Photosynthesis Responses of Tibetan Freshwater Algae Chlorella vulgaris to Herbicide Glyphosate
Abstract
1. Introduction
2. Materials and Methods
2.1. Algal Culture and Herbicide Treatment
2.2. Determination of Chlorophyll a Fluorescence
2.3. Evaluation of Photosynthetic Pigments
Cb = 20.13A646 − 5.03A663
Cc = (1000A470 − 3.27Ca − 104Cb)/229
2.4. Determination of ROS, SOD Activity and MDA and Protein Contents
2.5. Statistical Analysis
3. Results
3.1. The Effects of Glyphosate on Thephotosynthetic Activity
3.2. The Effects of Glyphosate on the Contents of Photosynthetic Pigments
3.3. The Effects of Glyphosate on Chl a Fluorescence Induction Kinetics
3.4. The Effects of Glyphosate on Antioxidant Systems
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wilson, M.C.; Smith, A.T. The pika and the watershed: The impact of small mammal poisoning on the ecohydrology of the Qinghai-Tibetan Plateau. Ambio 2015, 44, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Dong, L.; Zhao, Y.; Wang, Y. Effect of the Asian Water Tower over the Qinghai-Tibet Plateau and the characteristics of atmospheric water circulation. Chin. Sci. Bull. 2019, 64, 2830–2841. [Google Scholar]
- Chang, K.; Tao, J.; Fang, C.; Li, J.; Zhou, W.; Wang, X.; Yan, B.; Zeng, D.; Chen, G. Evolution of research topics on the Tibetan Plateau environment and ecology from 2000 to 2020: A paper mining. Environ. Sci. Pollut. Res. 2022, 29, 12933–12947. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Yang, L.; Shi, M.; Liu, G. Persistent organic pollutants in typical lake ecosystems. Ecotoxicol. Environ. Saf. 2019, 180, 668–678. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhang, Y. Emergy-Based Evaluation of Changes in Agrochemical Residues on the Qinghai-Tibet Plateau, China. Sustainability 2019, 11, 3652. [Google Scholar] [CrossRef]
- Qiong, P. The current situation, problems and countermeasures for the occurrence and prevention of grassland in farmland in the Tibet Autonomous Region. Tibet. Sci. Technol. 2018, 1, 9–12. [Google Scholar]
- Brovini, E.M.; Cardoso, S.J.; Quadra, G.R.; Vilas-Boas, J.A.; Paranaiba, J.R.; Pereira, R.d.O.; Mendonca, R.F. Glyphosate concentrations in global freshwaters: Are aquatic organisms at risk? Environ. Sci. Pollut. Res. 2021, 28, 60635–60648. [Google Scholar] [CrossRef]
