The Effect of Mealworm Frass on the Chemical and Microbiological Properties of Horticultural Peat in an Incubation Experiment
Abstract
1. Introduction
2. Materials and Methods
2.1. Characterization of Frass and Horticultural Peat
2.2. Experimental Design
2.3. Chemical Analysis of Frass and Horticultural Substrate
2.4. Quantitative PCR of Microbial Functional Genes
2.5. Statistical Analysis
3. Results
3.1. Chemical Changes in Deacidified Peat
3.2. Microbiological Changes in Deacidified Peat
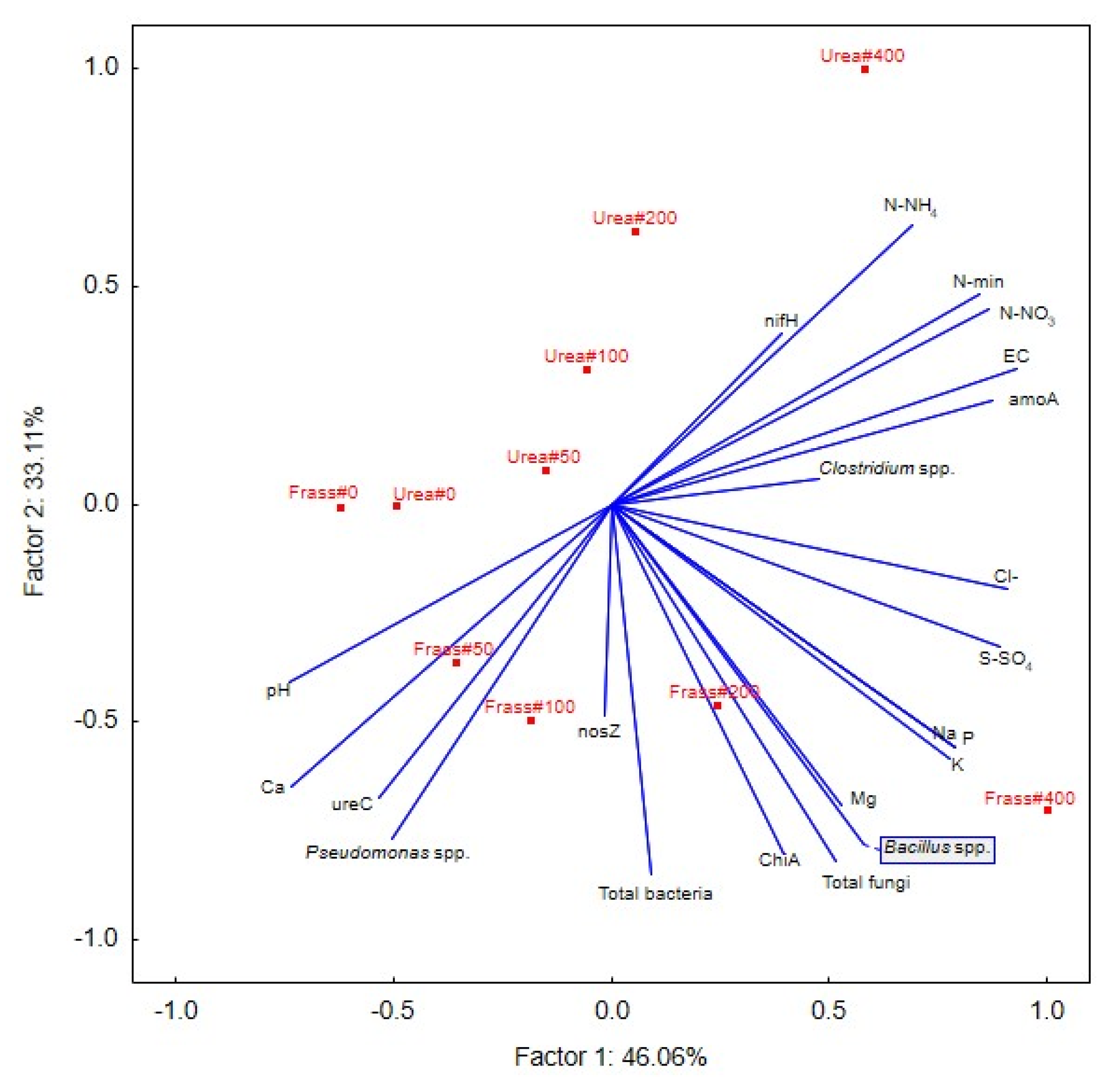
3.3. Relationships between the Chemical and Microbiological Parameters of Peat
4. Discussion
4.1. Chemical Changes in Deacidified Peat
4.2. The Effect of Frass and Urea on Functional Microorganisms in the Horticultural Substrate
4.3. The Relationship between the Chemical and Microbiological Parameters of Peat Fertilized with Different Nitrogen Rates
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- van Huis, A.; van Itterbeeck, J.; Klunder, H.; Mertens, E.; Halloran, A.; Muir, G.; Vantomme, P. Edible Insects: Future Prospects for Food and Feed Security; Food and Agriculture Organization of the United Nations: Rome, Italy, 2013; Volume 97, ISBN 9789251075951. [Google Scholar]
- Van Huis, A.; Dicke, M.; van Loon, J.J.A. Insects to Feed the World. J Insects Food Feed 2015, 1, 3–5. [Google Scholar] [CrossRef]
- FAO. Assessing the Potential of Insects as Food and Feed in Assuring Food Security. In Proceedings of the Action Plan Technical Consultation Meeting, Rome, Italy, 23–25 January 2012. [Google Scholar]
- Van Huis, A.; Halloran, A.; van Itterbeeck, J.; Klunder, H.; Vantomme, P. How Many People on Our Planet Eat Insects: 2 Billion? J. Insects Food Feed 2022, 8, 1–4. [Google Scholar] [CrossRef]
- Siemianowska, E.; Kosewska, A.; Aljewicz, M.; Skibniewska, K.A.; Polak-Juszczak, L.; Jarocki, A.; Jędras, M. Larvae of Mealworm (Tenebrio Molitor L.) as European Novel Food. Agric. Sci. 2013, 4, 287–291. [Google Scholar] [CrossRef]
- Raheem, D.; Carrascosa, C.; Oluwole, O.B.; Nieuwland, M.; Saraiva, A.; Millán, R.; Raposo, A. Traditional Consumption of and Rearing Edible Insects in Africa, Asia and Europe. Crit. Rev. Food Sci. Nutr. 2019, 59, 2169–2188. [Google Scholar] [CrossRef] [PubMed]
- Collins, C.M.; Vaskou, P.; Kountouris, Y. Insect Food Products in the Western World: Assessing the Potential of a New “Green” Market. Ann. Entomol. Soc. Am. 2019, 112, 518–528. [Google Scholar] [CrossRef]
