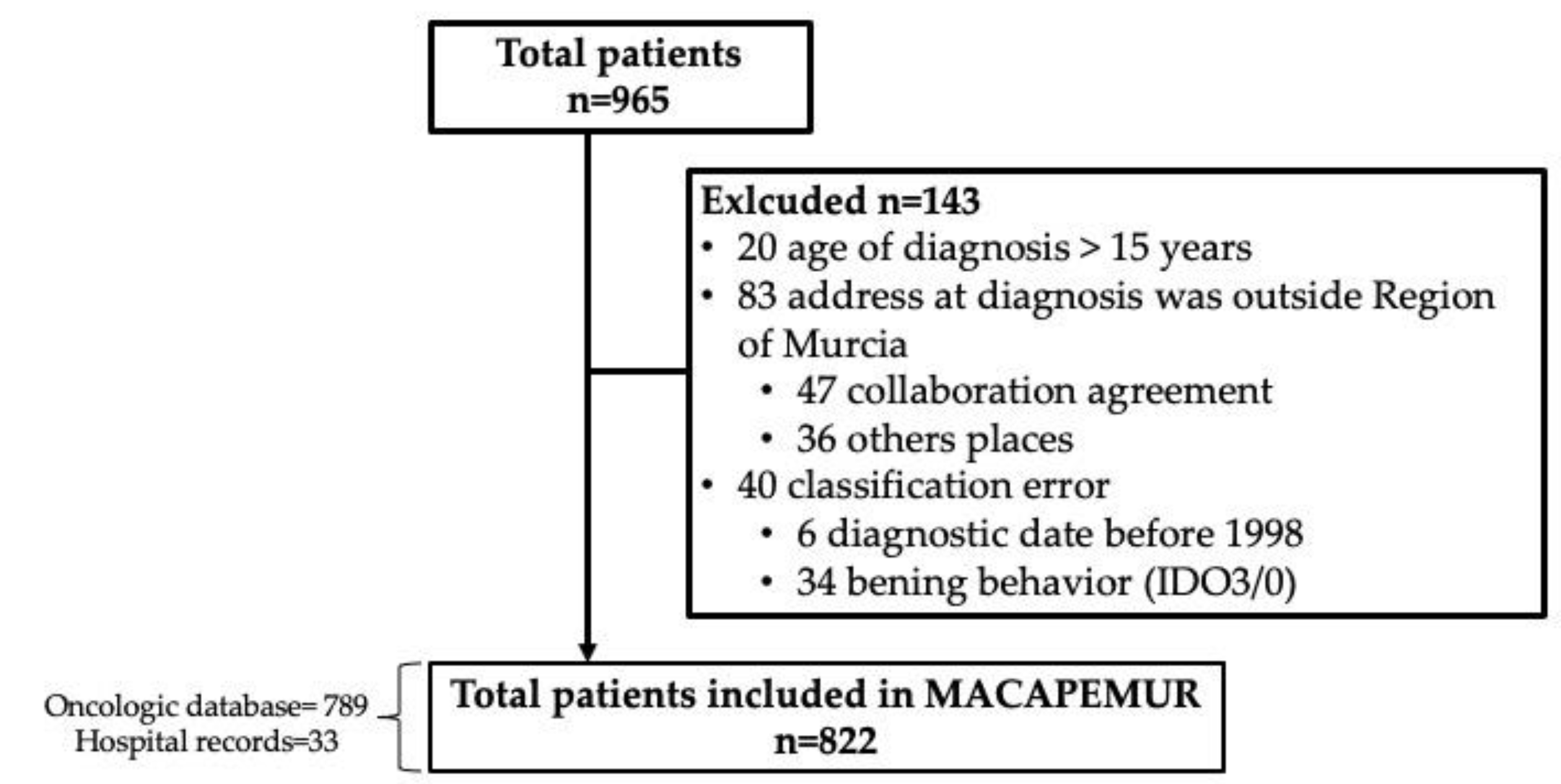
2. Materials and Methods
2.1. Data Collection
2.2. Environmental and Community Health Program for Longitudinal Follow-Up of Childhood and Adolescent Cancer Survivors
2.3. Pediatric Environmental History of Child and Adolescent Cancer Survivors
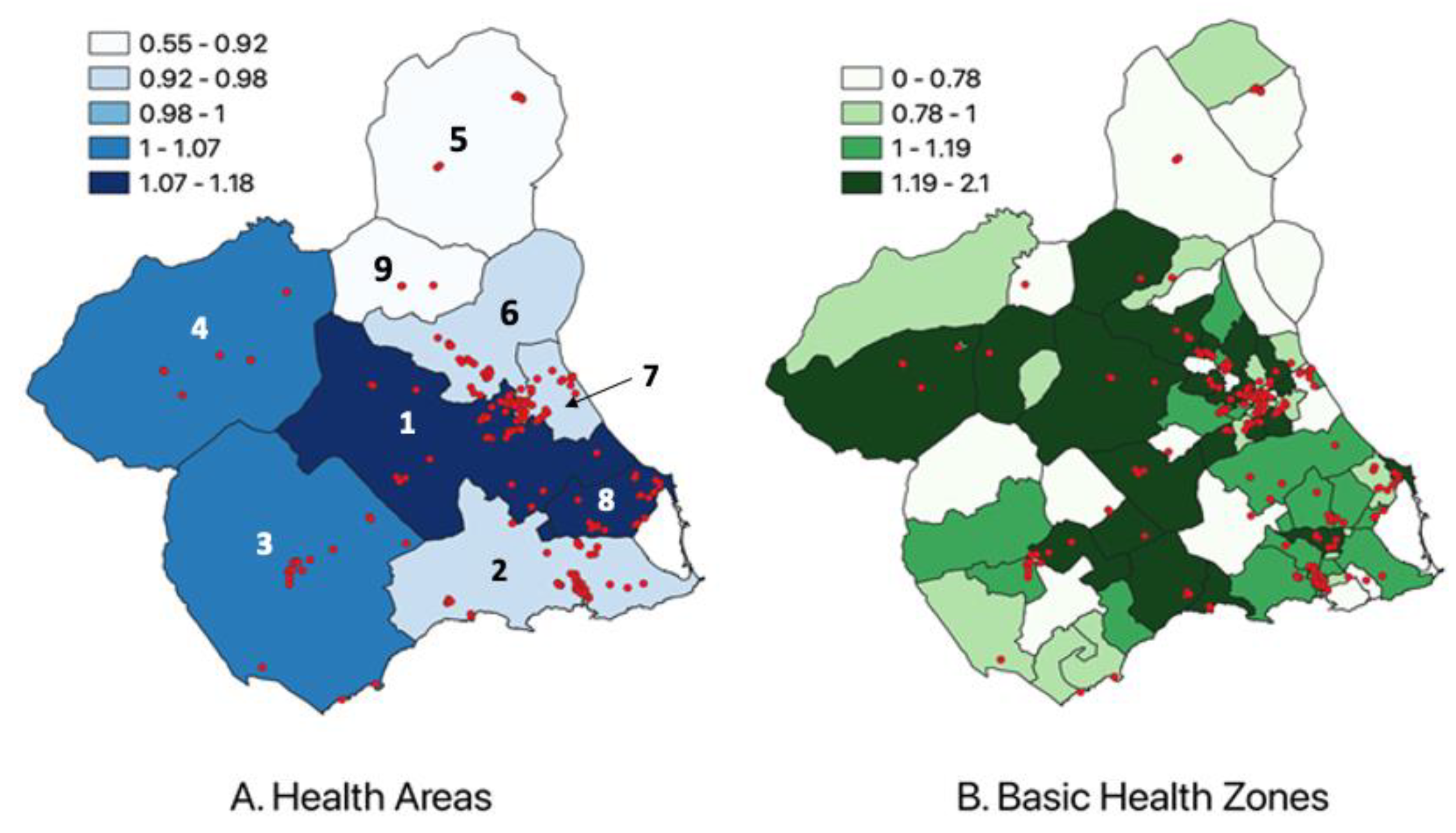
2.4. Descriptive and Geospatial Study
2.5. Spatial and Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer Statistics, 2021. CA A Cancer J. Clin. 2021, 71, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Ortega-García, J.A.; López-Hernández, F.A.; Cárceles-Álvarez, A.; Santiago-Rodríguez, E.J.; Sánchez, A.C.; Bermúdez-Cortes, M.; Fuster-Soler, J.L. Analysis of small areas of pediatric cancer in the municipality of Murcia (Spain). An. Pediatr. (Barc.) 2016, 84, 154–162. [Google Scholar] [CrossRef] [PubMed]
- Stiller, C.A.; Marcos-Gragera, R.; Ardanaz, E.; Pannelli, F.; Almar Marqués, E.; Cañada Martinez, A.; Steliarova-Foucher, E. Geographical Patterns of Childhood Cancer Incidence in Europe, 1988–1997. Report from the Automated Childhood Cancer Information System Project. Eur. J. Cancer 2006, 42, 1952–1960. [Google Scholar] [CrossRef] [PubMed]
- REDECAN Working Group; Galceran, J.; Ameijide, A.; Carulla, M.; Mateos, A.; Quirós, J.R.; Rojas, D.; Alemán, A.; Torrella, A.; Chico, M.; et al. Cancer Incidence in Spain, 2015. Clin. Transl. Oncol. 2017, 19, 799–825. [Google Scholar] [CrossRef]
- Magrath, I.; Steliarova-Foucher, E.; Epelman, S.; Ribeiro, R.C.; Harif, M.; Li, C.-K.; Kebudi, R.; Macfarlane, S.D.; Howard, S.C. Paediatric Cancer in Low-Income and Middle-Income Countries. Lancet Oncol. 2013, 14, e104–e116. [Google Scholar] [CrossRef]
- Lam, C.G.; Howard, S.C.; Bouffet, E.; Pritchard-Jones, K. Science and Health for All Children with Cancer. Science 2019, 363, 1182–1186. [Google Scholar] [CrossRef]
- Robison, L.L.; Hudson, M.M. Survivors of Childhood and Adolescent Cancer: Life-Long Risks and Responsibilities. Nat. Rev. Cancer 2014, 14, 61–70. [Google Scholar] [CrossRef]
- Oeffinger, K.C.; Mertens, A.C.; Sklar, C.A. Chronic Health Conditions in Adult Survivors of Childhood Cancer. Oncol. Times 2007, 29, 26. [Google Scholar] [CrossRef]