- Shuai, L.; Teng, W.; Ziye, W.; Fengkai, L. Water quality criteria derivation and ecological risk assessment for glyphosate. Asian J. Ecotoxicol. 2022, 1–18. [Google Scholar]
- Fan, J.; Geng, J.; Wang, X. Determination of Glyphosate in Taihu Lake by Ion Chromatography. In Proceedings of the 6th National Conference on Environmental Chemistry, 6th NCEC, Guangzhou, China, 5–8 November 2011. [Google Scholar]
- Sylwestrzak, Z.; Zgrundo, A.; Pniewski, F. Ecotoxicological Studies on the Effect of Roundup(R) (Glyphosate Formulation) on Marine Benthic Microalgae. Int. J. Environ. Res. Public Health 2021, 18, 884. [Google Scholar] [CrossRef]
- Gerdol, M.; Visintin, A.; Kaleb, S.; Spazzali, F.; Pallavicini, A.; Falace, A. Gene expression response of the alga Fucus virsoides (Fucales, Ochrophyta) to glyphosate solution exposure. Environ. Pollut. 2020, 267, 115483. [Google Scholar] [CrossRef]
- Gao, J.; Xiong, L.; Ruan, J.; Zeng, X.; Liang, X. Advances of aquatic environmental behaviors and toxicity of glyphosate to aquatic organisms. Asian J. Ecotoxicol. 2022, 17, 422–433. [Google Scholar]
- Carolina Sasal, M.; Demonte, L.; Cislaghi, A.; Gabioud, E.A.; Oszust, J.D.; Wilson, M.G.; Michlig, N.; Beldomenico, H.R.; Rosa Repetti, M. Glyphosate Loss by Runoff and Its Relationship with Phosphorus Fertilization. J. Agric. Food Chem. 2015, 63, 4444–4448. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Sun, W. Study advances on reproductive toxicity induced by glyphosate. Chin. J. Environ. Occup. Med. 2020, 37, 1230–1238. [Google Scholar]
- Camacho, A.; Mejia, D. The health consequences of aerial spraying illicit crops: The case of Colombia. J. Health Econ. 2017, 54, 147–160. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Liu, L.; Yang, X.; Yang, R.; Liu, H. Spatial-temporal Distribution Characteristics of Community Structure Periphytic Algae and Its Relationship with Physicochemical Factors of Water Environment in the Yarlung Zangbo River Basin. Acta Hydrobiol. Sin. 2022, 46, 1816–1831. [Google Scholar]
- Wang, J.; Li, B.; Feng, J.; Xie, S.-L.; Jia, Q.-X. Distribution and Floristic Characteristics of Algae in the Gongbu Nature Reserve, Tibet. Acta Bot. Boreali-Occident. Sin. 2012, 32, 807–814. [Google Scholar]
- Jie, W.; Bo, L.; Jia, F.; Shullian, X. Characteristics of algal flora and community in the southwestern Tibet. Acta Hydrobiol. Sin. 2015, 39, 837–844. [Google Scholar]