- van Huis, A.; Rumpold, B.A.; van der Fels-Klerx, H.J.; Tomberlin, J.K. Advancing Edible Insects as Food and Feed in a Circular Economy. J. Insects Food Feed 2021, 7, 935–948. [Google Scholar] [CrossRef]
- The European Commission. Commission Regulation (EU) 2017/893 of 24 May 2017 Amending Annexes I and IV to Regulation (EC) No 999/2001 of the European Parliament and of the Council and Annexes X, XIV and XV to Commission Regulation (EU) No 142/2011 as Regards the Provisions on Processed Animal Protein. Off. J. Eur. Union 2017, 138, 92–116. [Google Scholar]
- Commission Implementing Regulation (EU) 2021/882 of 1 June 2021 Authorising the Placing on the Market of Dried Tenebrio Molitor Larva as a Novel Food under Regulation (EU) 2015/2283 of the European Parliament and of the Council, and Amending Commission Implementing Regulation (EU) 2017/2470. Off. J. Eur. Union 2021, 194, 16–20.
- Oonincx, D.G.A.B.; van Itterbeeck, J.; Heetkamp, M.J.W.; van den Brand, H.; van Loon, J.J.A.; van Huis, A. An Exploration on Greenhouse Gas and Ammonia Production by Insect Species Suitable for Animal or Human Consumption. PLoS ONE 2010, 5, e14445. [Google Scholar] [CrossRef]
- Premalatha, M.; Abbasi, T.; Abbasi, T.; Abbasi, S.A. Energy-Efficient Food Production to Reduce Global Warming and Ecodegradation: The Use of Edible Insects. Renew. Sustain. Energy Rev. 2011, 15, 4357–4360. [Google Scholar] [CrossRef]
- Oonincx, D.G.A.B.; de Boer, I.J.M. Environmental Impact of the Production of Mealworms as a Protein Source for Humans—A Life Cycle Assessment. PLoS ONE 2012, 7, e51145. [Google Scholar] [CrossRef] [PubMed]
- Bueschke, M.; Kulczyński, B.; Gramza-Michałowska, A. Owady Jadalne w Żywieniu Człowieka (Edible Insects in Human Nutrition). Zeszyty Naukowe SGGW. Probl. Rol. Swiat. 2017, 17, 49–53. [Google Scholar]
- De Carvalho, N.M.; Walton, G.E.; Poveda, C.G.; Silva, S.N.; Amorim, M.; Madureira, A.R.; Pintado, M.E.; Gibson, G.R.; Jauregi, P. Study of in Vitro Digestion of Tenebrio Molitor Flour for Evaluation of Its Impact on the Human Gut Microbiota. J. Funct. Foods 2019, 59, 101–109. [Google Scholar] [CrossRef]
- Bordiean, A.; Krzyżaniak, M.; Stolarski, M.J.; Czachorowski, S.; Peni, D. Will Yellow Mealworm Become a Source of Safe Proteins for Europe? Agriculture 2020, 10, 233. [Google Scholar] [CrossRef]