- Pritchard-Jones, K.; Pieters, R.; Reaman, G.H.; Hjorth, L.; Downie, P.; Calaminus, G.; Naafs-Wilstra, M.C.; Steliarova-Foucher, E. Sustaining Innovation and Improvement in the Treatment of Childhood Cancer: Lessons from High-Income Countries. Lancet Oncol. 2013, 14, e95–e103. [Google Scholar] [CrossRef]
- Cancer (IARC), T.I.A. for R. on Global Cancer Observatory. Available online: https://gco.iarc.fr/ (accessed on 4 August 2022).
- Ortega-García, J.A.; Tellerías, L.; Ferrís-Tortajada, J.; Boldo, E.; Campillo-López, F.; van den Hazel, P.; Cortes-Arancibia, S.; Ramis, R.; Gaioli, M.; Monroy-Torres, R.; et al. Threats, challenges and opportunities for paediatric environmental health in Europe, Latin America and the Caribbean. An. Pediatr. (Barc.) 2019, 90, 124.e1–124.e11. [Google Scholar] [CrossRef]
- Force, L.M.; Abdollahpour, I.; Advani, S.M.; Agius, D.; Ahmadian, E.; Alahdab, F.; Alam, T.; Alebel, A.; Alipour, V.; Allen, C.A.; et al. The Global Burden of Childhood and Adolescent Cancer in 2017: An Analysis of the Global Burden of Disease Study 2017. Lancet Oncol. 2019, 20, 1211–1225. [Google Scholar] [CrossRef]
- Cárceles-Álvarez, A.; Ortega-García, J.A.; López-Hernández, F.A.; Orozco-Llamas, M.; Espinosa-López, B.; Tobarra-Sánchez, E.; Alvarez, L. Spatial Clustering of Childhood Leukaemia with the Integration of the Paediatric Environmental History. Environ. Res. 2017, 156, 605–612. [Google Scholar] [CrossRef] [PubMed]
- UNICEF; World Health Organization. Children in the New Millennium: Environmental Impact on Health; UNEP: Nairobi, Kenya, 2002. [Google Scholar]
- World Health Organization. Inheriting a Sustainable World? Atlas on Children’s Health and the Environment; World Health Organization: Geneva, Switzerland, 2017. [Google Scholar]
- Ortega-García, J.A. Llamando a La Tierra…Llamando a La Tierra, Una Aproximación Al Modelo De La Salud Medioambiental; Asociación Ecología y Salud: Cartagena, Spain, 2021. [Google Scholar]
- Chakraborty, P. Climate Change: A Potential Risk Factor for Cancer? Med. Res. 2021, 8, 154–163. [Google Scholar] [CrossRef]
- Nations, U. What Is Climate Change? Available online: https://www.un.org/en/climatechange/what-is-climate-change (accessed on 11 August 2022).