- Cruz de Carvalho, R.; Feijao, E.; Matos, A.R.; Cabrita, M.T.; Utkin, A.B.; Novais, S.C.; Lemos, M.F.L.; Cacador, I.; Marques, J.C.; Reis-Santos, P.; et al. Effects of Glyphosate-Based Herbicide on Primary Production and Physiological Fitness of the Macroalgae Ulva lactuca. Toxics 2022, 10, 430. [Google Scholar] [CrossRef]
- Wang, G.; Chen, L.; Hao, Z.; Li, X.; Liu, Y. Effects of salinity stress on the photosynthesis of Wolffia Arrhiza as probed by the OJIP test. Fresenius Environ. Bull. 2011, 20, 432–438. [Google Scholar]
- Strasser, R.J.; Tsimilli-Michael, M.; Srivastava, A. Analysis of the chlorophyll a fluorescence transient. Chlorophyll A Fluoerescence Signat. Photosynth. 2004, 19, 321–362. [Google Scholar]
- Deng, C.; Shao, H.; Pan, X.; Wang, S.; Zhang, D. Herbicidal effects of harmaline from Peganum harmala on photosynthesis of Chlorella pyrenoidosa: Probed by chlorophyll fluorescence and thermoluminescence. Pestic. Biochem. Physiol. 2014, 115, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Ye, C.; Liao, H.; Yang, Y. Allelopathic inhibition of photosynthesis in the red tide-causing marine alga, Scrippsiella trochoidea (Pyrrophyta), by the dried macroalga, Gracilaria lemaneiformis (Rhodophyta). J. Sea Res. 2014, 90, 10–15. [Google Scholar] [CrossRef]
- Strasser, R.J.; Srivastava, A. Polyphasic Chlorophyll-Alpha Fluorescence Transient in Plants and Cyanobacteria. Photochem. Photobiol. 1995, 61, 32–42. [Google Scholar] [CrossRef]
- Appenroth, K.J.; Stockel, J.; Srivastava, A.; Strasser, R.J. Multiple effects of chromate on the photosynthetic apparatus of Spirodela polyrhiza as probed by OJIP chlorophyll a fluorescence measurements. Environ. Pollut. 2001, 115, 49–64. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Ye, T.; Li, X.; Bian, P.; Liu, Y.; Wang, G. Survival of desert algae Chlorella exposed to Mars-like near space environment. Life Sci. Space Res. 2021, 29, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Zhao, K.; Li, Z.; He, D. Effect of Salinity Rising of Inland Water on Physioloqical Characteristics of Chlorella vulgaris. Curr. Biotechnol. 2022, 12, 894–899. [Google Scholar]
- Almutairi, A.W.; El-Sayed, E.; Marwa, R.M. Evaluation of high salinity adaptation for lipid bio-accumulation in the green microalga Chlorella vulgaris. Saudi J. Biol. Sci. 2021, 28, 3981–3988. [Google Scholar] [CrossRef]