- Hong, J.; Han, T.; Kim, Y.Y. Mealworm (Tenebrio Molitor Larvae) as an Alternative Protein Source for Monogastric Animal: A Review. Animals 2020, 10, 2068. [Google Scholar] [CrossRef]
- Gałęcki, R.; Zielonka, Ł.; Zasȩpa, M.; Gołȩbiowska, J.; Bakuła, T. Potential Utilization of Edible Insects as an Alternative Source of Protein in Animal Diets in Poland. Front. Sustain. Food Syst. 2021, 5, 675796. [Google Scholar] [CrossRef]
- Przemieniecki, S.W.; Kosewska, A.; Purwin, C.; Zapałowska, A.; Mastalerz, J.; Kotlarz, K.; Kolaczek, K. Biometric, Chemical, and Microbiological Evaluation of Common Wheat (Triticum aestivum L.) Seedlings Fertilized with Mealworm (Tenebrio Molitor L.) Larvae Meal. Appl. Soil Ecol. 2021, 167, 104037. [Google Scholar] [CrossRef]
- Houben, D.; Daoulas, G.; Faucon, M.P.; Dulaurent, A.M. Potential Use of Mealworm Frass as a Fertilizer: Impact on Crop Growth and Soil Properties. Sci. Rep. 2020, 10, 4659. [Google Scholar] [CrossRef]
- Houben, D.; Daoulas, G.; Dulaurent, A.M. Assessment of the Short-Term Fertilizer Potential of Mealworm Frass Using a Pot Experiment. Front. Sustain. Food Syst. 2021, 5, 714596. [Google Scholar] [CrossRef]
- Frost, C.J.; Hunter, M.D. Insect Canopy Herbivory and Frass Deposition Affect Soil Nutrient Dynamics and Export in Oak Mesocosms. Ecology 2004, 85, 3335–3347. [Google Scholar] [CrossRef]
- Kagata, H.; Ohgushi, T. Positive and Negative Impacts of Insect Frass Quality on Soil Nitrogen Availability and Plant Growth. Popul. Ecol. 2012, 54, 75–82. [Google Scholar] [CrossRef]
- Halloran, A.; Roos, N.; Eilenberg, J.; Cerutti, A.; Bruun, S. Life Cycle Assessment of Edible Insects for Food Protein: A Review. Agron. Sustain. Dev. 2016, 36, 57. [Google Scholar] [CrossRef] [PubMed]
- Poveda, J.; Jiménez-Gómez, A.; Saati-Santamaría, Z.; Usategui-Martín, R.; Rivas, R.; García-Fraile, P. Mealworm Frass as a Potential Biofertilizer and Abiotic Stress Tolerance-Inductor in Plants. Appl. Soil Ecol. 2019, 142, 110–122. [Google Scholar] [CrossRef]
- Kagata, H.; Ohgushi, T. Ingestion and Excretion of Nitrogen by Larvae of a Cabbage Armyworm: The Effects of Fertilizer Application. Agric. For. Entomol. 2011, 13, 143–148. [Google Scholar] [CrossRef]
- Lovett, G.M.; Ruesink, A.E. Carbon and Nitrogen Mineralization from Decomposing Gypsy Moth Frass. Oecologia 1995, 104, 133–138. [Google Scholar] [CrossRef] [PubMed]