- Chuvieco, E.; Burgui-Burgui, M.; Orellano, A.; Otón, G.; Ruíz-Benito, P. Links between Climate Change Knowledge, Perception and Action: Impacts on Personal Carbon Footprint. Sustainability 2021, 13, 8088. [Google Scholar] [CrossRef]
- Hertwich, E.G.; Peters, G.P. Carbon Footprint of Nations: A Global, Trade-Linked Analysis. Environ. Sci. Technol. 2009, 43, 6414–6420. [Google Scholar] [CrossRef] [PubMed]
- Ivanova, D.; Stadler, K.; Steen-Olsen, K.; Wood, R.; Vita, G.; Tukker, A.; Hertwich, E.G. Environmental Impact Assessment of Household Consumption: Environmental Impact Assessment of Household Consumption. J. Ind. Ecol. 2016, 20, 526–536. [Google Scholar] [CrossRef]
- What Is Your Carbon Footprint? Available online: https://www.nature.org/en-us/get-involved/how-to-help/carbon-footprint-calculator/ (accessed on 12 December 2022).
- What Is A Carbon Footprint? A Carbon Footprint Definition. Youmatter. Available online: https://youmatter.world/en/definition/definitions-carbon-footprint/ (accessed on 12 December 2022).
- Ritchie, H.; Roser, M.; Rosado, P. CO₂ and Greenhouse Gas Emissions. Available online: https://ourworldindata.org/co2-and-other-greenhouse-gas-emissions (accessed on 12 April 2022).
- Milner, J.; Hamilton, I.; Woodcock, J.; Williams, M.; Davies, M.; Wilkinson, P.; Haines, A. Health Benefits of Policies to Reduce Carbon Emissions. BMJ 2020, 368, l6758. [Google Scholar] [CrossRef] [PubMed]
- on behalf of the EUROCLUS project; Alexander, F.; Boyle, P.; Carli, P.-M.; Coebergh, J.; Draper, G.; Ekbom, A.; Levi, F.; McKinney, P.; McWhirter, W.; et al. Spatial Clustering of Childhood Leukaemia: Summary Results from the EUROCLUS Project. Br. J. Cancer 1998, 77, 818–824. [Google Scholar] [CrossRef]
- Demoury, C.; Goujon-Bellec, S.; Guyot-Goubin, A.; Hémon, D.; Clavel, J. Spatial Variations of Childhood Acute Leukaemia in France, 1990–2006. Eur. J. Cancer Prev. 2012, 21, 367–374. [Google Scholar] [CrossRef]
- Francis, S.S.; Enders, C.; Hyde, R.; Gao, X.; Wang, R.; Ma, X.; Wiemels, J.L.; Selvin, S.; Metayer, C. Spatial–Temporal Cluster Analysis of Childhood Cancer in California. Epidemiology 2020, 31, 214–223. [Google Scholar] [CrossRef]
- García-Pérez, J.; Morales-Piga, A.; Gómez, J.; Gómez-Barroso, D.; Tamayo-Uria, I.; Romaguera, E.P.; Fernández-Navarro, P.; López-Abente, G.; Ramis, R. Association between Residential Proximity to Environmental Pollution Sources and Childhood Renal Tumors. Environ. Res. 2016, 147, 405–414. [Google Scholar] [CrossRef] [PubMed]
- Knox, E.; Gilman, E. Spatial Clustering of Childhood Cancers in Great Britain. J. Epidemiol. Community Health 1996, 50, 313–319. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kreis, C.; Grotzer, M.; Hengartner, H.; Daniel Spycher, B. Swiss Paediatric Oncology Group and the Swiss National Cohort Study Group Space-time Clustering of Childhood Cancers in S Witzerland: A Nationwide Study. Int. J. Cancer 2016, 138, 2127–2135. [Google Scholar] [CrossRef] [PubMed]
- McNally, R.J.; Alexander, F.E.; Vincent, T.J.; Murphy, M.F. Spatial Clustering of Childhood Cancer in Great Britain during the Period 1969–1993. Int. J. Cancer 2009, 124, 932–936. [Google Scholar] [CrossRef] [PubMed]
- Ortega-García, J.; López-Hernández, F.; Sobrino-Najul, E.; Febo, I.; Fuster-Soler, J. Environment and paediatric cancer in the Region of Murcia (Spain): Integrating clinical and environmental history in a geographic information system. An. Pediatr. (Barc.) 2011, 74, 255–260. [Google Scholar] [CrossRef] [PubMed]
- Ramis, R.; Gomez-Barroso, D.; Tamayo, I.; Garcia-Perez, J.; Morales, A.; Pardo Romaguera, E.; Lopez-Abente, G. Spatial Analysis of Childhood Cancer: A Case/Control Study. PLoS ONE 2015, 10, e0127273. [Google Scholar] [CrossRef] [PubMed]
- Madero, L.; Lassaletta, A.; Sevilla, J. Efectos Secundarios Tardíos Del Cáncer Infantil. In Hematología y Oncología Pediátricas 3a Edición; Ergon: Madrid, Spain, 2015. [Google Scholar]
- National Institutes of Health NCI Dictionary of Cancer Terms-National Cancer Institute. Available online: https://www.cancer.gov/publications/dictionaries/cancer-terms (accessed on 1 March 2022).
- Steliarova-Foucher, E.; Stiller, C.; Lacour, B.; Kaatsch, P. International Classification of Childhood Cancer, Third Edition. Cancer 2005, 103, 1457–1467. [Google Scholar] [CrossRef]
- Fritz, A.; Percy, C.; Jack, A.; Shanmugaratnam, K.; Sobin, L.; Parkin, D.M.; Whelan, S. International classification of diseases for oncology (ICD-O), 3rd ed.; 1st revision; World Health Organization: Malta, 2013; Available online: https://apps.who.int/iris/handle/10665/96612 (accessed on 12 March 2022).