- Pan, W.W.Y.; Liu, Y.; Li, X. Effects of glyphosate on the growth and chlorophyII fluorescence induction dynamics of Microcystis aeruginosa PCC 7806. J. Henan Norm. Univ. 2018, 46, 89–94. [Google Scholar]
- Zhang, L.; Duan, S.; Kaifeng, S.; Xiaojia, Q. Hormesis effect of organophosphorus pesticide Glyphosate-isopropylammonium on Phaeocystis globosa. Ecol. Environ. 2010, 19, 51–56. [Google Scholar]
- Carlisle, S.M.; Trevors, J.T.; Cong, T. Glyphosate in the environment. World Pestic. 1990, 3, 409–420. [Google Scholar] [CrossRef]
- Zhang, C.; Xie, M.; Chen, J.; Zhang, Y.; Wei, S.; Ma, X.; Xiao, L.; Chen, L. UV-B radiation induces DEHP degradation and their combined toxicological effects on Scenedesmus acuminatus. Aquat. Toxicol. 2018, 203, 172–178. [Google Scholar] [CrossRef] [PubMed]
- Wellburn, A.R. The spectral determination of chlorophyll-A and chlorophhyll-B, as well as total carotenoids, using various solvents with spectrophotometers of different resolution. J. Plant Physiol. 1994, 144, 307–313. [Google Scholar] [CrossRef]
- Ye, T.; Wang, B.; Li, C.; Bian, P.; Chen, L.; Wang, G. Exposure of cyanobacterium Nostoc sp. to the Mars-like stratosphere environment. J. Photochem. Photobiol. B Biol. 2021, 224, 112307. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Ye, T.; Li, C.; Li, X.; Chen, L.; Wang, G. Cell damage repair mechanism in a desert green algae Chlorella sp. against UV-B radiation. Ecotoxicol. Environ. Saf. 2022, 242, 113916. [Google Scholar] [CrossRef]
- Chen, L.; Xie, M.; Bi, Y.; Wang, G.; Deng, S.; Liu, Y. The combined effects of UV-B radiation and herbicides on photosynthesis, antioxidant enzymes and DNA damage in two bloom-forming cyanobacteria. Ecotoxicol. Environ. Saf. 2012, 80, 224–230. [Google Scholar] [CrossRef]
- Ostera, J.M.; Puntarulo, S.; Malanga, G. Oxidative effects of glyphosate on the lipophobic intracellular environment in the microalgae Chlorella vulgaris. Biocell 2022, 46, 795–802. [Google Scholar] [CrossRef]
- Mercedes Iummato, M.; Fassiano, A.; Graziano, M.; Afonso, M.d.S.; Rios de Molina, M.d.C.; Beatriz Juarez, A. Effect of glyphosate on the growth, morphology, ultrastructure and metabolism of Scenedesmus vacuolatus. Ecotoxicol. Environ. Saf. 2019, 172, 471–479. [Google Scholar] [CrossRef]