- Gessner, M.O.; Swan, C.M.; Dang, C.K.; McKie, B.G.; Bardgett, R.D.; Wall, D.H.; Hättenschwiler, S. Diversity Meets Decomposition. Trends Ecol. Evol. 2010, 25, 372–380. [Google Scholar] [CrossRef] [PubMed]
- Berggren, Å.; Jansson, A.; Low, M. Approaching Ecological Sustainability in the Emerging Insects-as-Food Industry. Trends Ecol. Evol. 2019, 34, 132–138. [Google Scholar] [CrossRef] [PubMed]
- Nowosielski, O. Metody Oznaczania Potrzeb Nawozowych (Methods of Determining Fertilizer Requirements), 2nd ed.; PWRiL: Warszawa, Poland, 1974. [Google Scholar]
- Yu, Y.; Lee, C.; Kim, J.; Hwang, S. Group-Specific Primer and Probe Sets to Detect Methanogenic Communities Using Quantitative Real-Time Polymerase Chain Reaction. Biotechnol. Bioeng. 2005, 89, 670–679. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Liu, C.; Finegold, S.M. Real-Time PCR Quantitation of Clostridia in Feces of Autistic Children. Appl. Environ. Microbiol. 2004, 70, 6459–6465. [Google Scholar] [CrossRef]
- Hu, J.; Wei, Z.; Friman, V.-P.P.; Gu, S.H.; Wang, X.F.; Eisenhauer, N.; Yang, T.J.; Ma, J.; Shen, Q.R.; Xu, Y.C.; et al. Probiotic Diversity Enhances Rhizosphere Microbiome Function And. mBio 2016, 7, e01790-16. [Google Scholar] [CrossRef]
- Liu, C.M.; Kachur, S.; Dwan, M.G.; Abraham, A.G.; Aziz, M.; Hsueh, P.R.; Huang, Y.T.; Busch, J.D.; Lamit, L.J.; Gehring, C.A.; et al. FungiQuant: A Broad-Coverage Fungal Quantitative Real-Time PCR Assay. BMC Microbiol 2012, 12, 255. [Google Scholar] [CrossRef] [PubMed]
- Mora, I.; Cabrefiga, J.; Montesinos, E. Antimicrobial Peptide Genes in Bacillus Strains from Plant Environments. Int. Microbiol. 2011, 14, 213–223. [Google Scholar] [CrossRef] [PubMed]
- Rotthauwe, J.H.; Witzel, K.P.; Liesack, W. The Ammonia Monooxygenase Structural Gene Amoa as a Functional Marker: Molecular Fine-Scale Analysis of Natural Ammonia-Oxidizing Populations. Appl. Environ. Microbiol. 1997, 63, 4704–4712. [Google Scholar] [CrossRef] [PubMed]
- Rösch, C.; Bothe, H. Improved Assessment of Denitrifying, N2-Fixing, and Total-Community Bacteria by Terminal Restriction Fragment Length Polymorphism Analysis Using Multiple Restriction Enzymes. Appl. Environ. Microbiol. 2005, 71, 2026–2035. [Google Scholar] [CrossRef]
- Michotey, V.; Méjean, V.; Bonin, P. Comparison of Methods for Quantification of Cytochrome Cd1-Denitrifying Bacteria in Environmental Marine Samples. Appl. Environ. Microbiol. 2000, 66, 1564–1571. [Google Scholar] [CrossRef] [PubMed]