- Cañete-Nieto, A.; Pardo-Romaguera, E.; Muñoz-López, A.; Valero-Poveda, S.; Porta-Cebolla, S.; Barreda-Reine, M.S.; Bonet, R.P. Registro Español de Tumores Infantiles.(RETI-SEHOP). Estadística 1980–2021; Universidad de Valencia: Valencia, Spain, 2022. [Google Scholar]
- Cárceles-Álvarez, A.; Ortega-García, J.A.; Fuster-Soler, J.L.; Rivera-Pagán, G.A.; Bermúdez-Cortés, M.; Gomariz-Peñalver, V.; Monzó-Nuñez, E.; López-Hernández, F.A. Long-Term Follow-up of Childhood Cancer Survivors in the Murcia Region: Preferences and Attitudes of Primary Care Professionals. An. Pediatr. (Barc.) 2015, 83, 264–271. [Google Scholar] [CrossRef]
- Ferris-Tortajada, J.; Ortega-García, J.A.; Macián, M.; García-Castell, J. Environment and Pediatric Cancer. An. Pediatr. (Barc.) 2004, 61, 42–50. [Google Scholar] [CrossRef]
- Ortega-García, J.A.; Soldin, O.P.; López-Hernández, F.A.; Trasande, L.; Ferrís-Tortajada, J. Congenital Fibrosarcoma and History of Prenatal Exposure to Petroleum Derivatives. Pediatrics 2012, 130, e1019–e1025. [Google Scholar] [CrossRef]
- Ortega García, J.A. Programa de Salud Ambiental y Comunitaria de Largo Seguimiento de Supervivientes de Cáncer Pediátrico (PLASESCAP); Unidad de Salud Medioambiental Pediátrica, IMIB-Arrixaca: Murcia, Spain, 2005; Available online: https://pehsu.org/wp/?page_id=349 (accessed on 12 March 2022).
- Diaz-Rubio, E. Estrategia En Cáncer Del Sistema Nacional de Salud. Plan de Calidad para el Sistema Nacional de Salud; Centro de publicaciones, Madrid, Ministerio de Sanidad y Política Social, Gobierno de España: Madrid, Spain, 2006; Available online: https://www.sanidad.gob.es/organizacion/sns/planCalidadSNS/pdf/excelencia/cancer-cardiopatia/CANCER/opsc_est1.pdf.pdf (accessed on 12 December 2022).
- United Nations Carbon Offset Platform|UNFCCC. Available online: https://unfccc.int/climate-action/united-nations-carbon-offset-platform (accessed on 12 December 2022).
- Ortega-García, J.A. Manual de La Hoja Verde de Georreferenciación Del Cáncer Pediátrico. Salud y Medioambiente. Available online: https://pehsu.org/wp/wp-content/uploads/Manual_geoMACAPEMUR.pdf (accessed on 12 December 2022).
- Oglesby, L.; Künzli, N.; Monn, C.; Schindler, C.; Ackermann-Liebrich, U.; Leuenberger, P.; SAPALDIA Team. Validity of Annoyance Scores for Estimation of Long Term Air Pollution Exposure in Epidemiologic Studies: The Swiss Study on Air Pollution and Lung Diseases in Adults (SAPALDIA). Am. J. Epidemiol. 2000, 152, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Landier, W.; Bhatia, S.; Eshelman, D.A.; Forte, K.J.; Sweeney, T.; Hester, A.L.; Darling, J.; Armstrong, F.D.; Blatt, J.; Constine, L.S. Development of Risk-Based Guidelines for Pediatric Cancer Survivors: The Children’s Oncology Group Long-Term Follow-up Guidelines from the Children’s Oncology Group Late Effects Committee and Nursing Discipline. J. Clin. Oncol. 2004, 22, 4979–4990. [Google Scholar] [CrossRef]
- Hudson, M.M.; Ehrhardt, M.J.; Bhakta, N.; Baassiri, M.; Eissa, H.; Chemaitilly, W.; Green, D.M.; Mulrooney, D.A.; Armstrong, G.T.; Brinkman, T.M.; et al. Approach for Classification and Severity Grading of Long-Term and Late-Onset Health Events among Childhood Cancer Survivors in the St. Jude Lifetime Cohort. Cancer Epidemiol. Biomark. Prev. 2017, 26, 666–674. [Google Scholar] [CrossRef]
- Ministerio de Sanidad—Ciudadanos—Ministerio de Sanidad y Consumo—Ciudadanos—Sistema Nacional de Salud—Centros. Available online: https://www.sanidad.gob.es/ciudadanos/prestaciones/centrosServiciosSNS/hospitales/introduccionCentro.htm (accessed on 8 December 2022).