- Smedbol, E.; Lucotte, M.; Labrecque, M.; Lepage, L.; Juneau, P. Phytoplankton growth and PSII efficiency sensitivity to a glyphosate-based herbicide (Factor 540 (R)). Aquat. Toxicol. 2017, 192, 265–273. [Google Scholar] [CrossRef]
- Xiao, W.; Wang, H.; Liu, W.; Wang, X.; Guo, Y.; Strasser, R.J.; Qiang, S.; Chen, S.; Hu, Z. Action of alamethicin in photosystem II probed by the fast chlorophyll fluorescence rise kinetics and the JIP-test. Photosynthetica 2020, 58, 358–368. [Google Scholar] [CrossRef]
- Krause, G.H.; Weis, E. Chlorophyll Fluorescence and Photosynthesis: The Basics. Annu. Rev. Plant Physiol. 1991, 42, 313–349. [Google Scholar] [CrossRef]
- Qu, M.; Wang, L.; Xu, Q.; An, J.; Mei, Y.; Liu, G. Influence of glyphosate and its metabolite aminomethylphosphonic acid on aquatic plants in different ecological niches. Ecotoxicol. Environ. Saf. 2022, 246, 114155. [Google Scholar] [CrossRef] [PubMed]
- Dabney, B.L.; Patino, R. Low-dose stimulation of growth of the harmful alga, Prymnesium parvum, by glyphosate and glyphosate-based herbicides. Harmful Algae 2018, 80, 130–139. [Google Scholar] [CrossRef]
- Xia, J.R.; Li, Y.J.; Zou, D.H. Effects of salinity stress on PSII in Ulva lactuca as probed by chlorophyll fluorescence measurements. Aquat. Bot. 2004, 80, 129–137. [Google Scholar] [CrossRef]
- Lu, C.M.; Qiu, N.W.; Wang, B.S.; Zhang, J.H. Salinity treatment shows no effects on photosystem II photochemistry, but increases the resistance of photosystem II to heat stress in halophyte Suaeda salsa. J. Exp. Bot. 2003, 54, 851–860. [Google Scholar] [CrossRef] [PubMed]
- Singh, D.V.; Bhat, R.A.; Upadhyay, A.K.; Singh, R.; Singh, D.P. Microalgae in aquatic environs: A sustainable approach for remediation of heavy metals and emerging contaminants. Environ. Technol. Innov. 2021, 21, 101340. [Google Scholar] [CrossRef]
- Maroli, A.S.; Nandula, V.K.; Dayan, F.E.; Duke, S.O.; Gerard, P.; Tharayil, N. Metabolic Profiling and Enzyme Analyses Indicate a Potential Role of Antioxidant Systems in Complementing Glyphosate Resistance in an Amaranthus palmeri Biotype. J. Agric. Food Chem. 2015, 63, 9199–9209. [Google Scholar] [CrossRef]
- Falace, A.; Tamburello, L.; Guarnieri, G.; Kaleb, S.; Papa, L.; Fraschetti, S. Effects of a glyphosate-based herbicide on Fucus virsoides (Fucales, Ochrophyta) photosynthetic efficiency. Environ. Pollut. 2018, 243, 912–918. [Google Scholar] [CrossRef] [PubMed]