- Throbäck, I.N.; Enwall, K.; Jarvis, Å.; Hallin, S. Reassessing PCR Primers Targeting NirS, NirK and NosZ Genes for Community Surveys of Denitrifying Bacteria with DGGE. FEMS Microbiol. Ecol. 2004, 49, 401–417. [Google Scholar] [CrossRef]
- Kloos, K.; Mergel, A.; Rösch, C.; Botthe, H. Denitrification within the Genus Azospirillum and Other Associative Bacteria. Funct. Plant Biol. 2001, 28, 991–998. [Google Scholar] [CrossRef]
- Koper, T.E.; El-sheikh, A.F.; Norton, J.M.; Klotz, M.G. Urease-Encoding Genes in Ammonia-Oxidizing Bacteria. Appl. Environ. Microbiol. Environ. Microbiol 2004, 70, 2342–2348. [Google Scholar] [CrossRef]
- Przemieniecki, S.W.; Kosewska, A.; Kosewska, O.; Purwin, C.; Lipiński, K.; Ciesielski, S. Polyethylene, Polystyrene and Lignocellulose Wastes as Mealworm (Tenebrio Molitor L.) Diets and Their Impact on the Breeding Condition, Biometric Parameters, Metabolism, and Digestive Microbiome. Sci. Total Environ. 2022, 832, 154758. [Google Scholar] [CrossRef]
- TIBCO Software Inc. STATISTICA (Data Analysis Software System); TIBCO Software Inc.: Palo Alto, CA, USA, 2017. [Google Scholar]
- Iman, R.L.; Davenport, J.M. Approximations of the Critical Region of the Friedman Statistic. Commun. Stat. 1980, 9, 571–595. [Google Scholar] [CrossRef]
- Breś, W.; Golcz, A.; Komosa, A.; Kozik, E. Żywienie Roślin Ogrodniczych. Podstawy i Perspektywy (Nutritional Requirements of Horticultural Plants. Introduction and Prospects); Komosa, A., Ed.; PWRiL: Poznań, Poland, 2012. [Google Scholar]
- Kowalska, I. Przemiany Azotu w Podłożach z Uprawą Pomidora Szklarniowego (Nitrogen Conversion in Substrates for Greenhouse-Grown Tomatoes). Zesz. Nauk. Akad. Rol. W Krakowie. Ogrod. 1997, 23, 81–91. [Google Scholar]
- Poveda, J. Insect Frass in the Development of Sustainable Agriculture. A Review. Agron Sustain Dev 2021, 41, 5. [Google Scholar] [CrossRef]
- Przemieniecki, S.W.; Zapałowska, A.; Skwiercz, A.; Damszel, M.; Telesiński, A.; Sierota, Z.; Gorczyca, A. An Evaluation of Selected Chemical, Biochemical, and Biological Parameters of Soil Enriched with Vermicompost. Environ. Sci. Pollut. Res. 2021, 28, 8117–8127. [Google Scholar] [CrossRef] [PubMed]
| Fertilizer | Nitrogen Rates (mg dm−3) | |||||
|---|---|---|---|---|---|---|
| 0 | 50 | 100 | 200 | 400 | Mean | |