- Gatta, G.; Botta, L.; Rossi, S.; Aareleid, T.; Bielska-Lasota, M.; Clavel, J.; Dimitrova, N.; Jakab, Z.; Kaatsch, P.; Lacour, B.; et al. Childhood Cancer Survival in Europe 1999–2007: Results of EUROCARE-5—A Population-Based Study. Lancet Oncol. 2014, 15, 35–47. [Google Scholar] [CrossRef] [PubMed]
- Demoor-Goldschmidt, C.; Tabone, M.-D.; Bernier, V.; de Vathaire, F.; Berger, C. Long-Term Follow-up after Childhood Cancer in France Supported by the SFCE—Force and Weakness—Current State, Results of a Questionnaire and Perspectives. Br. J. Radiol. 2018, 91, 20170819. [Google Scholar] [CrossRef]
- Fedorovsky, J.M.; Cuervo, L.G.; Luciani, S. Pediatric Cancer Registries in Latin America: The Case of Argentina’s Pediatric Cancer Registry. Rev. Panam. Salud. Publica 2017, 41, e152. [Google Scholar] [CrossRef] [PubMed]
- Ssenyonga, N.; Stiller, C.; Nakata, K.; Shalkow, J.; Redmond, S.; Bulliard, J.-L.; Girardi, F.; Fowler, C.; Marcos-Gragera, R.; Bonaventure, A.; et al. Worldwide Trends in Population-Based Survival for Children, Adolescents, and Young Adults Diagnosed with Leukaemia, by Subtype, during 2000–14 (CONCORD-3): Analysis of Individual Data from 258 Cancer Registries in 61 Countries. Lancet Child Adolesc. Health 2022, 6, 409–431. [Google Scholar] [CrossRef] [PubMed]
- Mirutse, M.K.; Tolla, M.T.; Memirie, S.T.; Palm, M.T.; Hailu, D.; Abdi, K.A.; Buli, E.D.; Norheim, O.F. The Magnitude and Perceived Reasons for Childhood Cancer Treatment Abandonment in Ethiopia: From Health Care Providers’ Perspective. BMC Health Serv. Res. 2022, 22, 1014. [Google Scholar] [CrossRef] [PubMed]
- Williams, L.A.; Richardson, M.; Marcotte, E.L.; Poynter, J.N.; Spector, L.G. Sex Ratio among Childhood Cancers by Single Year of Age. Pediatr. Blood Cancer 2019, 66, e27620. [Google Scholar] [CrossRef] [PubMed]
- Spector, L.G.; Pankratz, N.; Marcotte, E.L. Genetic and Nongenetic Risk Factors for Childhood Cancer. Pediatr. Clin. N. Am. 2015, 62, 11–25. [Google Scholar] [CrossRef]
- Chan, C.W.H.; Choi, K.C.; Chien, W.T.; Sit, J.W.H.; Wong, R.; Cheng, K.K.F.; Li, C.K.; Yuen, H.L.; Li, C.K. Health Behaviors of Chinese Childhood Cancer Survivors: A Comparison Study with Their Siblings. Int. J. Environ. Res. Public Health 2020, 17, 6136. [Google Scholar] [CrossRef] [PubMed]
- Kadan-Lottick, N.S.; Robison, L.L.; Gurney, J.G.; Neglia, J.P.; Yasui, Y.; Hayashi, R.; Hudson, M.; Greenberg, M.; Mertens, A.C. Childhood Cancer Survivors’ Knowledge About Their Past Diagnosis and Treatment: Childhood Cancer Survivor Study. JAMA 2002, 287, 1832. [Google Scholar] [CrossRef] [PubMed]
- Schindler, M.; Spycher, B.D.; Ammann, R.A.; Ansari, M.; Michel, G.; Kuehni, C.E.; for the Swiss Paediatric Oncology Group (SPOG). Cause-specific Long-term Mortality in Survivors of Childhood Cancer in S Witzerland: A Population-based Study. Int. J. Cancer 2016, 139, 322–333. [Google Scholar] [CrossRef] [PubMed]
- Reulen, R.C. Long-Term Cause-Specific Mortality Among Survivors of Childhood Cancer. JAMA 2010, 304, 172. [Google Scholar] [CrossRef]
- Filippini, T.; Heck, J.E.; Malagoli, C.; Giovane, C.D.; Vinceti, M. A Review and Meta-Analysis of Outdoor Air Pollution and Risk of Childhood Leukemia. J. Environ. Sci. Health Part C 2015, 33, 36–66. [Google Scholar] [CrossRef]