- Mercurio, P.; Flores, F.; Mueller, J.F.; Carter, S.; Negri, A.P. Glyphosate persistence in seawater. Mar. Pollut. Bull. 2014, 85, 385–390. [Google Scholar] [CrossRef]
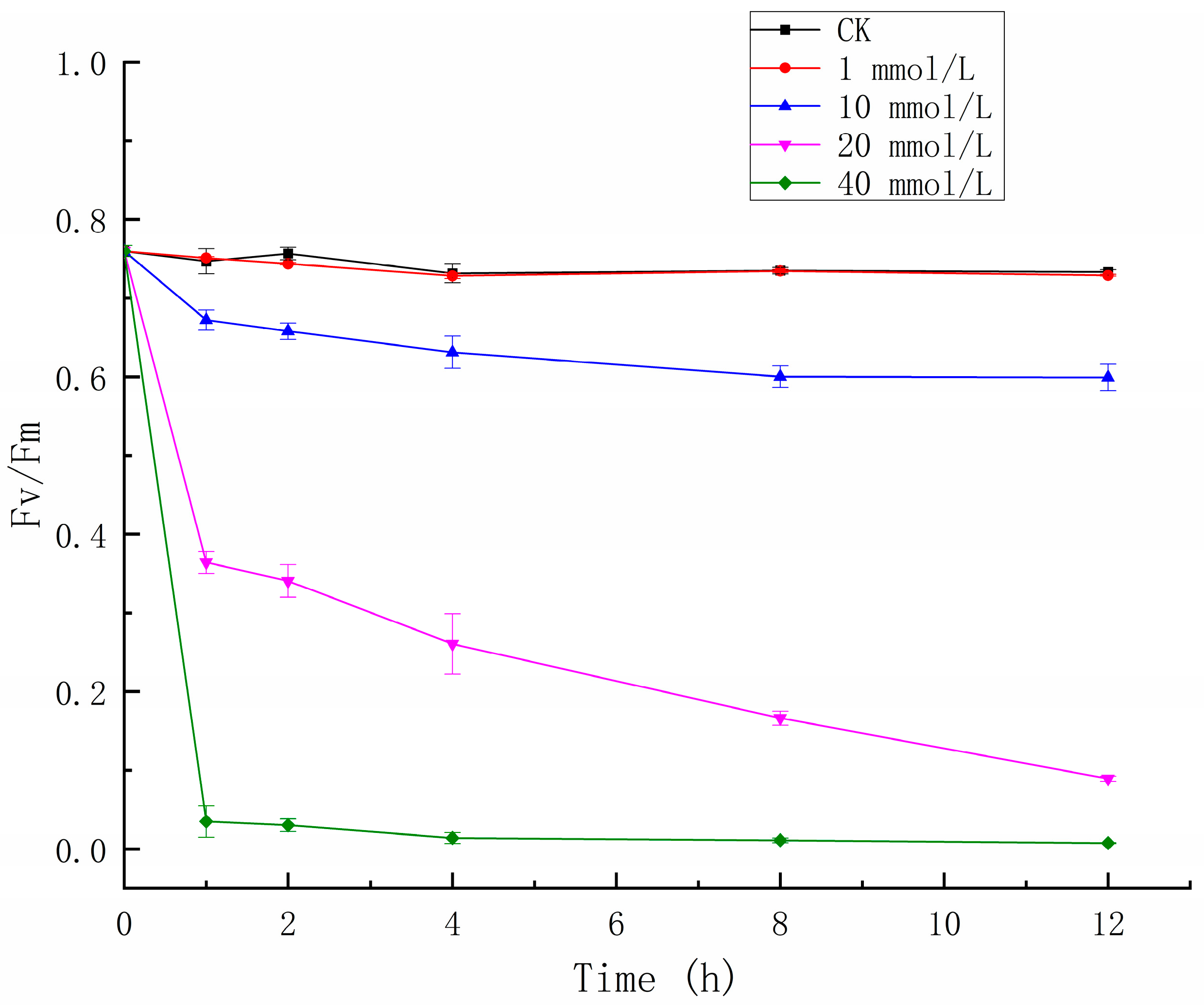

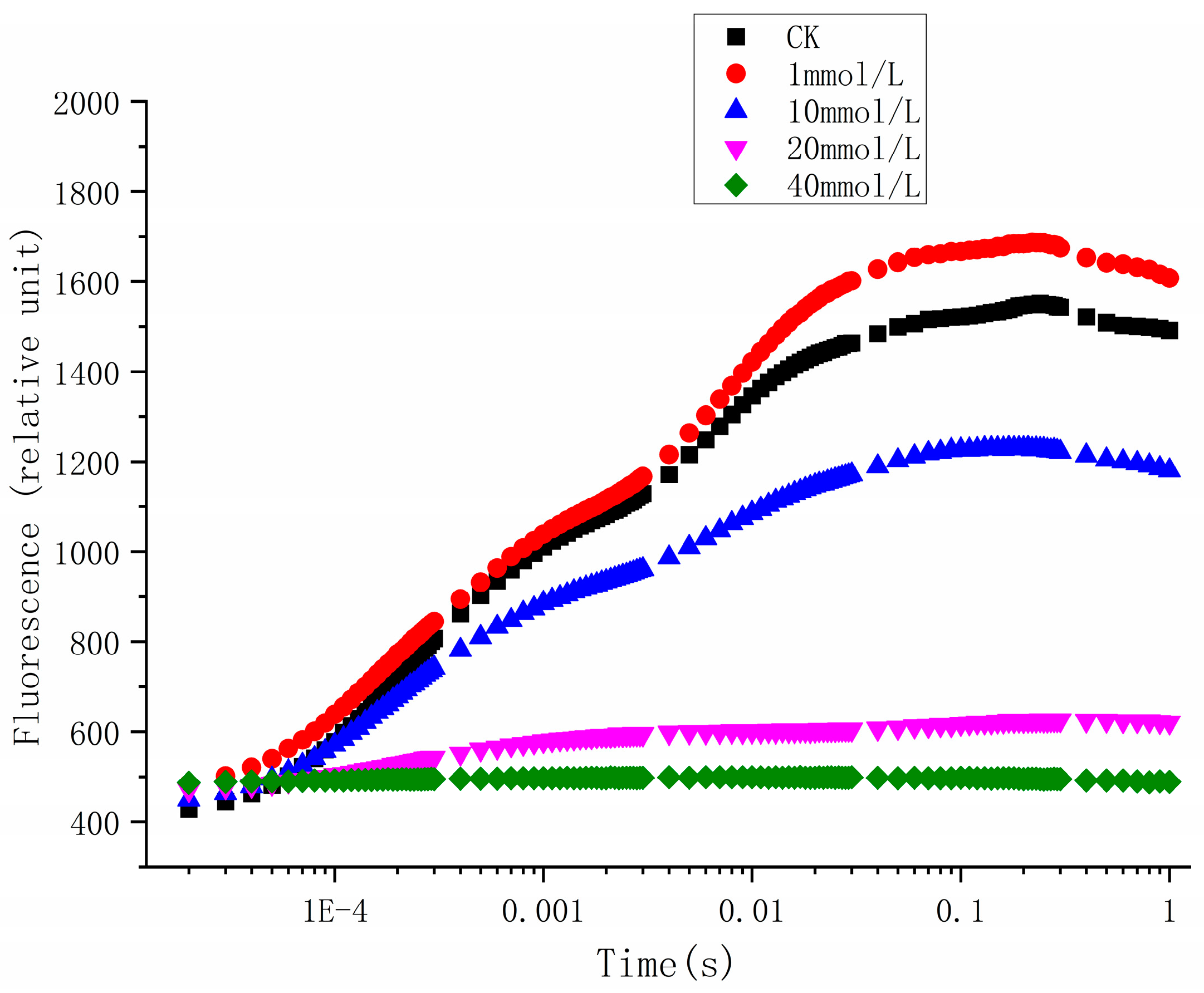

| Parameters | CK | 1 mmol/L | 10 mmol/L | 20 mmol/L | 40 mmol/L |
|---|---|---|---|---|---|
| VJ | 0.595 ± 0.004 | 0.528 ± 0.006 * | 0.734 ± 0.090 * | 0.765 ± 0.012 * | 0.956 ± 0.255 * |
| Mo | 1.425 ± 0.015 | 1.228 ± 0.028 * | 1.840 ± 0.247 * | 1.880 ± 0.021 * | 2.915 ± 0.335 * |
| ABS/RC | 2.729 ± 0.049 | 2.663 ± 0.035 | 3.311 ± 0.175 * | 7.954 ± 1.030 * | 242.425 ± 128.689 * |
| TRo/RC | 1.996 ± 0.008 | 1.940 ± 0.022 | 2.088 ± 0.044 * | 2.048 ± 0.053 * | 2.642 ± 0.668 * |
| ETo/RC | 0.808 ± 0.006 | 0.918 ± 0.003 * | 0.611 ± 0.216 | 0.499 ± 0.025 * | 0.569 ± 0.138 * |
| DIo/RC | 0.732 ± 0.045 | 0.723 ± 0.016 | 1.223 ± 0.134 * | 5.906 ± 1.058 * | 239.783 ± 128.293 * |
| φp0 | 0.732 ± 0.012 | 0.728 ± 0.003 | 0.631 ± 0.021 * | 0.261 ± 0.039 * | 0.013 ± 0.008 * |
| φE0 | 0.296 ± 0.006 | 0.344 ± 0.005 * | 0.169 ± 0.062 * | 0.061 ± 0.011 * | 0.001 ± 0.003 * |
| φD0 | 0.268 ± 0.012 | 0.272 ± 0.003 | 0.369 ± 0.021 * | 0.739 ± 0.039 * | 0.987 ± 0.008 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Chen, Z.; Li, X.; Wu, X.; Chen, L.; Wang, G. Photosynthesis Responses of Tibetan Freshwater Algae Chlorella vulgaris to Herbicide Glyphosate. Int. J. Environ. Res. Public Health 2023, 20, 386. https://doi.org/10.3390/ijerph20010386
Zhang Y, Chen Z, Li X, Wu X, Chen L, Wang G. Photosynthesis Responses of Tibetan Freshwater Algae Chlorella vulgaris to Herbicide Glyphosate. International Journal of Environmental Research and Public Health. 2023; 20(1):386. https://doi.org/10.3390/ijerph20010386
Chicago/Turabian StyleZhang, Yixiao, Zixu Chen, Xiaoyan Li, Xinguo Wu, Lanzhou Chen, and Gaohong Wang. 2023. "Photosynthesis Responses of Tibetan Freshwater Algae Chlorella vulgaris to Herbicide Glyphosate" International Journal of Environmental Research and Public Health 20, no. 1: 386. https://doi.org/10.3390/ijerph20010386
APA StyleZhang, Y., Chen, Z., Li, X., Wu, X., Chen, L., & Wang, G. (2023). Photosynthesis Responses of Tibetan Freshwater Algae Chlorella vulgaris to Herbicide Glyphosate. International Journal of Environmental Research and Public Health, 20(1), 386. https://doi.org/10.3390/ijerph20010386