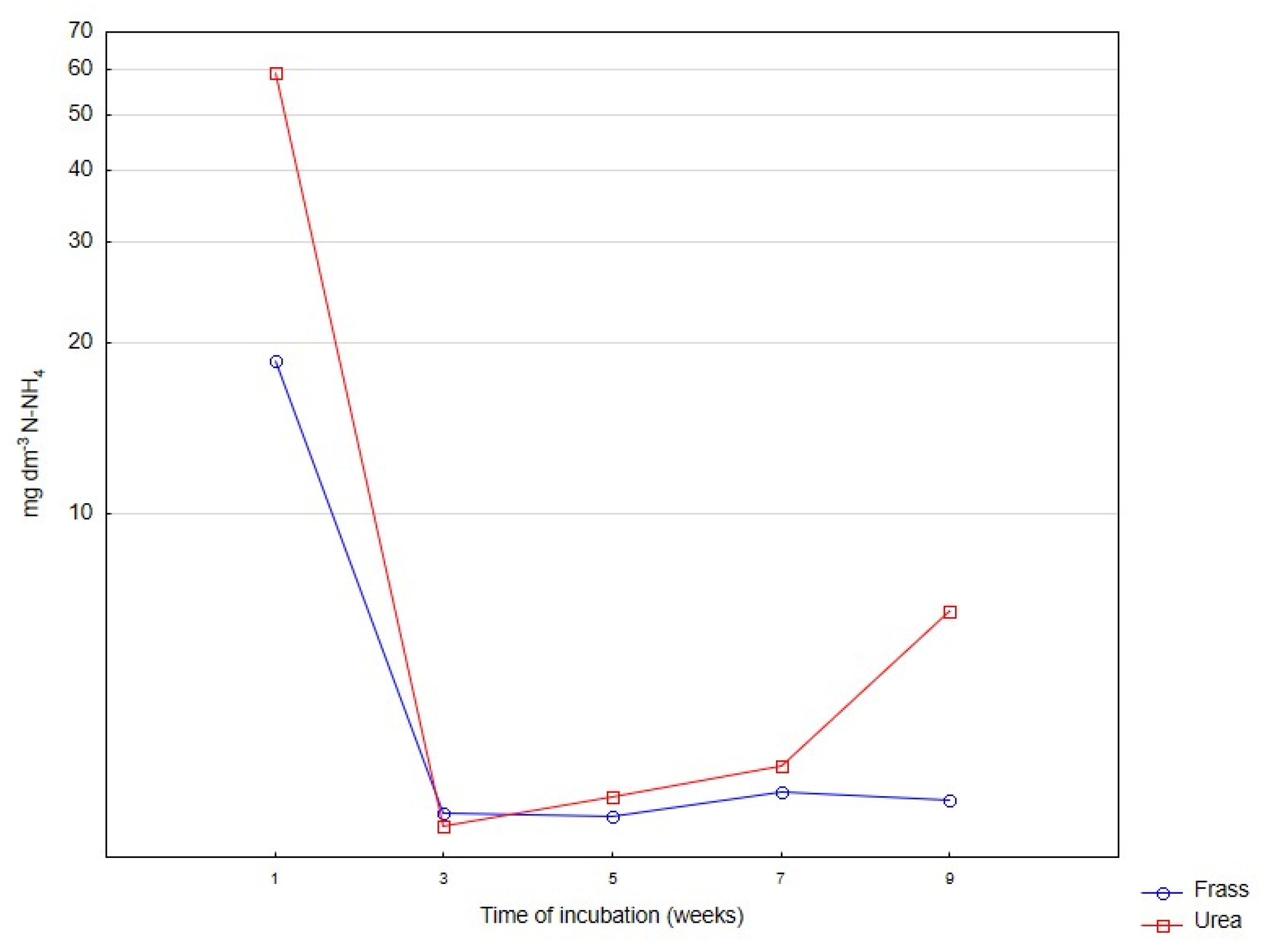
| N-NH4 | ||||||
| Frass | 2.11 a | 2.36 a | 3.53 b | 6.42 c | 16.45 d | 6.17 |
| Urea | 2.40 a | 8.00 b | 10.35 c | 13.54 d | 41.69 e | 15.19 |
| Significant | ns | * | * | ns | ns | *** |
| Mean | 2.25 A | 5.18 B | 6.94 C | 9.98 D | 29.07 E | |
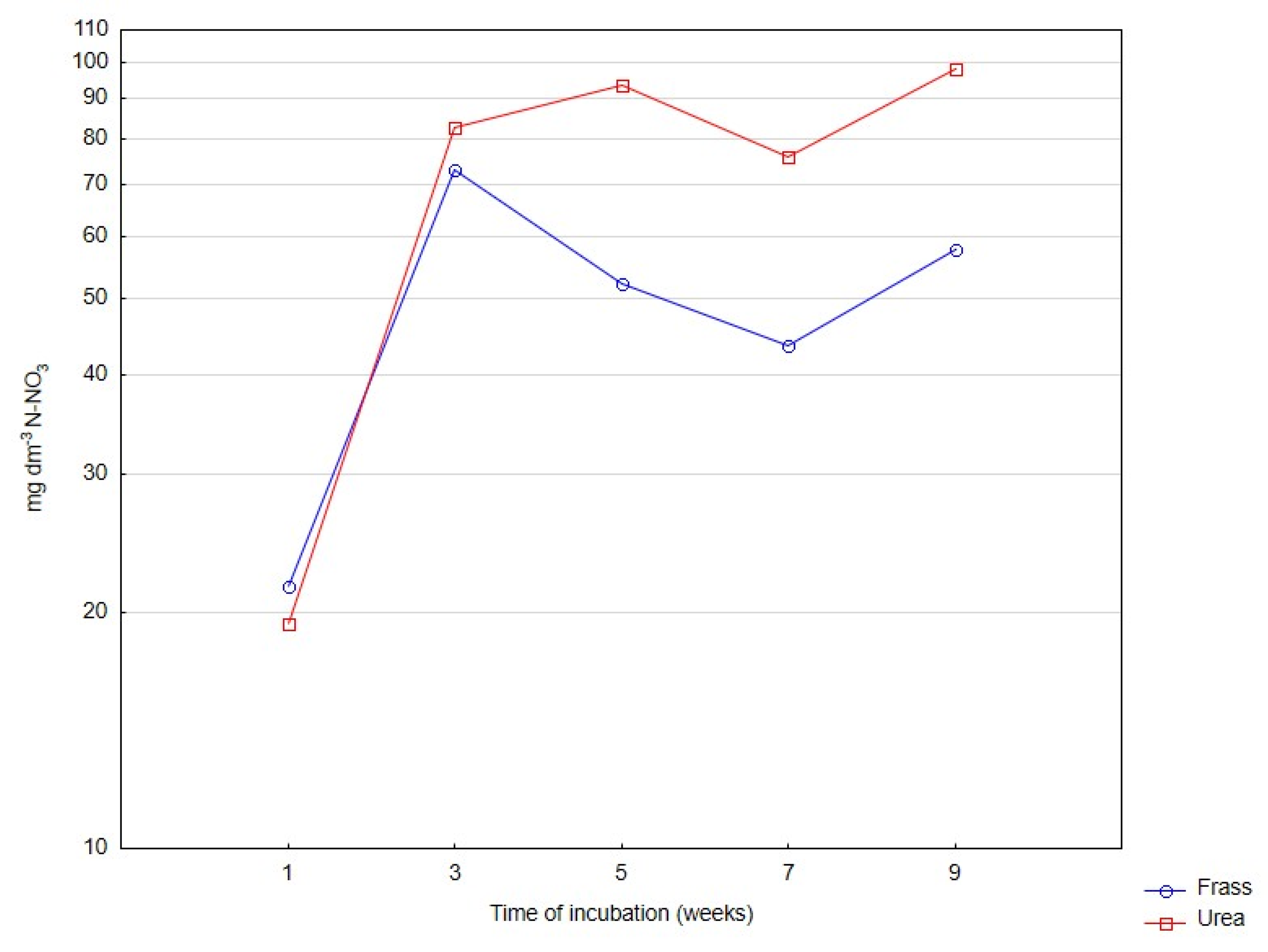
| N-NO3 | ||||||
| Frass | 7.90 a | 16.34 b | 28.27 c | 72.96 d | 122.90 e | 49.7 |
| Urea | 3.95 a | 26.67 b | 58.31 c | 92.74 d | 188.05 e | 73.9 |
| Significant | ns | * | * | * | * | ns |
| Mean | 5.93 A | 21.51 B | 43.29 C | 82.85 D | 155.47 E | |
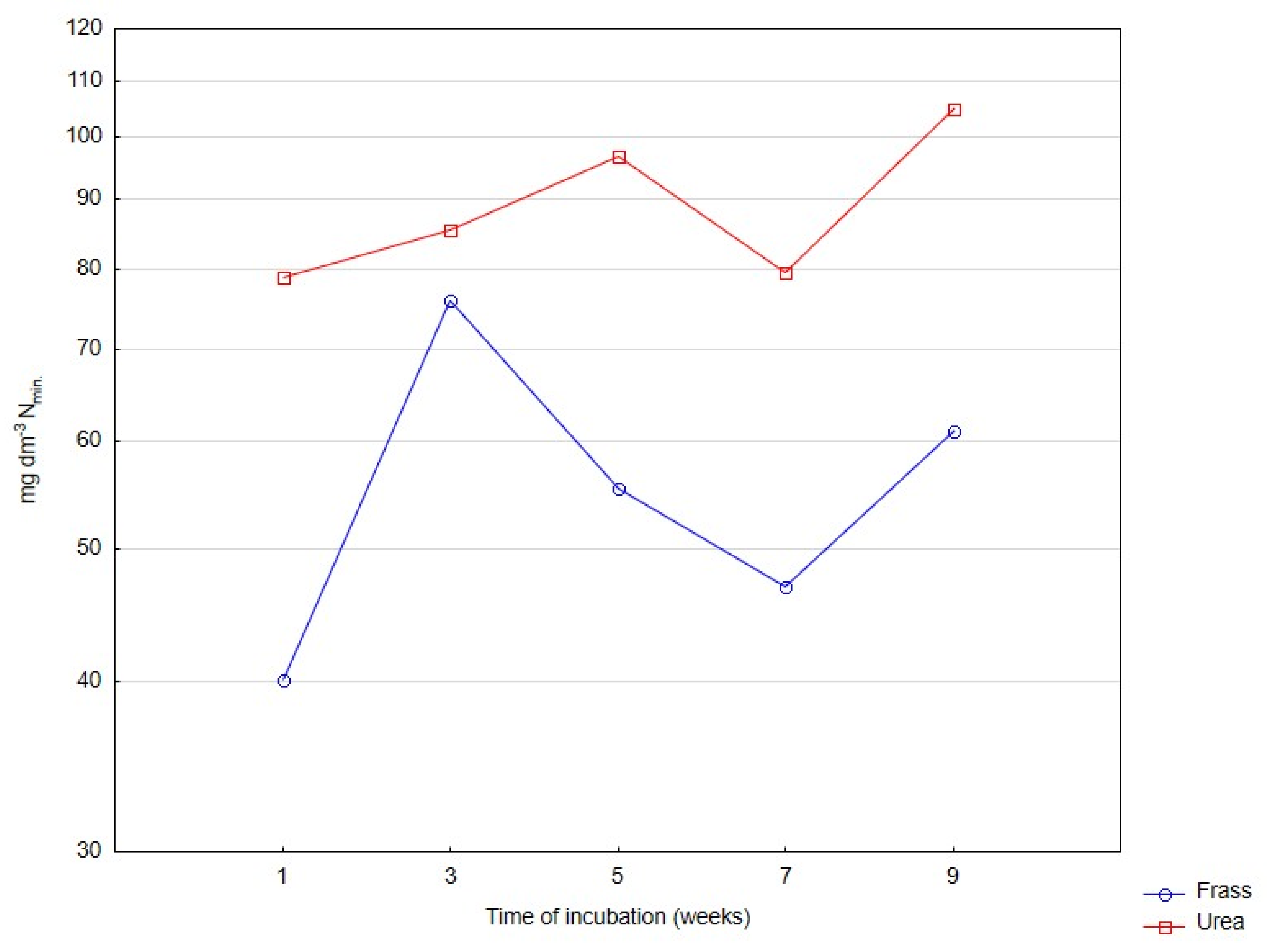
| Nmin | ||||||
| Frass | 10.01 a | 18.70 b | 31.80 c | 79.38 d | 139.34 e | 55.8 |
| Urea | 6.35 a | 34.67 b | 68.66 c | 106.28 d | 229.73 e | 89.1 |
| Significant | ns | * | ** | ** | **** | * |
| Mean | 8.18 A | 26.68 B | 50.23 C | 92.83 D | 184.54 E | |
| Fertilizer | Nitrogen Rate (mg dm−3) | Mean | ||||
|---|---|---|---|---|---|---|
| 0 | 50 | 100 | 200 | 400 | ||
| S-SO4 | ||||||
| Frass | 20.4 | 22.8 | 28.3 | 35.5 | 55.7 | 32.5 |
| Urea | 24.1 | 24.6 | 28.7 | 32.2 | 30.6 | 28.0 |
| Significant | ns | ns | ns | ns | *** | ns |
| Mean | 22.2 A | 23.7 A | 28.5 AB | 33.8 B | 43.1 C | |
| Cl | ||||||
| Frass | 13.2 | 17.6 | 20.4 | 21.2 | 24.9 | 19.5 |
| Urea | 13.0 | 17.1 | 18.6 | 18.5 | 21.5 | 17.7 |
| Significant | ns | ns | ns | ns | ns | ns |
| Mean | 13.1 A | 17.4 B | 19.5 C | 19.9 C | 23.2 D | |
| P | ||||||
| Frass | 19.6 | 23.7 | 32.7 | 50.8 | 91.1 | 43.6 |
| Urea | 23.2 | 22.6 | 21.7 | 25.5 | 28.2 | 24.2 |
| Significant | ns | ns | *** | **** | **** | **** |
| Mean | 21.4 A | 23.2 B | 27.2 C | 38.1 D | 59.7 E | |
| Ca | ||||||
| Frass | 1228 | 1239 | 1243 | 1149 | 1049 | 1182 |
| Urea | 1214 | 1128 | 1056 | 1019 | 937 | 1071 |
| Significant | ns | ns | * | ns | * | ** |
| Mean | 1221 E | 1183 D | 1149 C | 1084 B | 993 A | |
| Mg | ||||||
| Frass | 80.5 | 78.5 | 80.5 | 84.4 | 92.8 | 83.3 |
| Urea | 72.6 | 83.5 | 77.5 | 74.4 | 74.9 | 76.6 |
| Significant | ns | ns | ns | * | ** | ** |
| Mean | 76.5 A | 81. 0 B | 79.0 B | 79.4 B | 83.8 C | |
| K | ||||||
| Frass | 43.8 | 58.5 | 76.2 | 107.1 | 170.6 | 91.2 |
| Urea | 46.7 | 80.3 | 52.5 | 57.5 | 60.5 | 59.5 |
| Significant | ns | ns | * | *** | **** | **** |
| Mean | 45.3 A | 69.4 C | 64.4 B | 82.3 D | 115.6 E | |
| Na | ||||||
| Frass | 17.5 | 19.0 | 21.7 | 27.5 | 33.3 | 23.8 |