- Jiang, X.-Q.; Mei, X.-D.; Feng, D. Air Pollution and Chronic Airway Diseases: What Should People Know and Do? J. Thorac. Dis. 2016, 8, E31. [Google Scholar]
- Cárceles-Álvarez, A.; Ortega-García, J.A.; López-Hernández, F.A.; Fuster-Soler, J.L.; Ramis, R.; Kloosterman, N.; Castillo, L.; Sánchez-Solís, M.; Claudio, L.; Ferris-Tortajada, J. Secondhand Smoke: A New and Modifiable Prognostic Factor in Childhood Acute Lymphoblastic Leukemias. Environ. Res. 2019, 178, 108689. [Google Scholar] [CrossRef]
- Alavanja, M.C.R.; Bonner, M.R. Occupational Pesticide Exposures and Cancer Risk: A Review. J. Toxicol. Environ. Health Part B 2012, 15, 238–263. [Google Scholar] [CrossRef]
- Frumkin, H.; Haines, A. Global Environmental Change and Noncommunicable Disease Risks. Annu. Rev. Public Health 2019, 40, 261–282. [Google Scholar] [CrossRef]
- Coleman, N.C.; Ezzati, M.; Marshall, J.D.; Robinson, A.L.; Burnett, R.T.; Pope, C.A. Fine Particulate Matter Air Pollution and Mortality Risk Among US Cancer Patients and Survivors. JNCI Cancer Spectr. 2021, 5, pkab001. [Google Scholar] [CrossRef]
- Ou, J.Y.; Kirchhoff, A.C.; Hanson, H.A. Air Pollution across the Cancer Continuum: Extending Our Understanding of the Relationship between Environmental Exposures and Cancer. Cancer Epidemiol. Biomark. Prev. 2020, 29, 1876–1879. [Google Scholar] [CrossRef] [PubMed]
- Ebrahimi, M.; Khalili, N.; Razi, S.; Keshavarz-Fathi, M.; Khalili, N.; Rezaei, N. Effects of Lead and Cadmium on the Immune System and Cancer Progression. J. Environ. Health Sci. Engineer. 2020, 18, 335–343. [Google Scholar] [CrossRef] [PubMed]
- García-Pérez, J.; Gómez-Barroso, D.; Tamayo-Uria, I.; Ramis, R. Methodological Approaches to the Study of Cancer Risk in the Vicinity of Pollution Sources: The Experience of a Population-Based Case–Control Study of Childhood Cancer. Int. J. Health Geogr. 2019, 18, 12. [Google Scholar] [CrossRef] [PubMed]
- Cox, C.L.; Nolan, V.G.; Leisenring, W.; Yasui, Y.; Ogg, S.W.; Mertens, A.C.; Neglia, J.P.; Ness, K.K.; Armstrong, G.T.; Robison, L.L. Noncancer-Related Mortality Risks in Adult Survivors of Pediatric Malignancies: The Childhood Cancer Survivor Study. J. Cancer Surviv. 2014, 8, 460–471. [Google Scholar] [CrossRef] [PubMed]
- AMERICAN ACADEMY OF PEDIATRICS Section on Hematology/Oncology CHILDREN’S ONCOLOGY GROUP. Long-Term Follow-up Care for Pediatric Cancer Survivors. Pediatrics 2009, 123, 906–915. [Google Scholar] [CrossRef]

| Name | When? | Description |
|---|---|---|
| Individual Carbon Footprint | At diagnosis |
|
| Green Page | At the time of diagnosis |
|
| Risk Assessment | 1–4 months after diagnosis |
|
| Transition Report “green passport” | At the end of the treatment |
|
| Long-Term Follow-Up Plan | During the CACS’ lifespan |
|
| % Survival (Typical Error) | |||||||
|---|---|---|---|---|---|---|---|
| 2 Years | 5 Years | 10 Years | p-Value * | ||||
| Sex | |||||||
| Male | 82.8 | (1.8) | 82.0 | (1.9) | 81.2 | (1.9) | 0.651 |
| Female | 86.3 | (1.8) | 83.3 | (2.0) | 81.6 | (2.1) | |
| Income | |||||||
| <2000 | 87.6 | (1.6) | 86.5 | (1.6) | 85.3 | (1.8) | 0.581 |
| 2000–3500 | 86.7 | (2.8) | 85.0 | (3.0) | 83.9 | (3.1) | |
| >3500 | 90.9 | (4.3) | - | - | - | - | |
| Family history of cancer 1st degree | |||||||
| Yes | 89.0 | (4.7) | - | - | - | - | 0.759 |
| No | 87.3 | (1.3) | 85.9 | (1.4) | 84.7 | (1.5) | |
| Family history of cancer 1st degree or 2nd degree (younger than 55 years old) | |||||||
| Yes | 90.4 | (2.8) | 88.3 | (3.1) | 86.9 | (3.3) | 0.465 |
| No | 86.8 | (1.5) | 85.4 | (1.5) | 84.4 | (1.6) | |
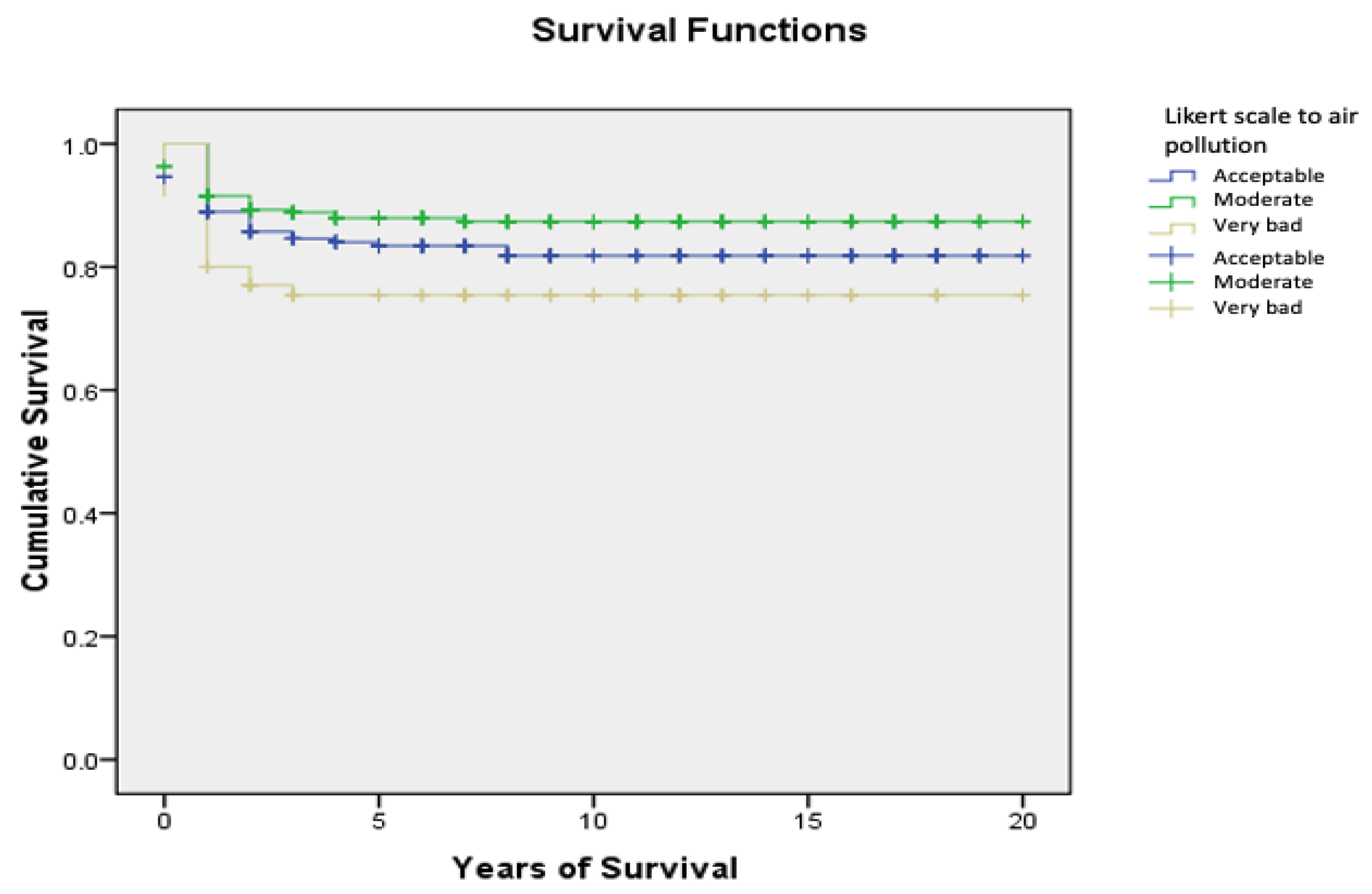
| Likert scale of air pollution in address at the time of diagnosis | |||||||
| Acceptable | 85.7 | (2.5) | 83.4 | (2.7) | 81.8 | (2.9) | <0.05 |
| Moderate | 89.3 | (1.8) | 87.9 | (2.0) | - | - | |
| Very bad | 77.0 | (5.0) | - | - | - | - | |
| Exposure to pesticides inside the residence | |||||||
| Yes | 86.9 | (1.7) | 85.7 | (1.8) | 84.4 | (1.9) | 0.57 |
| No | 88.0 | (2.0) | 86.6 | (2.2) | - | - | |
| Contact with nature | |||||||
| Every day | 88.7 | (1.8) | 87.5 | (1.9) | 87.0 | (2.0) | 0.937 |
| Once a week | 88.6 | (1.9) | 86.7 | (2.1) | 85.2 | (2.3) | |
| Once a month | 87.9 | (5.1) | - | - | - | - | |
| Only in vacations | - | - | - | - | - | - | |
| Never | - | - | - | - | - | - | |
| Physical activity frequency | |||||||
| Never | 90.0 | (3.4) | 88.5 | (3.6) | - | - | 0.283 |
| 1–2 days/week | 90.1 | (1.8) | 88.9 | (1.9) | - | - | |
| 3–4 days/week | - | - | - | - | 91.7 | (2.7) | |
| > 5 days/week | 96.3 | (2.6) | - | - | - | - | |
| Maternal smoking during pregnancy | |||||||
| Yes | 88.1 | (1.9) | - | - | 85.2 | (2.2) | 0.824 |
| No | 86.8 | (1.8) | 85.7 | (1.9) | - | - | |
| Paternal smoking during pregnancy | |||||||
| Yes | 86.1 | (1.8) | 84.8 | (1.9) | 83.4 | (2.0) | 0.231 |
| No | 89.0 | (1.9) | 88.2 | (1.9) | - | - | |
| Maternal smoking at diagnosis | |||||||
| Yes | 85.9 | (2.4) | 85.3 | (2.5) | 83.5 | (2.7) | 0.352 |
| No | 88.9 | (1.5) | 87.5 | (1.7) | - | - | |
| Paternal smoking at diagnosis | |||||||
| Yes | 85.9 | (2.2) | 85.0 | (2.3) | 83.6 | (2.4) | 0.327 |
| No | 88.5 | (1.7) | 87.1 | (1.8) | - | - | |
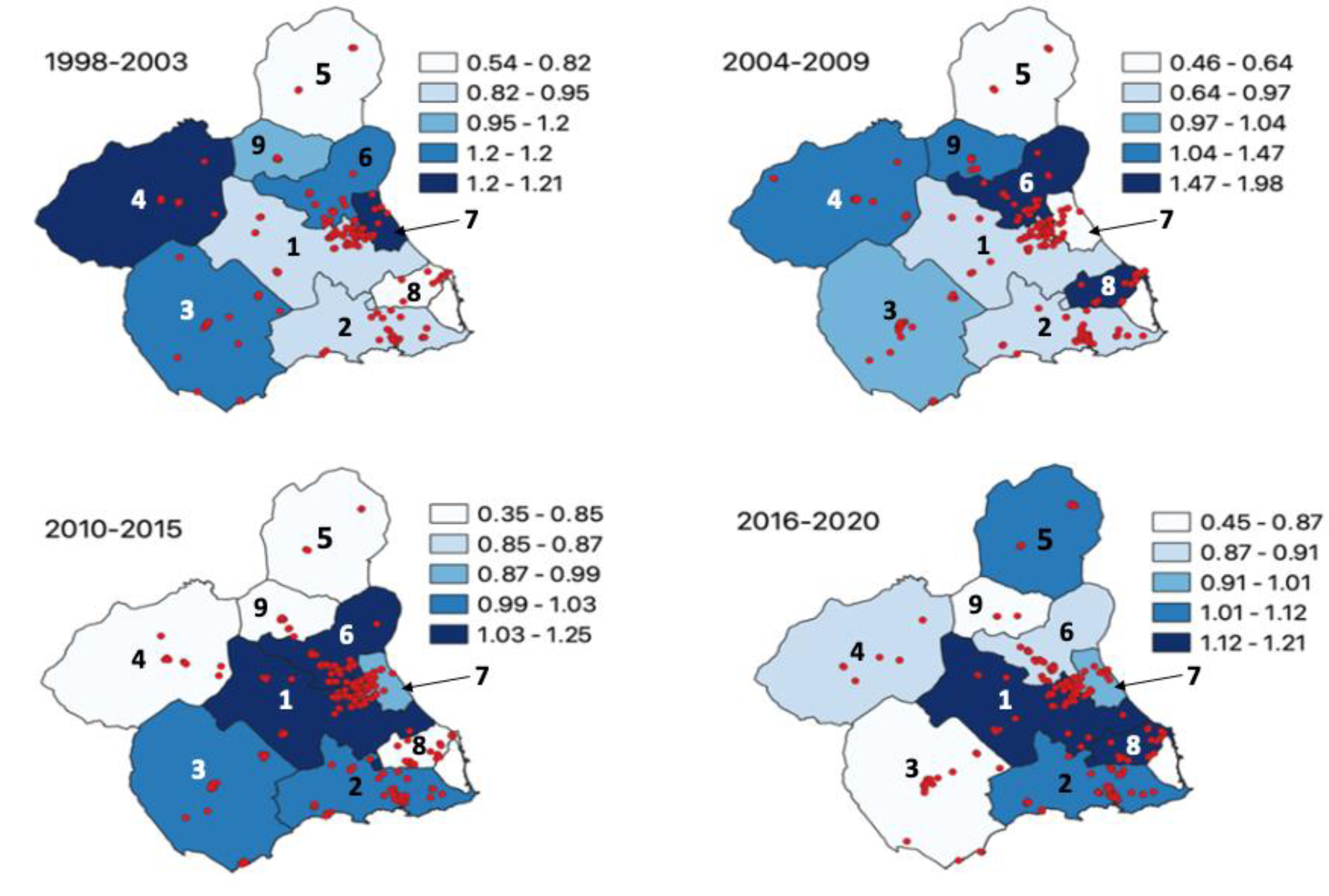
| Cases by Period | CR/ASRw | SIR (CI 95%) by Type and Period | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 98–03 | 04–09 | 10–15 | 16–20 | Total Periods | % | MACAPE MUR 98-20 | Spain 00–16 | S. Europe 01-10 a | 1998–2003 | 2004–2009 | 2010–2015 | 2016–2020 | ||
| Leukemia | 43 | 64 | 72 | 62 | 241 | 29.2 | 43.8 | 46.4/47.5 | /51.1 | 0.81 (0.62–1.05) | 1.01 (0.81–1.24) | 1.04 (0.85–1.26) | 1.12 (0.90–1.39) | |
| ALL | 33 | 51 | 56 | 47 | 187 | 34.0 | 36.4/37.4 | --- | 0.81 (0.59–1.08) | 1.04 (0.81–1.31) | 1.04 (0.82–1.30) | 1.10 (0.85–1.40) | ||
| AML | 8 | 11 | 12 | 12 | 43 | 7.8 | 8.0/8.1 | --- | 0.84 (0.42–1.51) | 0.97 (0.55–1.61) | 0.98 (0.56–1.58) | 1.22 (0.70–1.97) | ||
| Lymphomas | 21 | 12 | 33 | 19 | 85 | 10.3 | 15.4 | 20.2/19.7 | /21.6 | 1.08 (0.73–1.56) | 0.56 (0.32–0.91) | 1.38 (1.01–1.85) | 0.93 (0.61–1.37) | |
| HL | 9 | 5 | 9 | 12 | 35 | 6.4 | 7.4/7.0 | --- | 1.12 (0.59–1.08) | 0.57 (0.81–1.31) | 0.92 (0.82–1.30) | 1.43 (0.85–1.40) | ||
| NHL | 12 | 7 | 24 | 7 | 50 | 9.1 | 6.8/6.7 | --- | 1.06 (0.58–1.95) | 0.56 (0.22–1.20) | 1.70 (1.09–2.54) | 0.59 (0.82–2.31) | ||
| CNST | 41 | 47 | 65 | 42 | 195 | 23.7 | 35.4 | 35.8/36.1 | /37.6 | 0.95 (0.72–1.23) | 0.92 (0.71–1.18) | 1.16 (0.94–1.43) | 0.93 (0.71–1.21) | |
| SNST | 14 | 13 | 27 | 16 | 70 | 8.5 | 12.7 | 13.1/14.0 | /14.5 | 0.92 (0.56–1.44) | 0.68 (0.40–1.08) | 1.33 (0.94–1.84) | 1.04 (0.66–1.59) | |