| Urea | 18.9 | 21.4 | 20.0 | 20.4 | 20.0 | 20.1 |
| Significant | ns | ns | ns | * | ** | * |
| Mean | 18.2 A | 20.2 B | 20.8 B | 23.9 C | 26.6 D | |
| pH | ||||||
| Frass | 5.68 | 5.60 | 5.70 | 5.54 | 5.52 | 5.61 |
| Urea | 5.72 | 5.54 | 5.52 | 5.54 | 5.46 | 5.56 |
| Significant | ns | * | ** | ns | * | * |
| Mean | 5.7 D | 5.57 B | 5.61 C | 5.54 B | 5.49 A | |
| EC | ||||||
| Frass | 0.10 | 0.15 | 0.23 | 0.54 | 0.95 | 0.40 |
| Urea | 0.11 | 0.22 | 0.38 | 0.65 | 1.07 | 0.49 |
| Significant | ns | * | **** | ** | * | * |
| Mean | 0.11 A | 0.19 B | 0.31 C | 0.59 D | 1.01 E | |
| Fertilizer | Nitrogen Rate (mg dm−3) | Mean | ||||
|---|---|---|---|---|---|---|
| 0 | 50 | 100 | 200 | 400 | ||
| Total bacteria | ||||||
| Frass | 8.61 b | 8.71 a | 8.69 a | 8.70 a | 8.71 a | 8.68 |
| Urea | 8.69 a | 8.63 ab | 8.61 ab | 8.57 b | 8.61 ab | 8.62 |
| Significant | *** | * | * | ** | ** | *** |
| Mean | 8.65 NS | 8.67 NS | 8.65 NS | 8.64 NS | 8.66 NS | |
| Total fungi | ||||||
| Frass | 11.44 d | 11.64 b | 11.56 c | 11.69 b | 11.86 a | 11.64 |
| Urea | 11.48 a | 11.48 a | 11.46 a | 11.43 ab | 11.40 b | 11.45 |
| Significant | ns | ** | ns | ** | **** | **** |
| Mean | 11.46 D | 11.56 B | 11.51 C | 11.56 B | 11.63 A | |
| Bacillus spp. | ||||||
| Frass | 6.91 d | 6.96 cd | 7.00 c | 7.05 b | 7.14 a | 7.01 |
| Urea | 6.90 a | 6.92 ab | 6.96 b | 6.88 c | 6.89 c | 6.91 |
| significant | ns | ns | ns | * | * | ** |
| Mean | 6.91 B | 6.94 B | 6.98 AB | 6.96 AB | 7.02 A | |
| Clostridium spp. | ||||||
| Frass | 7.87 b | 8.03 ab | 8.14 a | 8.00 ab | 8.12 a | 8.03 |
| Urea | 8.03 b | 8.15 a | 5.14 a | 7.98 b | 8.14 a | 8.09 |
| Significant | ns | ns | ns | ns | ns | ns |
| Mean | 7.95 C | 8.09 B | 8.14 A | 7.99 BC | 8.13 A | |
| Pseudomonas spp. | ||||||
| Frass | 6.63 c | 6.87 b | 7.04 a | 6.82 b | 6.37 d | 6.74 |
| Urea | 6.57 a | 6.45 a | 6.47 a | 6.19 b | 5.95 c | 6.33 |
| Significant | ns | * | ** | ** | ** | **** |
| Mean | 6.60 C | 6.66 B | 6.75 A | 6.51 D | 6.16 E | |
| chiA | ||||||
| Frass | 4.31 c | 4.37 bc | 4.42 b | 4.40 b | 4.63 a | 4.43 |
| Urea | 4.41 a | 4.32 b | 4.21 c | 4.27 c | 4.24 c | 4.29 |
| Significant | ns | ns | * | ns | *** | ** |
| Mean | 4.36 NS | 4.35 NS | 4.31 NS | 4.34 NS | 4.44 NS | |
| nifH | ||||||
| Frass | 7.46 c | 7.85 b | 8.00 a | 8.06 a | 7.92 ab | 7.86 |
| Urea | 8.18 c | 7.91 d | 8.27 b | 7.88 d | 8.40 a | 8.13 |