| Others | 44 | 60 | 72 | 57 | 233 | 28.3 | 42.3 | 41.4/ | --- | --- | --- | --- | --- | |
| TOTAL | 163 | 196 | 269 | 196 | 824 | 100 | 149.6 | 157.0/159.4 | --- | 0.89 (0.78–1.01) | 0.91 (0.80–1.02) | 1.14 (1.03–1.26) | 1.03 (0.92–1.16) | |
| CR | 131.3 | 157.9 | 216.7 | 157.9 | 149.6 | |||||||||
| Incidence Cohort | % Survival (Typical Error) | ||||
|---|---|---|---|---|---|
| 1 Year | 3 Years | 5 Years | 10 Years | ||
| All Cancer (n = 733) | 98–03 | 84.7 (2.8) | 79.1 (3.2) | 76.1 (3.3) | 76.1 (3.3) |
| 04–08 | 83.5 (2.9) | 80.4 (3.2) | 80.4 (3.2) | 79.1 (3.2) | |
| 09–13 | 86.2 (2.3) | 82.5 (2.6) | 82.0 (2.6) | 79.7 (2.8) | |
| 14–18 | 91.8 (2.0) | 87.6 (2.4) | 85.5 (2.6) | --- | |
| 98–18 | 86.8 (1.3) | 82.7 (1.4) | 81.3 (1.4) | 80.1 (1.5) | |
| Leukemia (n = 207) | 98–03 | 83.7 (5.6) | 79.1 (6.2) | 76.7 (6.4) | 76.7 (6.4) |
| 04–08 | 92.2 (3.8) | 92.2 (3.8) | 92.2 (3.8) | 92.2 (3.8) | |
| 09–13 | 83.9 (4.7) | 80.6 (5.0) | 79.0 (5.2) | 75.8 (5.4) | |
| 14–18 | 94.1 (3.3) | 90.2 (4.2) | 90.2 (4.2) | --- | |
| 98–18 | 88.4 (2.2) | 85.5 (2.4) | 84.5 (2.5) | 83.3 (2.6) | |
| CNST (n = 79) | 98–03 | 78.0 (6.5) | 73.2 (6.9) | 68.3 (7.3) | 68.3 (7.3) |
| 04–08 | 71.1 (7.4) | 71.1 (7.4) | 71.1 (7.4) | 68.4 (7.5) | |
| 09–13 | 79.6 (5.5) | 77.8 (5.7) | 77.8 (5.7) | 71.6 (6.9) | |
| 14–18 | 87.0 (5.0) | 78.2 (6.1) | 78.2 (6.1) | --- | |
| 98–18 | 78.0 (6.5) | 73.2 (6.9) | 68.3 (7.3) | 68.3 (7.3) | |
| Lymphoma (n = 79) | 98–03 | 81.0 (8.6) | 81.0 (8.6) | 81.0 (8.6) | 81.0 (8.6) |
| 04–08 | 85.7 (13.2) | 85.7 (13.2) | 85.7 (13.2) | 85.7 (13.2) | |
| 09–13 | 91.3 (5.9) | 91.3 (5.9) | 91.3 (5.9) | 91.3 (5.9) | |
| 14–18 | 96.4 (3.5) | 92.7 (5.0) | 92.7 (5.0) | --- | |
| 98–18 | 89.9 (3.4) | 88.6 (3.6) | 88.6 (3.6) | 88.6 (3.6) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cabrera-Rivera, L.T.; Sweetser, B.; Fuster-Soler, J.L.; Ramis, R.; López-Hernández, F.A.; Pérez-Martínez, A.; Ortega-García, J.A. Looking Towards 2030: Strengthening the Environmental Health in Childhood–Adolescent Cancer Survivor Programs. Int. J. Environ. Res. Public Health 2023, 20, 443. https://doi.org/10.3390/ijerph20010443
Cabrera-Rivera LT, Sweetser B, Fuster-Soler JL, Ramis R, López-Hernández FA, Pérez-Martínez A, Ortega-García JA. Looking Towards 2030: Strengthening the Environmental Health in Childhood–Adolescent Cancer Survivor Programs. International Journal of Environmental Research and Public Health. 2023; 20(1):443. https://doi.org/10.3390/ijerph20010443
Chicago/Turabian StyleCabrera-Rivera, Laura T., Brittney Sweetser, José L. Fuster-Soler, Rebeca Ramis, Fernando A. López-Hernández, Antonio Pérez-Martínez, and Juan A. Ortega-García. 2023. "Looking Towards 2030: Strengthening the Environmental Health in Childhood–Adolescent Cancer Survivor Programs" International Journal of Environmental Research and Public Health 20, no. 1: 443. https://doi.org/10.3390/ijerph20010443
APA StyleCabrera-Rivera, L. T., Sweetser, B., Fuster-Soler, J. L., Ramis, R., López-Hernández, F. A., Pérez-Martínez, A., & Ortega-García, J. A. (2023). Looking Towards 2030: Strengthening the Environmental Health in Childhood–Adolescent Cancer Survivor Programs. International Journal of Environmental Research and Public Health, 20(1), 443. https://doi.org/10.3390/ijerph20010443