| Significant | ns | ns | ns | ns | ns | ns |
| Mean | 7.82 B | 7.88 B | 8.14 A | 7.97 AB | 8.16 A | |
| amoA | ||||||
| Frass | 5.32 d | 5.41 c | 5.56 b | 5.59 b | 5.67 a | 5.51 |
| Urea | 5.39 d | 5.42 c | 5.47 c | 5.56 b | 5.80 a | 5.53 |
| Significant | ns | ns | ns | ns | ns | ns |
| Mean | 5.36 C | 5.41 C | 5.51 B | 5.57 B | 5.73 A | |
| nosZ | ||||||
| Frass | 4.75 | 4.82 | 4.83 | 4.79 | 4.77 | 4.79 |
| Urea | 4.73 | 4.75 | 4.79 | 4.75 | 4.76 | 4.76 |
| Significant | ns | ** | ns | ns | ns | ** |
| Mean | 4.74 B | 4.78 AB | 4.81 A | 4.77 AB | 4.76 AB | |
| ureC | ||||||
| Frass | 6.80 ab | 6.84 a | 6.85 a | 6.82 a | 6.74 b | 6.81 |
| Urea | 6.80 a | 6.78 a | 6.78 a | 6.68 b | 6.71 b | 6.75 |
| Significant | ns | ns | * | ** | ns | ** |
| Mean | 6.80 A | 6.81 A | 6.82 A | 6.75 B | 6.73 B | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nogalska, A.; Przemieniecki, S.W.; Krzebietke, S.J.; Załuski, D.; Kosewska, A.; Skwierawska, M.; Sienkiewicz, S. The Effect of Mealworm Frass on the Chemical and Microbiological Properties of Horticultural Peat in an Incubation Experiment. Int. J. Environ. Res. Public Health 2023, 20, 21. https://doi.org/10.3390/ijerph20010021
Nogalska A, Przemieniecki SW, Krzebietke SJ, Załuski D, Kosewska A, Skwierawska M, Sienkiewicz S. The Effect of Mealworm Frass on the Chemical and Microbiological Properties of Horticultural Peat in an Incubation Experiment. International Journal of Environmental Research and Public Health. 2023; 20(1):21. https://doi.org/10.3390/ijerph20010021
Chicago/Turabian StyleNogalska, Anna, Sebastian Wojciech Przemieniecki, Sławomir Józef Krzebietke, Dariusz Załuski, Agnieszka Kosewska, Małgorzata Skwierawska, and Stanisław Sienkiewicz. 2023. "The Effect of Mealworm Frass on the Chemical and Microbiological Properties of Horticultural Peat in an Incubation Experiment" International Journal of Environmental Research and Public Health 20, no. 1: 21. https://doi.org/10.3390/ijerph20010021
APA StyleNogalska, A., Przemieniecki, S. W., Krzebietke, S. J., Załuski, D., Kosewska, A., Skwierawska, M., & Sienkiewicz, S. (2023). The Effect of Mealworm Frass on the Chemical and Microbiological Properties of Horticultural Peat in an Incubation Experiment. International Journal of Environmental Research and Public Health, 20(1), 21. https://doi.org/10.3390/ijerph20010021









